Journal of Animal Health and Production
Research Article
Tuberculosis Lesions of Bovine Carcasses in Algerian Municipal Abattoirs and Associated Risk Factors
Faїza Belakehal1*, Irmgard Moser3,4, Malek Naim2, Safia Zenia1, Taha-Mossadak Hamdi1
1Laboratory of Food Hygiene and Quality Insurance System, High National Veterinary School, Rue Issad Abbes, 16111 El Alia, Oued Smar, Algiers, Algeria; 2Department of Microbiology, Central Military Hospital, 16050 Kouba, Algiers, Algeria; 3Friedrich-Loeffler-Institut/Federal Research Institute for Animal Health, Institute of Molecular Pathogenesis, Naumburger Str. 96a, D-07743 Jena, Germany; 4National Reference Laboratory for Bovine Tuberculosis, at Friedrich-Loeffler-Institut/Federal Research Institute for Animal Health, Institute of Molecular Pathogenesis, Naumburger Str. 96a, D-07743 Jena, Germany.
Abstract | Bovine tuberculosis (bTB) is a transmissible disease of livestock with high economic consequences. To estimate the prevalence and risk factors associated to bTB, a study was conducted in Algerian abattoirs involving detailed inspection, microscopic examination, culture of tissue samples and molecular investigation. Out of 3848 bovine carcasses examined, 184 (4.78%) exhibited visible lesions suggestive of tuberculosis (TB). Anatomically 84.8% of the lesions were found in thoracic cavity. Lesions were predominant in tracheobronchial lymph nodes (71.7%), lung tissue (4.9%) and retropharyngeal lymph nodes (4.9%), followed by hepatic tissue (3.8%) and retrohepatic lymph nodes (3.8%). Less frequently, lesions were observed in prescapular lymph nodes (1.6%), digestive tract (0.5%) and kidney (0.5%). The study revealed statistically high (p<0.0001) infection rate in males (78.8%) than females (21.2%). A Significant difference (p<0.05) in TB infection rate was recorded between the three age/animal categories: 2 to 6 years (42.9%), <2 years (39.1%) and ≥6 years (17.9%). Analysis of data recorded in the present study showed a high significant variation (p<0.0001) in seasonal prevalence of bTB with a higher frequency during dry season than in rainy season. Microscopic examination of 105 samples revealed that 60 (57.1%) harbored acid-fast-bacilli (AFB) and 45(42.9%) were negative (p˃0.05). Culture yielded 60 (57.1%) positive, 43(41%) negative and only 2(1.9%) samples were contaminated. In PCR RD9 analysis, all isolated strains were belong to the Mycobacterium tuberculosis complex (MTC). The present findings can serve as background information to elaborate an adequate eradication program for bTB in Algeria.
Keywords | Bovine tuberculosis, Lesions, Slaughterhouses, Prevalence, Risk factor
Received | August 05, 2021; Accepted | September 05, 2021; Published | November 15, 2021
*Correspondence | Faiza Belakehal, Laboratory of Food Hygiene and Quality Insurance System, High National Veterinary School, Rue Issad Abbes, 16111 El Alia, Oued Smar, Algiers, Algeria; Email: [email protected]
Citation | Belakehal F, Moser I, Naim M, Zenia S, Hamdi TM (2021). Tuberculosis lesions of bovine carcasses in algerian municipal abattoirs and associated risk factors. J. Anim. Health Prod. 9(4): 479-486.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.479.486
ISSN | 2308-2801
Copyright © 2021 Belakehal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In Algeria, livestock production is an important part of agriculture, it account for a significant proportion of gross national agricultural income. Livestock farming is predominantly extensive (Bengouga et al., 2019) and mainly distributed in the North part of the country. Tuberculosis (TB) is an ancient disease, discovered in the relics of ancient Egypt, India and China (Good et al., 2018). In 1882, Robert Koch presented his findings in his famous lecture at the Berliner Physiologischen Gesellschaft in Berlin, where he described the isolation of the causative agent from human and animal samples. In developing countries, TB represents a major cause of organ condemnation according to meat inspection of cattle carcasses at slaughterhouses, after fasciolosis, hydatidosis and cysticercosis. Therefore, TB is of great economic importance in these countries (Addis, 2017). In Algeria, the animal TB status is not clear, due to the lack of an official data, in addition, the scale of zoonotic transmission is not well documented. Nevertheless, the cultural practices that exist could facilitate the elucidation of transmission between cattle and humans. Meat inspection in slaughterhouses play an important role to fight against bTB in Algeria. The aim of this study is to generate data on the prevalence of bTB in Algeria, through a risk factor study of abattoir records and thus to contribute to the knowledge on the epidemiology of the disease. This study can serve as a background information for guidelines for control measures.
Materials and methods
Postmortem inspection procedures
The TB detection was conducted by the abattoir assistant meat inspector, according to the guidelines recommended by the Algerian Ministry of Agriculture and Rural Development (MADRP). Inspection of carcasses for the presence of visible lesions involves palpation and incision into thin sections of lymph nodes (Ln). The cut surfaces were examined carefully for the presence of abscess, caseous mass and tubercles.
Collection of samples
The samples (n = 184) were collected from bovine carcasses (n = 3848) after meat inspection in four slaughterhouses, during the rainy season from October to March and the dry season from April to September. Samples of approximately 50g from each organ or Ln are randomly collected using a sterile single-use scalpel. These samples were placed in hermetically sealed, sterile, single-use vials and labeled with the age, the sex of animal and the collection date.
Ziehl-Neelsen acid fast staining and culture
Samples are transported to the Reference Laboratory of Mycobacteria and Control of Drug Resistance at Pasteur Institute of Algeria (IPA). From 184 positive carcasses a sample of 105 tissues lesions are examined by culture and direct smear microscopy using Ziehl-Neelsen staining for recording the presence of AFB. The tissue samples are decontaminated according to modified Petroff’s method (Tripathi et al., 2014). Briefly, an amount of 5ml of 4% NaOH solution was added to about 10g of tissue minced by a mortar in 4ml of sterile distilled water, and incubated for 15min. The suspension is neutralized by 10 ml of sterile distilled water and then centrifuged at 3000rpm for 20 min. The pellet was used as an inoculum to Löwenstein-Jensen as solid culture medium. The culture of mycobacteria was performed in a minimum of 8 to 12 weeks at 37°C (OIE, 2008).
Molecular investigation
Bacterial DNA is extracted using thermal lysis method following the protocol of Berg et al. (2009). The PCR was performed on 60 strains for the identification of Mycobacterium tuberculosis complex (MTC) members using oligonucleotide primers for detection of the RD9 deletion. Mycobacterium bovis (M. bovis) BCG (DSMZ 43990, ATCC 27289) and Mycobacterium tuberculosis (M. tuberculosis) H37Rv strains served as positive controls (Table 1).
Statistical analysis
The data obtained were entered into Microsoft Excel 2010 spread sheets and analyzed using the SPSS-Version 20.0. Chi-square test was used to analyze the data for disease occurrence and associated risk factors of sex, age and season. Interval of confidence is of 95% for the determination of prevalences and for the study of risk factors. P-value was held at <0.05 for significance.
Results
Results obtained are reported in tables 2 to 8. From 3848 inspected carcasses, 184 (4.78%), presented suspected bovine tuberculosis lesions (Table 2). Among 184 carcasses with visible TB lesions, 39 (21.2%) were females and 145 (78.8%) were males (Table 3). Out of 184 carcasses presenting visible lesions of TB, 72(39.1%) were less than 2 years old, 33(17.9%) were older than 6 years, and 79 (42.9%) were between 2 and 6 years old (Table 4).
TB suspected lesions were mainly detected in thoracic cavity, with high prevalence in tracheobronchial Ln 132 (71.7%) (Table 5). Lesions were also observed in some other Ln and organ tissues (Table 5).Below are some examples of bovine TB lesions encountered in slaughterhouses (Figure 1), showing pleura with solid spherical formations covered with yellowish nodules (Pearl disease) (Figure 1a), tracheobronchial Ln section with yellowish caseous consistence (Figure 1b), digestive tract showing TB lesion in the rumen wall (Figure 1c). Higher prevalence of TB suspected lesions were recorded during dry season 140(76.09%) (Table 6). Results of microscopic examination revealed that 60 samples were positive and 45 were negative (Table 7; Figure 2). Culture of mycobacteria indicate that 60/105 samples had characteristic colonies of M. bovis (Table 8). Molecular identification by PCR RD9 revealed that all 60 strains analyzed are members of MTC (Figure 3).
Table 1: Oligonucleotide primers used for molecular identification of mycobacteria isolates and sizes of the PCR products.
| Locus | Primer name | Primer sequence |
Present (M.tuberculosis) |
Deleted (M.bovis) |
| RD 9 | RD9_FlankF | AACACGGTCACGTTGTCGTG | 396 | 575 |
| RD9_FlankR | CAAACCAGCAGCTGTCGTTG | |||
| RD9_InternalR |
TTGCTTCCCCGGTTCGTCTG |
Table 2: Prevalence of bTB in four slaughterhouses from North Algeria
| Abattoir |
Cattle Examined (No.) |
Positive (No.) |
Mean (%) |
Prevalence (%) |
IC (%) |
p |
| Hussein Dey | 2207 | 98 | 53.3 | 4.44 | 0.46-0.60 | <0.0001 |
| El Harrach | 1167 | 62 | 33.7 | 5.31 | 0.26-0.40 | |
| Hadjout | 344 | 17 | 9.2 | 4.94 | 0.05-0.13 | |
| Dellys | 130 | 7 | 3.8 | 5.38 | 0.02-0.08 | |
| Total | 3848 | 184 | 25 | 4.78 |
0.04-0.05 |
IC: Interval of confidence is of 95%
Table 3: Prevalence of bTB with respect to sex
| Sex | Positive(No.) | Prevalence (%) | IC (%) | p |
| Female | 39 | 21.2 | 0.16-0.28 | |
| Male | 145 | 78.8 | 0.72-0.84 | <0.0001 |
| Total | 184 | 100 |
IC: Interval of confidence is of 95%
Table 4: Prevalence of bTB with respect to age
| Age | Positive(No.) | Prevalence (%) | IC (%) | p |
| < 2 years | 72 | 39.1 | 0.32-0.46 | <0.0001 |
| 2 to 6 years | 79 | 42.9 | 0.36-0.50 | |
| ≥6 years | 33 | 17.9 | 0.13-0.24 | |
| Total | 184 | 100 |
IC: Interval of confidence is of 95%
Table 5: Distribution of TB lesions in slaughtered cattle
| Localization | Positive(No.) | Prevalence(%) | IC (%) | p |
| Sternal Ln | 1 | 0.5 | 0.001-0.03 | <0.0001 |
| Retrohepatic Ln | 7 | 3.8 | 0.02-0.08 | |
| Pulmonary (Inspector) Ln | 4 | 2.2 | 0.01-0.05 | |
| Mediastinal Ln | 6 | 3.3 | 0.02-0.07 | |
| Prescapular Ln | 3 | 1.6 | 0.01-0.05 | |
| Retropharyngial Ln | 9 | 4.9 | 0.03-0.09 | |
| Tracheobronchial Ln | 132 | 71.7 | 0.65-0.78 | |
| Pleura | 4 | 2.2 | 0.01-0.05 | |
| Kidney | 1 | 0.5 | 0.001-0.03 | |
| Digestif tract | 1 | 0.5 | 0.001-0.03 | |
| Hepatic tissue | 7 | 3.8 | 0.02-0.08 | |
| Lung tissue | 9 | 4.9 | 0.03-0.09 | |
| Total | 184 | 100 |
IC: Interval of confidence is of 95%, Ln: Lymph node
Table 6: Prevalence of bTB with respect to season
| Season | Positive(No.) | Prevalence(%) | IC(%) | p |
| Dry | 140 | 76.09 | 0.7-082 | <0.0001 |
| Rainy | 44 | 23.91 | 0.17-0.30 | |
| Total | 184 | 100 |
IC: Interval of confidence is of 95%
Table 7: Prevalence of bTB in bovine carcasses analyzed by direct smear microscopy
| Microscopy | Prevalence(No.) | Prevalence(%) | IC(%) | p |
| Positive | 60 | 57.1 | 0.48-0.66 |
˃0.05 |
| Negative | 45 | 42.9 | 0.34-0.52 | |
| Total | 105 | 100 |
IC: Interval of confidence is of 95%
Table 8: Prevalence of bTB in bovine carcasses analyzed by culture technique
| Culture | Prevalence (No.) | Prevalence(%) | IC(%) | P |
| Contaminated | 2 | 1.9 | 0.01-0.07 | <0.05 |
| Positive | 60 | 57.1 | 0.48-0.66 | |
| Negative | 43 | 41.0 | 0.32-0.51 | |
| Total | 105 | 100 |
IC: Interval of confidence is of 95%
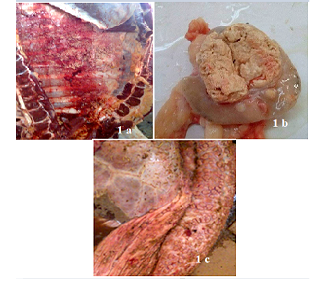
Figure 1: Bovine tuberculosis lesions discovered in slaughterhouses. Pleura with solid spherical formations covered with yellowish nodules (Pearl disease) (a), tracheobronchial Ln section with yellowish caseous consistence (b), digestive tract showing TB lesion in the rumen wall(c).
Discussion
Of 3848 bovine carcasses inspected, 184 (4.78%) presented suspicious lesions of bTB (Table 2). This prevalence is higher than those reported in Algeria by Sahraoui et al. in 2008 (3.58%) and Ayad et al. in 2020 (2.06%), however, it
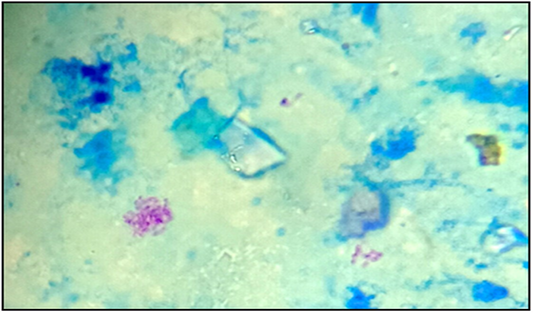
Figure 2: Acid-fast bacilli appeared as pinkish coccobacilli in a bluish background detected in lymph nodes from calve (x100).
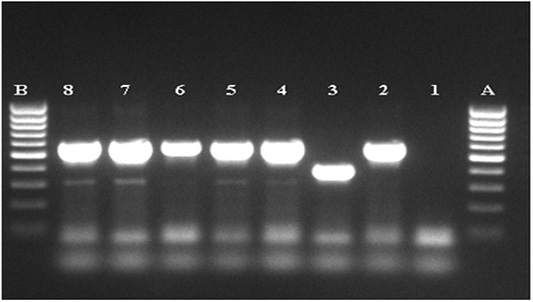
Figure 3: Agarose gel electrophoresis of the amplified MTC product. A, B: DNA marker (80-1000pb), 1: negative, control, 2: positive control M.bovis (BCG), 3: positive control M.tuberculosis (H37Rv), 4-8: MTC PCR product.
is lower than that recorded in a recent study by Damene et al. in 2020 (6.5%). Hamid et al. in 2019 in Morocco recorded a similar prevalence (4.6%). Several studies in Africa reported higher prevalences; including Pal et al. in 2017 (5.7%), Terefe, in 2014 (6.79%) and Aylate et al. in 2013 in Ethiopia (8.4%), Egbe et al. in 2016 in Cameroon (12-17%), Cleaveland et al. in 2007 in Tanzania (19.8%) and Atiadeve et al. in 2014 in Ghana (34%). This may indicate the enzootic nature of the disease in the prevalent country. In addition, the non-respect or the absence of concept of border barriers may explain the high prevalence of the disease in these countries.
Our results show a statistically significant difference (p<0.05) in infection rates between male and female carcasses, the highest rate recorded in males (78.8%) as compared to females (21.2%) (Table 3). Our results differ from those of Nalapa et al. (2017) (12.4%) in Uganda and Nuru et al. (2015) (87.9%) in Ethiopia, who reported that females had a higher risk of developing bTB-like lesions compared to males.
Our results show a significant difference in susceptibility to TB infection between the different categories of cattle: bulls, cows and calves (Table 4). A Significant difference (p<0.05) in rate was recorded among the three age categories of animals. Age was found to be related to the distribution of bTB reactors with low prevalence in younger age groups and high values in older ones (Munyeme et al., 2009). Whereas, in the present study, young (<2 years) and adult animals (2 to 6 years) seems to be more frequently infected by TB than older ones (≥6 years). This seemed to be due to the fairly regular flow of cattle sent out to slaughterhouse at the end of their productive cycle, on average at 5 years of age (Rossi et al., 2015). In contrast, the survey by Traoré et al. (2004), shows that a cattle aged ˃6 years are more reactive to TB than a younger one.
Patterns of Mekonnen et al. (2019), indicate that bTB reactors increase to a peak at 5 to 7 years of age. This increase could be linked to a longer exposure time to the M. bovis. Overall, the observation of lesion in calves could be explained by congenital transmission in utero. Pseudo-vertical transmission (like ingestion of contaminated colostrums) is often referred to the close contact between cows and their calves.
Calves and bulls are destined earlier for slaughter in order to cover the population’s demand for meat consumption, while females are kept until an advanced age for reproductive reasons and to exploit maximum milk production during their lifetime. Brooks-Pollock et al. (2014) showed that reactor rates to the comparative cervical test for bTB increased with age for the first 2-3 years for beef, but also for dairy cattle in Great Britain. In fact, this was expected for cow-calf herds, since calves spend most of their time with their dam before being weaned and moved away (Rossi et al., 2019).
Most of bTB like lesions were anatomically located in the thoracic cavity (84.8%) (Table 5, Figure 1a), mainly in respiratory tract. These were mainly in the tracheobronchial Ln (71.7%) (Figure 1b), lung tissue (4.9%), mediastinal Ln (3.3%), pleura (2.2%), pulmonary (Inspector) Ln (2.2%) and sternal Ln (0.5%). Another localization was observed in the liver tissue and associated (retrohepatic) Ln (7.6%) and retropharyngeal Ln (4.9%). The TB lesions were less noticed in the prescapular Ln (1.6%), in the digestive tract (0.5%) (Figure 1c) and in the kidney (0.5%).
The route of transmission determines the location and spectrum of lesions observed in bTB (Serrano et al., 2018). According to Michel et al. (2010), overall, the TB lesions most often occur in the lungs and the retropharyngeal, bronchial, mediastinal and mesenteric Ln. Our results are in agreement with those of Menzies and Neill (2000), in which TB lesions are observed mostly in thoracic cavity 57%. Similarly, Boukary et al. (2012) identified 92.77% of gross lesions in the lungs. Detection of lesions in the thoracic cavity (Ln) suggests that the respiratory tract is the entry point for M. bovis infection.
On the other hand, ingestion of M. bovis from contaminated pasture, feed, or water usually results in mesenteric Ln lesions (Menzies and Neill, 2000) and few or no visible lesions on the intestinal wall (Neill et al., 2001), which could explain the presence of this lesion in our study at a very low frequency (0.5%) (Figure 1c). The presence of lesions in the liver could be related to transplacental transmission to the fetus probably as a result of TB endometritis from the cow (Domingo et al., 2014). Corner et al. (1990) observed that the most frequently involved organ was the medial retropharyngeal Ln (29.4%), followed by mediastinal Ln (28.2%), tracheobronchial Ln (18.0%), lung tissue (8.0%), mesenteric Ln (2.9%), parotid Ln (2.4%) and cervical caudal Ln (2.4%). In summary, although the aerogenous route is the most common pathway of infection in both domestic ruminants and human beings and most cases of bTB involve the lungs and respiratory Ln (O’Reilly and Daborn, 1995), the oropharyngeal route is also a frequent pathway of entry that is probably more relevant than previously thought (Domingo et al., 2014).
Analysis of data recorded in the present study shows that bTB is present during all seasons, but with high frequency during dry season (p<0.05) particularly July and August (Table 6). According to Awah-Ndukum et al. (2010) from Cameroon, the detection of TB lesions is not influenced by season but were high during stressful periods such as inter season and peak-season periods. Boukary et al. (2012), Raufu and Ameh (2010) and, Sa’idu et al. (2017) from Nigeria reported that the frequency of lesions was higher at the beginning of the rainy season (July and August).
The high frequency of TB lesions during the dry season (76.09%), observed in our study, could be attributed to the massive destocking of animals by farmers during this season, and the high number of cattle presented for slaughter especially during summer which corresponds to the period of traditional celebrations and thus a high demand for meat.The period of winter concurs with the return of animals from transhumance. During the winter season, animals are confined to their farms because of the rains, thus, so close contact between animals promotes the transmission of TB infection. Animals are infected during the cold season and express the disease after a long incubation period during the dry season.
In current investigation, 60/105 (57.1%) samples tested were AFB positive by smear microscopy, 45 (42.9%) were negative (Table 7). The number of positive samples for AFB was greater than the negative ones with no significant difference (p˃0.05). This analysis demonstrates that Ziehl-Neelsen technique gives a good sensitivity for the detection of AFB in our samples.
Results of Aljameel et al. (2014) showed that out of 163 analyzed samples, 124 (76.1%) were smear positive while 39 (23.9%) were AFB negative. One of the explanation supplied could be related to the small number of samples examined in our study. Three modes of bacilli appearance are distinguished in the present study, most of them are single or grouped into two or three bacilli long filaments, resembling the M. tuberculosis species. Other forms, such as small shaped bacilli were observed, but rarely coccobacilli (Figure 2). The bacilli were usually pink colored. Nevertheless, conventional methods for TB detection using AFB microscopic staining are insensitive due to the potential confusion with other AFB genera such as Gordonia, Norcadia, Rhodococcus and Tsukamurella (Talip et al., 2013). From a total of 105 samples cultured, 60 were positives (57.1%), 43 were negative (41.0%) and 2 were (1.9%) contaminated (Table 8). The positive cultures (n=60) analyzed by PCR RD9, belonging to the MTC (Figure 3). Courcoul et al. (2015) study shows, that PCR is more sensitive and specific than bacteriology. This makes PCR a useful tool that could potentially become an official bTB diagnostic test in Algeria.
Conclusion
The post-mortem examination results revealed that the prevalence of bTB in Algeria is relatively low (4.78%). It is suggested that on the basis of this finding, the TB could not be underestimated, because in some cases lesions may not be sufficiently developed to be able to detect during routine inspection at the slaughterhouses. Gender, age and season were recognized as important risk factors associated with bTB infection. Males (78.8%) were found more susceptible to infection than females (21.2%); young (39.1%) and adult (42.9%) animals were more susceptible to infection than older one (17.9%); and dry season (76.09%) seems to be the most favorable period for the spread of the disease as compared to rainy season (23.91%). These results should interpolate the competent health authorities to adopt effective control strategies and appropriate measures for the prevention of bTB in Algeria, and development of laboratories specialized in the diagnosis of animal TB as well.
Acknowledgments
The authors are grateful to the veterinarians experts of the abattoir of Hussein Dey, El Harrach, Hadjout and Dellys; Dr Seyad F, Dr Boussakssou H, Dr Haouassine R, Dr Ismail S, Dr Baka A, Dr Kouidri A and Dr El Farci S, for their excellent support to perform the post mortem examination.
Conflict of interest
The authors declare no conflict of interest.
authors contribution
Conceptualization: FB, MN, TMH
Data curation: FB
Formal analysis: SZ, FB
Investigation: FB
Methodology: FB
Supervision: TMH, MN
Visualization: IM, FB
Writing – original draft: FB
FB conceived, designed the study and drafted the manuscript under the supervision of TMH and MN. FB designed the experiment protocol. FB collected and analyzed samples. FB and SZ performed the statistical analysis. FB revised the manuscript under the supervision of IM and TMH. All authors read and approved the final manuscript.
References






