Journal of Animal Health and Production
Research Article
Reproductive Efficiency, Milk Production, Health Status, Antioxidant Capacity, Lipid Profile, and Metabolic Hormones of Lactating Cows Treated with Coenzyme Q10 and L-Carnitine
Wafa W.M.*, H.A. El-Nagar
Animal Production Research Institute, Agricultural Research Center, Giza, Egypt.
Abstract | The present study aimed to investigate the effect of Coenzyme Q10 (CoQ10) and L-carnitine (LC) on the reproductive performance, milk production, and health status of lactating cows. Multiparous Friesian cows (n=21) with 2-4 parities at late pregnancy were divided into three groups (7 in each). Cows in the first group were fed a basal ration (control, G1), while those in G2 and G3 were fed the same diet and received an oral dose of 1.5 mg CoQ10 and 100 mg LC per kg BW, respectively. Treatment lasted for 30 d prepartum to 120 d postpartum. Results show that LC increased (P<0.05) daily milk yield, body weight of dams and their calves at calving, serum total proteins, albumin, globulin, glucose, total lipids, total cholesterol, triglycerides, triiodothyronine (T3), insulin-like growth factor-I (IGF-I), insulin, glutathione (GSH), and superoxide dismutase (SOD) activity, erythrocyte, and leukocyte counts, as well as hemoglobin and packed cell volume (PCV) concentration. On the other hand, placental drop, uterine involution, days to cervical closer, postpartum 1st estrus interval, service period and the number of services/conception, days open, calving interval, serum cortisol, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were decreased (P<0.05) by LC treatment. Treatment of CoQ10 followed the beneficial effects of LC treatment on most parameters studied. In conclusion, oral pre-and post-partum administration of LC (100 mg/kg BW) may use as a useful tool for improving milk production and reproductive efficiencies, energy metabolism, and antioxidant status of lactating cows during the early postpartum period.
Keywords | Metabolic agent, Energy metabolism, Lactating cattle, Milk, Reproduction.
Received | May 17, 2021; Accepted | May 21, 2021; Published | September 25, 2021
*Correspondence | Wafa WM, Animal Production Research Institute, Agricultural Research Center, Giza, Egypt; Email: [email protected]
Citation | Wafa WM, El-Nagar HA (2021).Reproductive efficiency, milk production, health status, antioxidant capacity, lipid profile, and metabolic hormones of lactating cows treated with coenzyme q10 and l-carnitine. J. Anim. Health Prod. 9(4): 380-390.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.380.390
ISSN | 2308-2801
Copyright © 2021 Wafa and El-Nagar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Reproductive regularity is the most important factor affecting the productive performance of dairy cattle. Improving cow reproductive efficiency can achieve the main goal of dairy herd breeders for getting healthy calves every year. Nutrition, management and climate are the main factors affecting the reproduction of dairy cows. Calving interval reduction can increase the yearly income of the herd (Shamsuddin et al., 2006). The transition period in dairy cows is a critical period because the high increase in energy requirement for milk production beginning while the feed intake is inadequate to confront the negative energy balance (LaCount et al., 1995). Also, the calving process induce stress on cows leading to reduction in ration consumption and energy intake (Hydbring et al., 1999).
At post-partum, the pancreatic insulin production decrease followed by reducing in glucose utilization (Drackley et al., 2001). In addition, the mammary gland require more glucose to produce milk (Komatsu et al., 2005). Energy lack induces mobilization in body fat to recovery this deficiency. In this case, the liver cannot metabolize this fat and ketone production exceeds the utilized then ketosis occurs (Grummer, 1993). Treatment of dairy cows with L-carnitine (LC) decreased liver triglycerides accumulation as a sign of lipid and energy adjustment (Carlson et al., 2007).
The LC is an important substance (vitamin-like) that can be synthesized in animal body from lysine and methionine by the metabolic process in the liver and kidneys. It had a vital role in cell mitochondria energy produce (Greenwood et al., 2001). Therefore, LC has an important function by transport the medium-, short- and long-chain fatty acids through the mitochondria membrane in the oxidation process that strengthening animal immunity, improving antioxidant status and increasing reproductive performance (Pirestani et al., 2009).
The redox-active quinone Coenzyme-Q (CoQ) had numerous isoprene units the predominant one in bovine is CoQ10 (Lass et al., 1997). The major source of intracellular O2 in the cell is autoxidation of CoQ10 in mitochondria (Giulivi et al., 1995). The CoQ10 is found in the inner mitochondria membrane to help mitochondria in energy production via electron transport mechanism (Bentov et al., 2014; Balercia et al., 2017; Shukla and Dubey, 2018). CoQ10 had an important role in the process of ATP production in mitochondria based on its antioxidant properties that can control the cellular redox (Quinzii et al., 2010). It has beneficial effects on improving the health of many diseases like obesity, diabetes, and aging (Sohet et al., 2009), and antioxidant properties (Showell et al., 2013) to prevent the reproduction process from free oxygen radicals, which causes obstructed in oocyte maturation, embryo development and lower embryo viability (Caamaño et al., 1996; Rakhit et al., 2013). Generally, the positive impact of CoQ10 on the pregnancy rate was indicated by Bentov et al. (2014). In recent years, CoQ10 was indicated to use as a food supplement because of its two functions as a key role in mitochondrial bioenergetics and as an antioxidant (Littarru et al., 2017).
The present study aimed to investigate if treatment of dairy cows with CoQ10 or LC, as metabolic agents, can improve the reproductive performance, milk production, and health status of lactating cows during the postpartum period.
MATERIALS AND METHODS
This study achieved the EU standards for the protection of animals used for scientific purposes and feed legislation (2010/63/EU). The experimental work of this study was conducted in El-Gemmeza Animal Production Experimental Station in the middle Delta of Egypt belonged to Animal Production Research Institute (APRI), Agricultural Research Center, Ministry of Agriculture and Land Reclamation.
Animals, feeding system, and experimental design
Twenty-one multiparous Friesian cows (2-4 parity) at late pregnancy with an average body weight of 493±20.1 kg were used in this experiment. All animals were housed in semi-open sheds. At 30 days before the expected parturition date, the animals were divided to three experimental groups (7 in each).
Cows in the first group were fed on a basal diet without treatment (G1), while those in the second group (G2) were fed the same diet, and orally treated with Coenzyme Q10 (CoQ10, Mepaco-Medifood, Enshas El Raml, Sharkeia, Egypt) at a level of 1.5 mg/kg BW (Shoukheba and Elgendy, 2016). The CoQ10 might not be degraded by ruminal microbes and thus might have the potential to be by-passed to the lower digestive tract and be absorbed (Bae et al., 2018). Cows in the third group (G3) were fed the same diet, but orally treated with L-Carnitine (LC, Mepaco-Medifood, Enshas El Raml, Sharkeia, Egypt) at a level of 100 mg/kg BW according to Pirestani et al. (2009).
The basal diet contained 7 kg concentrate feed mixture (65% un-corticated cotton seed cake, 9% wheat bran, 20% rice polish, 3% molasses, 2% limestone, and 1% common salt), 2 kg berseem hay (BH; 2nd cut) and 3 kg rice straw as a basal diet without treatment and served as a control group. The chemical composition of different feedstuffs on DM basis is presented in Table 1. Cows were fed twice daily at 8 a.m. and 4 p.m., while clean drinking water was available all day times. Amounts of feeds were weekly adjusted according to milk yield and reproductive stage.
The handling and management of animals were conduct according to the Directive 2010/63/EU for animal protection which used for scientific purposes (Official Journal of the European Union, 2010).
Experimental procedures
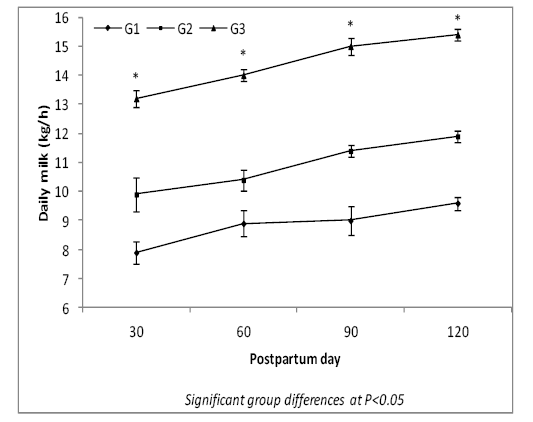
Cows in all experimental groups were milked twice daily at 5 a.m. and 5 p.m. using a milking machine. Average daily milk yield was recorded for morning and evening milking during the treatment period up to 120-day postpartum
Reproductive traits
After calving, uterine involution in terms of symmetric uterine horns and cervical closer was conducted by rectal palpation from 7 days of parturition until the occurrence of symmetric uterine horns and cervical closer. Estrus was detected twice daily and cows that exhibited estrus were inseminated. Pregnancy was diagnosed by rectal palpation after 50 days of insemination. After calving, cows and newborn calves were weighted and dams were monitored to determine the duration of fetal membranes drop.
Table 1: Chemical composition on DM basis (%) of different feedstuffs of the basal ration.
| Item | DM | OM | CP | CF | EE | NFE | Ash |
| CFM | 90.63 | 90.65 | 17.04 | 9.21 | 3.11 | 61.09 | 9.35 |
| Rice straw | 92.14 | 81.71 | 3.42 | 36.13 | 1.28 | 40.88 | 18.29 |
| Berseem hay | 90.89 | 83.93 | 14.50 | 26.82 | 2.74 | 39.87 | 16.07 |
DM: Dry matter OM: Organic matter CP: Crude protein CF: Crude fiber
EE: Ether extract NFE: Nitrogen free extract CFM: Concentrate feed mixture
Blood samples
Using jugular venipuncture, two blood samples were collected from all animals in each group before morning feed ing at 90 day-postpartum. The first sample (5 ml) with EDTA, as an anticoagulant, was used for hematological parameters examination including count of red blood cells (RBCs) and white blood cells (WBCs) and its differentiation, hemoglobin (Hb) concentration, and percentage of packed cell volume (PCV). Hematological parameters were determined by using a veterinary haematology analyzer (Exigo, Boule medical AB., Sweden).
The second blood samples (5 ml) without anticoagulant were left for 2-3 h and centrifuged at 4000 rpm for 15 minutes then serum was harvested and stored frozen at -20ºC until analyzed for biochemicals, enzymes, and hormones.
Analytical methods
Blood serum of cows was analyzed for concentrations of total proteins (Henry, 1964), albumin (Doumas et al., 1997), total lipids (Zöllner and Kirsch, 1962), total cholesterol (Richmond, 1973), triglycerides (McGowan et al., 1983), and glucose (Trinder, 1969) by using commercial kits (Nanjing Jiancheng Biochemical Reagent Co., China). Globulin concentration was calculated by subtraction of albumin from total proteins concentration. Serum concentration of insulin, cortisol, insulin-like growth factor-I (IGF-I), triiodothyronine (T3) and thyroxin (T4), estradiol-17β (E2) and progesterone (P4) were estimated by radioimmunoassay (RIA) using commercial kits (Coat-A-Count® Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). The activities of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assessed according to Reitman and Frankel (1957). Antioxidant status was determined as glutathione (GSH) and superoxide dismutase (SOD) levels by the method of Prins and Loos (1969) and Madesh and Balasubramanian (1998), respectively.
Statistical analysis
Data were recorded using Excel software and statistically analyzed by one-way analysis of variance to evaluate the effect of treatment using IBM SPSS statistical program version 25 in a completely random design. The significant differences were checked using Duncan’s multiple range test (Duncan, 1955).
RESULTS
Milk yield
Results illustrated in Figure-1 show that the daily milk yield of cows was higher (P<0.05) in treatment groups (G2 and G3) than in G1 (control) during four lactation months. Also, daily milk yield was higher (P<0.05) in G3 than in G2 at different lactation months.
Reproductive performance
Data of reproductive performance parameters presented in Table 2 clear significant (P<0.05) improvement in body weight of dams and their calves at birth in G3, as compared to G1 and G2. Body weight of calves at birth in G3 increased by 20.7 and 5.5% compared with G1 and G2, being in association with LBW of their dams.
The reproductive performance parameters including duration of fetal membranes drop, uterine horn symmetry, uterine involution, days to cervical closer, postpartum 1st estrus interval, service period and the number of services per conception, days open, and calving interval significantly (P<0.05) decreased in G2 and G3 as compared to G1, being the lowest in G3. During the 1st 90-day postpar tum period, the conception rate was significantly (P<0.05)
Table 2: Effect of treatments on reproductive performance parameters of lactating cows.
| Item | G1 (control) | G2 (CoQ10) | G3 (LC) |
| Cow body weight at calving (kg) |
463.86±13.54b |
478.57±12.52b |
537.14±13.18a |
| Body weight of born calves (kg) |
33.72±1.87b |
38.57±2.15ab |
40.70±2.09a |
| Fetal membrane drop (h) |
7.29±0.79a |
4.71±0.47b |
4.07±0.40b |
| Uterine horn symmetry (d) |
37.57±2.76a |
31.00±2.89b |
22.29±2.69c |
| Uterine involution (d) |
28.00±0.49a |
22.71±0.68b |
16.71±0.60c |
| Cervical closer (d) |
34.43±1.13a |
27.86±1.01b |
24.14±1.70b |
|
Postpartum 1st estrus interval (d) |
66.14±1.16a |
42.86±1.26b |
35.29±1.12c |
| Service period length (d) |
36.14±3.79a |
9.14±4.31b |
3.14±3.14b |
| Number of services/conception |
2.14±0.34a |
1.43±0.20ab |
1.14±0.14b |
| Days open |
102.29±4.31a |
52.00±3.82b |
38.43±2.37c |
|
Conception rate# |
28.6b |
100a |
100a |
| Calving interval (d) |
379.43±5.14a |
327.57±5.80b |
312.29±2.93c |
Means in the same raw with different superscripts are significantly different at P<0.05.
# Conception rate of cows during a postpartum period of three months.
Table 3: Effect of treatments on some biochemical variables in blood serum of lactating cows.
| Item | G1 (control) | G2 (CoQ10) | G3 (LC) |
| Total proteins (g/dl) |
6.24±0.06c |
6.82±0.03b |
8.04±0.12a |
| Albumin (g/dl) |
3.34±0.07b |
3.48±0.05b |
4.39±0.12a |
| Globulin (g/dl) |
2.90±0.02c |
3.34±0.02b |
3.65±0.01a |
| Albumin/globulin ratio | 1.15±0.04 | 1.04±0.02 | 1.20±0.03 |
|
Glucose (mg/dl) |
65.11±1.20c |
72.10±1.53b |
80.74±1.61a |
| Total lipids (mg/dl) |
550.43±9.64c |
600.86±9.39b |
872.14±8.04a |
| Total cholesterol (mg/dl) |
125.14±6.24b |
127.29±7.16b |
158.44±8.69a |
| Triglycerides (mg/dl) |
13.27±0.05c |
14.43±0.04b |
15.93±0.03a |
Means in the same raw with different superscripts are significantly different at P<0.05.
higher in cows of both treatment groups (100% for each) than in the control (28.6%).
Concentrations of blood biochemicals
The effect of treatment on all serum biochemicals studied was significant (Table 3). Concentrations of total proteins, globulin, glucose, total lipids, and triglycerides were higher (P<0.05) in treatment groups (G2 and G3) than in G1. Albumin and total cholesterol concentrations were higher (P<0.05) in G3 than in G1 and G2. Albumin/globulin ratio was nearly similar in all groups indicating a similar trend in the level of albumin and globulin in each group.
Hormonal profiles
The effect of treatment was significant on concentrations of T3, insulin, cortisol, IGF-1, and E2, and non-significant on T4 and P4 in the serum of cows (Table 4). Treatment of LC in G3 increased (P<0.05) concentrations of T3, insulin, IGF-1, and E2, while decreased (P<0.05) cortisol concentration as compared to control (G1). Treatment of CoQ10 in G2 increased (P<0.05) IGF-1 and E2, while decreased cortisol as compared to G1. The significant increase observed in insulin level in G3 was associated with marked elevation in IGF-1 and reduction in cortisol. These results indicated a remarkable effect of LC treatment rather than CoQ10 on the level of metabolic hormones. On the other hand, the concentration of E2 was higher (P<0.05) in G2 and G3 than in G1.
Enzyme activity and antioxidant status
The effect of treatment on the activity of AST and ALT as well as levels of glutathione (GSH) and superoxide dismutase (SOD) was significant (Table 5). The activity of AST and ALT decreased (P<0.05) in G3 as compared to G2 and G1, while levels of GSH and SOD improved in G2 and G3 as compared to G1. The levels of GSH and SOD were higher (P<0.05) in G3 than in G2.
Hematological parameters
The effect of treatment on the count of red blood cells (RBCs) and white blood cells (WBCs), the concentration of hemoglobin (Hb), and value of packed cell volume (PCV) was significant. However, erythrocytic indices (MCV, MCH, and MCHC) and leukocytic indices (neutrophil, lymphocytes, monocytes, eosinophils, and basophils) were not affected significantly by treatments (Table 6). Count of RBCs and WBCs, and Hb increased (P<0.05) in G3 than in G2 and G1, while PCV value increased in G2 and G3 as compared to G1. The PCV value was higher (P<0.05) in G3 than in G2.
Table 4: Effect of treatments on concentrations of some hormones in blood serum of lactating cows.
| Item | G1 (control) | G2 (CoQ10) | G3 (LC) |
| Metabolic hormones | |||
|
T3 (ng/ml) |
1.82±0.05b |
1.96±0.08b |
2.17±0.06a |
|
T4 (µg/dl) |
6.09±1.04 | 6.63±1.01 | 7.10±1.09 |
| Insulin (µU/L) |
0.20±0.03b |
0.25±0.05b |
0.51±0.06a |
| Cortisol (mg/dl) |
2.17±0.04a |
1.96±0.05b |
1.61±0.07c |
| IGF-1 (ng/ml) |
106.00±3.24c |
121.57±3.46b |
154.29±4.34a |
| Reproductive hormones | |||
|
P4 (ng/ml) |
0.57±0.04 | 0.60±0.07 | 0.63±0.08 |
|
E2 (pg/ml) |
5.15±0.19b |
5.86±0.16a |
6.04±0.14a |
Means in the same raw with different superscripts are significantly different at P<0.05.
Table 5: Effect of treatments on enzyme activity and antioxidant status in serum of lactating cows.
| Item | G1 (control) | G2 (CoQ10) | G3 (LC) |
| Aspartate aminotransferase, AST (IU/L) |
59.29±2.19a |
56.29±2.13a |
44.86±2.25b |
| Alanine aminotransferase, ALT (IU/L) |
20.14±0.80a |
19.29±0.52a |
15.14±0.63b |
| Glutathione, GSH (mg/dl) |
6.94±0.60c |
9.01±0.44b |
13.40±0.51a |
| Superoxide dismutase, SOD (mg/dl) |
10.36±0.39c |
13.40±0.72b |
17.26±0.60a |
Means in the same raw with different superscripts are significantly different at P<0.05.
Table 6: Effect of treatments on hematological parameters of lactating cows.
| Item | G1 (control) | G2 (CoQ10) | G3 (LC) |
|
RBC (x106/mm3) |
7.96±0.23b |
8.81±0.44ab |
9.77±0.31a |
| Hb (g/dl) |
9.91±0.39b |
10.34±0.46b |
11.59±0.32a |
| PCV (%) |
31.50±0.96c |
34.79±1.20b |
38.64±0.80a |
| MCV (FL/cell) | 39.98±2.35 | 40.32±3.11 | 39.71±1.19 |
| MCH (Pg/cell) | 12.52±0.59 | 11.99±1.03 | 11.89±0.36 |
|
MCHC (g/dl) |
31.65±1.60 | 29.71±0.75 | 30.06±1.05 |
|
WBC (x103/mm3) |
7.53±0.59b |
8.21±0.74ab |
9.11±0.49a |
| Neutrophil (%) | 29.66±1.92 | 26.29±2.86 | 23.97±2.19 |
| Lymphocytes (%) | 65.81±2.05 | 68.36±3.02 | 71.70±2.49 |
| Monocytes (%) | 2.96±0.18 | 3.50±0.30 | 2.73±0.29 |
| Eosinophils (%) | 1.19±0.19 | 1.46±0.20 | 1.16±0.15 |
| Basophils (%) | 0.39±0.02 | 0.40±0.01 |
0.44±0.03 |
Means in the same raw with different superscripts are significantly different at P<0.05.
DISCUSSION
In accordance with the obtained results of lactating cattle daily milk yield, Abou El- Ela et al. (2017) indicated that dietary supplementation of L-Carnitine (LC) and Coenzyme Q10 (CoQ10) significantly increased milk production of goats in terms of marked improvement in milk yield and composition. They indicated that LC treatment produced more milk than those treated with CoQ10, but the difference was not significant. Moreover, Abu El Ella et al. (2014) reported similar results on Zaraibi females treated with L-tyrosine. The improvement observed in milk yield of LC group may be due to improved blood supply of mammary glands and energy sources (Mepham, 1982; Wurtman, 1982) or/and is in association with increasing body weight and body condition score of lactating cows. Generally, LC and CoQ10 treatments were reported to have a positive effect on improving cow performance by increasing insulin hormone (Ruggenenti et al., 2009) as proved in our study.
The results of reproductive performance parameters were in agreement with Ramanau et al. (2004) who found an increase in body weight of piglets born from saws treated with LC during pregnancy and postpartum, and this may be attributed to the influence of LC on the metabolism of glucose under IGF-1 secretion and protein turnover (Vaz and Wanders, 2002). In dairy Holstein cows, Pirestani et al. (2011) indicated that treatment with LC from 7 days prepartum until 4 wk postpartum significantly decreased days open. Abou El-Ela et al. (2017) indicated that the fertility rate of Damascus goat does treated with LC increased to 130% compared to 70% in non-treated goats.
During postpartum period of dairy cows, the negative energy balance inhibits LH secretion then decreased glucose and insulin concentration that affected directly on the growth and development of ovarian follicles (Butler, 2000). The significant (P<0.05) improvement observed in most reproductive parameters of cows treated with CoQ10 in our study was reported by several authors. In this way, Abou El-Ela et al. (2017) found that the fertility rate was 100 and 70% in Damascus goat does treated with CoQ10 and controls, respectively. Improving the reproductive efficiency of cows treated with CoQ10 may be attributed to increased CoQ10 concentration in reproductive tissues that induced ATP production from cell mitochondria (Conley et al., 2007). In this respect, several studies indicated a significant increase in ATP production in genitalia tissue by CoQ10 supplementation (Ozcan et al., 2016). The addition of CoQ10 in oocyte media improved their development by increasing mitochondrial ATP production (Gendelman and Roth, 2012). Also, CoQ10 had an important role in folliculogenesis, embryo fertilization, and embryo quality (Caso et al., 2007). Supplementation of CoQ10 to the in vitro culture of bovine embryos increased the rates of early embryo cleavage, blastocyst formation, hatching, the expanding blastocysts, and the size of the inner cell mass. These results were attributed to increased ATP production in the treated group (Stojkovic et al., 1999). In humans, a significant relationship was found between CoQ10 level in the follicular fluid and each of oocyte fertilization and embryo grade (Turi et al., 2012). In rats, supplementation of CoQ10 can restore oocyte mitochondrial gene expression, which improved the mitochondria activity then the treated mice produced more oocytes with good developmental potential that increased the number of born offspring compared to non-treated old animals (Ben-Meir et al., 2015).
Generally, the supplementation of amino acids was reported to improve dairy cow reproductive performance. In this respect, Stevenson et al. (1997) indicated that tyrosine supplementation reduced the first postpartum ovulation interval of dairy cows. Also, El-Amrawi, et al. (1992) recorded that the treatment of buffalo heifers with a single dose of L-tyrosine decreased the interval of the 1st estrus after treatment (3-4 days). Each of LC and CoQ10 as metabolic agents may increase ATP production. In this context, Conley et al. (2007) reported that the increase in ATP production in mitochondria of different cells can improve the energy balance of postpartum dairy cows. This improvement in energy balance led to reducing the interval from parturition to uterine involution complementation and rapid the onset of the ovarian cycle then decreased the number of services per conception and subsequently reduced the service period (Tyagi et al., 2010).
The blood biochemistry contents could be used as indicators for animal health status and productive status as mentioned by Puppel and Kuczyńska (2016). The overall values of blood biochemicals recorded in this study for all groups are within the normal ranges of lactating cows. However, the obvious increase in blood metabolites in G2 and G3 may indicate additional effects of CoQ10 and LC on the metabolism of protein, carbohydrates, and lipids of lactating cows. In this respect, the dietary addition of LC and CoQ10 increased blood total proteins and albumin concentrations in lactating goats (Abou El-Ela et al., 2017) and rats (Ali et al., 2010). This increase may be an indicator to the increase the synthesis of protein under the regulation of anabolic hormones (EI-Masry and Habeeb, 1989; Abou El-Ela et al., 2014). Also, there was a clear increase in blood total proteins concentration in animals treated with CoQ10 which may indicate improvement in the animal health (Dawood et al., 2016). However, Jain and Singh (2015) reported no effect for LC treatment on blood proteins concentration in Albino rats. Lee et al. (2016) recorded a significant increase in blood cholesterol of LC treated cows, while in humans, Huang et al. (2013) found no effect of LC treatment was observed on blood cholesterol or triglycerides. Mohamed et al. (2015) noticed a decrease in blood triglycerides with increasing the amount of milk production which required triglycerides withdrawal by the mammary gland for synthesis the milk fat (Mantovani et al., 2010). It is of interest to note that improving milk production in both treatment groups was associated with increasing triglycerides level in blood serum. This finding may indicate the positive role of LC and CoQ10 on lipid profile and energy metabolism. In this context, CoQ10 plays an important role in the Krebs cycle of carbohydrates, protein, and lipids metabolism (Shukla and Dubey, 2018). The positive role of CoQ10 on improving animal performance is in relation to CoQ10 effects on insulin, glucagon, and cortisone hormones (Henriksen et al., 1999). CoQ10 is an important to energy consumption (Rosenfeldt et al., 2003), and acts a main role in bio-energy (Littarru, 1994). Also, it has an important role in controlling oxidation-reduction in the cell (Kaltschmidt et al., 1999), and acts as a key element in the chain of oxidative phosphorylation through ATP production as well as its property as an antioxidant (Mancini et al., 2011).
The thyroid hormones play an important role in ketosis because of their low levels in dairy cows with decreasing energy metabolism during the transition period (Djokvic et al., 2014). In dairy cattle, there is a relationship between blood thyroid hormone levels and the energy balance (Cassar-Malek et al., 2001), and thyroid hormones have an inverse correlation with CoQ10 levels (Bianchi et al., 1999). Although the present results indicated an insignificant effect of CoQ10 on T3 and insulin as compared to control, Mancini et al. (2005) reported high T3 level with the low concentration CoQ10 in the blood. In fish, El Basuini et al. (2020) found that dietary CoQ10 supplementation decreased blood glucose (an indicator of increasing insulin level, Modi et al., 2006) and cortisol levels, which are in accordance with the obtained results in G2. It is clear to note that, LC and CoQ10 treatments had positive effects on improving cow performance by increasing insulin hormone (Ruggenenti et al., 2009) as proved in cows treated with LC in our study. General, the average of cow blood cortisol in the presented study was in the normal range (1-10 mg/dl) as described by Hopster et al. (2002), but the observed reduction in cortisol profile in G2 and G3 may reflect more welfare of treated cows than the control ones. These results may suggest that LC may acts as an anti-stress factor.
The significant reduction in AST activity in cows treated with LC in the present study is in agreement with the effect of LC on decreasing the activity of blood AST in newborn buffaloes (Gabr, 2020), and rats (Ali et al., 2010). Although Sohet et al. (2009) indicated decreased inflammatory stress in liver of mice received an oral dose of CoQ10 and this may be in association with a slight reduction in AST and ALT activities in cows treated with CoQ10 in or study. Also, Castaneda-Gutierrez et al. (2009) indicated no injury in hepatic cells was observed due to insignificant reduction of ALT or AST activities in dairy cows during the postpartum period. These findings may reflect the absence of toxicity in cows treated with LC or CoQ10.
Incomparable with the present results, Abou El-Ela et al. (2017) observed that the activity of AST could be increase as a response to LC or CoQ10 supplementation during the transition period, while ALT activity was not affected by goat does treatments.
The increase of reactive oxygen species (ROS) which was attributed to a decrease in its disposal led to an increase in animal oxidative stress (Castro and Tafalla, 2015). It is clear to note that SOD is one of the most important antioxidant defenses that had a major role in ROS withdrawal from the cell (Aruoma, 1998). In this respect, CoQ10 has antioxidant potential and acts as a free radical scavenger that can improve animal health status (Varela-López et al., 2016). In addition, LC has the same antioxidants potential as CoQ10 (Bloomer et al., 2009), and can increase body energy balance. In ruminants, animals expose to very stressful periods during pregnancy and lactation due to high levels of oxidative damage, so they want to consume more enzymatic and non-enzymatic antioxidants (Castillo et al., 2005). Both LC and CoQ10 treatments increased the activity of blood GSH and SOD, as enzymatic antioxidants. Similarly, Al-Hassan et al. (2016) and Abou El-Ela et al. (2017) indicated that LC or CoQ10 supplementation could increase the concentration of total antioxidants in blood compared to control lactating goats. Also, Cigliano et al. (2014) found a significant increase in the activity of GSH and SOD in the blood plasma of lactating cows. Liu et al. (2016) found that daily intake of CoQ10 at a level of 300 mg improved the antioxidant capacity in hepatocellular carcinoma patients which indicated that CoQ10 can alleviate the high oxidative stress levels.
It is of interest to note that both treatments had positive effects on erythrocytic indices, but the effect of LC was more pronounced than CoQ10. Increasing RBCs count was associated with a marked increase in Hb, and PCV. On the other hand, the CoQ10 treatment had a tendency of improving erythrocytic indices as compared to the control, but the differences were not significant. In contrast, Gabr (2020) indicated that the treatment of buffalo calves with CoQ10 or LC had no effect on blood hematological parameters. The observed increase in WBCs count by LC in our study was reported by Chen et al. (2008), who observe an increase in the count of WBCs when LC was included in diet of finishing pigs. However, the tendency of increasing WBCs as affected by CoQ10 is in parallel with Abbasi et al. (2014), who recorded a slight increase in leukocytes, neutrophils, MCHC, and RBC in athletes treated with CoQ10 compared with the control group. They indicated that CoQ10 could be had anti-inflammatory and antioxidant properties that suitable to protect the muscle by involved in energy production.
Conclusion
The presented results indicated the beneficial effects of oral pre-and post-partum administration of LC or CoQ10 on improving milk production, reproductive performance, energy metabolism, hematological parameters and improving the antioxidant status and immunity of lactating cows. The administration of LC at a level of 100 mg/kg BW showed the best results.
acknowledgements
The authors thank the El-Gemmezah Animal Production Experimental Station, APRI, Egypt for allowing animals that used in this.
Conflict of interest
The authors declare no conflicts of interest.
authors contribution
Wael Mohamed Wafa, Hamdy Abdala El-Nagar contributed to design the experimental work, conducted the experimental procedures and collected data. Wael Mohamed Wafa critically revised the manuscript.
REFERENCES