Journal of Animal Health and Production
Research Article
Experimental Infection of Broilers with H9n2 Avian Influenza Virus Local Isolate
Amer Khazaal Al-Azaway*, Karim Sadun Al-Ajeeli, Duaa Al-Salihi
Department of Microbiology, College of Veterinary Medicine, University of Diyala, Iraq.
Abstract | The present study was an attempt to assess the pathogenicity of local isolate of H9N2 avian influenza virus (AIV) in Diyala province, Iraq in the experimentally infected vaccinated and unvaccinated commercial broiler chickens with available commercial H9N2 vaccine. The virus was propagated in allantoic cavity of hen’s embryonated eggs and gave a titer (1024HAU/0.1) by HA test and a (1010.5EID50/0.1 ml) of stock virus. One hundred and 80 broilers of one day old were used and subdivided into 3 groups (60 birds each) as group A, B, and C. La-Sota Newcastle disease virus (NDV) vaccine was used to vaccinate groups A, group B was further vaccinated with bivalent inactivated H9N2 and NDV commercial vaccine, whereas group C was used as control (unvaccinated). Groups A and B at the age of 28 days were infected by intranasal dropping with 0.3 ml of stock virus of a titer (1010.5EID50/0.1 ml). The mean titre of maternal antibodies measured by enzyme linked immunosorbent assay (ELISA) at 3 days of age appeared as 6541.66. Levels of anti AIV antibodies reduced significantly (P≤0.05) at age of 14 and 35 days when compared to level of maternal antibody. The mean titres of antibodies at 14 days of age appeared 1173.16, 2503.77 and 373.94 for groups A, B and C respectively. The mean titres of IgG against AIV at the age of 35 days appeared as 337.77, 2303.22 and 174.27 for group A, B, and C respectively. Mild clinical signs were observed in A and B groups at 4 and 12 days post infection (PI) respectively. The morbidity was too low and the mortality was reported in group A only and not be exceeded 3.3%. The virus was detected in tissue samples only of group A collected from trachea, liver and lung by real time PCR using specific primers and probe. Histopathological changes were observed in trachea, liver and lung, like degeneration, necrosis and infiltration of inflammatory cells. In conclusion, available commercial inactivated H9N2 vaccine, could not completely protect broilers from the infection of same virus H9N2 local isolate.
Keywords | Influenza virus, Broilers, H9N2, Vaccine, Isolate
Received | June 20, 2019; Accepted | September 02, 2019; Published | December 28, 2019
*Correspondence | Amer Khazaal Al-Azaway, Department of Microbiology, College of Veterinary Medicine, University of Diyala, Iraq; Email: [email protected]
Citation | Al-Azaway AK, Karim Sadun Al-Ajeeli KS, Al-Salihi D (2019). Experimental infection of broilers with H9N2 avian influenza virus local isolate. J. Anim. Health Prod. 7(4): 147-157.
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.4.147.157
ISSN | 2308-2801
Copyright © 2019 Al-Azaway et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Poultry industry is greatly affected by outbreaks due to avian influenza viruses that spread worldwide. These outbreaks are associated with high morbidity and mortality or high morbidity and low mortality, as it was related to virus strain and subtype (Capua and Alexander, 2008; Goudrazi et al., 2013).
Avian influenza viruses are RNA genome viruses, which is single stranded and negative sense RNA. The genome is segmented into 8 segments and their total length of 13.5 kilo base. The virions are enveloped and classified within the family Orthomyxoviridae (Lamb, 2001). All avian influenza viruses are grouped within the genus Influenza A of the family Orthopmyxoviridae, and this family have another four genera which are, Influenza B, Influenza C, Thogotovirus, and Isavirus (MacLachlan and Dubovi 2010). This classification is attributed to the differences in genetic structures of both matrix proteins (MP) and nucleoproteins (NP) of these viruses (MacLachlan and Dubovi 2010).
The genus Influenza A included many viruses, these are although related to each other serologically and from genetic point of view, but differences are also detected between each other depending on the structure of their glycoprotein spikes, accordingly viruses of Influenza A are divided into subtypes (Tzarum et al., 2017). These glycoprotein surface spikes of the virus are hemagglutinin (HA) and neuraminidase (NA). Attachment of viruses to specific receptors of host is mediated by HA, whereas NA is mediated the release of the virus from infected host cell after viral replication (Pales et al., 2007). Such genetic differences between HA and NA distinguished 18 H subtypes (H1 to H18) and 11 NA subtypes (N1 to N11) including those isolated from bats (H17 and H18, N10 and N11). The name of each subtype is a combination of HA subtype and NA subtype (Ellakany et al., 2018; Wang et al., 2018). Aquatic birds and migratory birds like gulls, waterfowl and shorebirds are the main reservoirs of Influenza A viruses. New Influenza A viruses those are detected in bats and classified as H17N10 and H18N11 led to a suggestion that wild and aquatic birds are not the only reservoir for Influenza A viruses (Tong et al., 2013). Avian influenza viruses are not only infected birds, as many reports mentioned the transmission of such viruses to humans and some other mammalian (MacLachlan and Dubovi 2010).
Some of the influenza A viruses are characterized by low pathogenicity indices, mild or no clinical signs, and low mortality are grouped or known as low pathogenic avian influenza viruses (LPAIVs) (Swayne et al., 2013). Genetic reassortment (genetic shift) between two avian and human influenza viruses was mentioned as the cause of many outbreaks of influenza in Europe, Asia, and Africa. These reassortments might result in emerging of highly contagious and virulent strains that caused human pandemic infections among the years, 1918, 1957, 1968, 1977 and 2009 (Smith et al., 2009; Liu et al., 2013). Many avian influenza virus outbreaks had hit poultry industry of Asian and Middle East poultry farms during late 1990s and early 2000s. These outbreaks are reported to be due to LPAI subtype H9N2 (AL-Natour et al., 2005).
In Iraq, avian influenza virus was firstly isolated from flocks of layers by Al-Nasraoui (2002), and this was followed by the isolation of subtype H9N2 (AMR.ANT/Iraq/2005) from broilers. Therefore, the present study was an attempt to assess the pathogenicity of local isolate of H9N2 avian influenza virus in experimentally infected vaccinated and unvaccinated commercial broiler chickens with available commercial H9N2 vaccine.
Materials and Methods
Study Design
This study was conducted in Diyala province from the period of September 2018 to August 2019. The main objectives of this study is to investigate the susceptibility of vaccinated and unvaccinated broiler flocks with available commercial H9N2 vaccine to H9N2 AIV local isolate and to study pathogenicity of H9N2 AIV local isolate in broiler flocks of Diyala province.
Scientific Ethical Committee decided to approve the research methodology and give the Ethical Number (Vet Medicine (30) September 2018 D, A and K).
Reference Virus
Influenza virus isolate H9N2 that registered in National Central for Biotechnology Information NCBI with accession number (MH368755.1) was kindly provided as a courtesy from Mohammed Abdulkadhim Hussein and his Supervisor Prof. Dr. Emad J. Khammas from College of Veterinary Medicine, University of Baghdad.
Propagation of the Stock Virus in Eggs
Reference virus H9N2 the above-mentioned was used in this study. Hen eggs of 9-10 days old embryo were inoculated with 200 µl of reference virus in allantoic cavity. All inoculated eggs including the control at 37°C, observed daily and any dead embryo 24 hrs post infection (PI) was discarded as non-specific death. Eggs that showed dead embryo 24-72 hrs PI are removed from incubator, chilled in refrigerator at 40C for few hrs and checked for virus growth in allantoic cavity by slide hemagglutination (HA) test using 4% avian RBCs in sterile normal saline. Positive allantoic virus from inoculated embryonated eggs was collected and pooled together, checked for sterility on blood agar, PPLO media, and sabouraud agar as usual, redistributed in 1ml capacity sterile Eppendorf tubes, and kept in -300C until further use.
Titration of Propagated Virus
Propagated stock H9N2 virus local isolate was titrated by HA according to OIE manual (OIE, 2012) and by embryo infectious dose 50 (EID50) according to method described by Reed and Muench (1938).
Preparation of Hyper Immune Serum
The method described by Horwitz and Scharff (1969) was used to prepare hyperimmune serum against Influenza NDV. Five rabbits are used, three for hyperimmune serum preparation and 2 used as control. An amount of one ml of inactivated H9N2 vaccine (Nobilis Influenza, MSD, Holland) was inoculated intramuscularly. This was repeated weekly for 4 successful weeks, and on the fifth week, the rabbits are bled and the resulted serum was collected in sterile tubes and centrifuged at 4000 rpm for 10 minutes using cold centrifuge. The supernatant serum was collected and distributed in sterile one Eppendorf tubes and kept frozen until be used. For the purpose of hyperimmune serum titration, hemagglutination inhibition (HI) test used following the procedures of Brugh et al. (1978).
Grouping of Experimental Broilers
The virulence and the infectivity of the stock virus H9N2 are checked in susceptible broiler chicks as follows: one hundred eighty (day-old broiler chicks Ross 308) obtained from a commercial hatchery, subjected to a completely randomized design, by allocating them in to three equal treatments (60 chicks/treatment). All treatments were reared in the experimental farm of the College of Veterinary Medicine, University of Diyala in Diyala Province, Iraq. The farm was equipped with all requirements, feeders, thermometers, water and light source. All birds in the farm were offered feed and water ad libitum. The broilers were grouped into three equal groups (A, B and C), 60 birds each (Table 1). During the experimental study, the birds were provided daily with 16 hours of light and 8 hours of darkness. Nutrient requirements were provided evenly to birds in all the three groups, following periodic scheduling of diets according to the Ross 308 catalogue for Nutrition Specifications (2014), namely starter, (day 0-15), grower (day 16-28), and finisher (day 29-42).
Table 1: Experimental broilers grouping
| Group | Number of Birds | Description | Treatment |
| A | 60 | Vaccinated with LaSota NDV vaccine only (2 times) | Challenged with H9N2 local isolate |
| B | 60 | Vaccinated with AIV (H9N2) bivalent vaccine (Nobilis®) at 3 days and also with NDV La-Sota | Challenged with H9N2 local isolate |
| *C | 60 | Unvaccinated |
Not challenged |
*Group C was moved far from other groups A and B, to avoid the possibility of transmission of infection from infected groups.
Measurement of Maternal Immunity to AIV
Blood samples were collected from three days old chicken randomly from the three groups, serum was extracted and subjected to ELISA (Synbiotics ProFLOK® PLUS, Avian Influenza Virus) according to instruction manual of the manufacturer to determine maternal antibodies to avian influenza virus.
Vaccination Program of Experimental Chickens
Groups A, is vaccinated with La-Sota NDV (MSD/Holland) vaccine strain at age of 7 and 14 days by drinking water method. Group B was vaccinated at 3 days old with the inactivated vaccine for the immunization of chickens against Avian Influenza virus type A, subtype H9 in a water in oil emulsion (NOBILIS INFLUENZA H9N2 vaccine) subcutaneously in 0.3ml/chick, also vaccinated with La-Sota NDV vaccine in a drink water at 14 days old. For all water vaccination, the birds were fastened from water for 3 hours. This was followed by dissolving a vial of La-Sota vaccine of 1000 dose in 10 liters of boiled and cooled tap water, whereas group C left as control unvaccinated group.
Challenge of Broilers
Broiler groups (A and B) were challenged by the H9N2 stock virus at day 28 of age. This was done by intranasal dropping of 0.3 ml with a titer of 1010.5 EID50 /0.1 ml the virus for each bird. Control birds in group C were inoculated by the same method using one ml sterile PBS for each bird. All the infected groups were watched daily for 15 days.
Measurement of Immunity to AIV after Challenge with H9N2
At day 35, just one week post challenge with the H9N2, 18 blood samples from each groups were collected for detection the IgG antibodies against AIV by ELISA (Synbiotics ProFLOK® PLUS, Avian Influenza Virus).
Collection of Tissue Samples from Infected Birds
Tissue samples were collected from lung, trachea, liver, spleen and kidney of experimentally infected chickens and divided into two groups. The first group was subjected for virus detection by Real-Time PCR, whereas the second group of tissue samples was subjected to hisopathological study.
Detection of the Virus in Infected Chickens by Real-Time PCR
The viral RNA was extracted from the tissue samples using a (iNtRON, Patho-Gene-SpinTM DNA/RNA Extraction Kit, USA) according to the manufacturer’s instructions manual. Extracted RNA from samples was subjected to one step real-time PCR and according to the method described by the manufacturer by using of master mix (GoTaq® 1-Step Master Mix. Promega/USA), Forward primer (M25F) 5′AGATGAGTCTTCTAACCGAGGTCG3′, Reverse primer (M124R) 5′TGCAAAAACATCTTCAAGTCTCTG3′, and Probe FAM M64 5′TCAGGCCCCCTCAAAGCCGA-TAMRA3′ (all from Alpha/USA). The mixture was run for 40 cycle according to the manufacturer’s manual.
Histopathology of Collected Samples
Tissue samples are collected from three chickens from each group at day 7 PI. These samples are included kidneys, spleen, lung and trachea. The collected samples are fixed in 10% formalin neutral buffer, and embedded in paraffin according to the method described by Kiernan, (1999). Thin sections (4-6 µm) are made from paraffin embedded tissues, stained by haematoxylin and Eosin, and examined by light microscope.
Statistical Analysis
Data were calculated by SPSS for windows TM version 22.0 using Analysis of variance (ANOVA), and P≤ 0.05 was considered to be significant (Steel and Tarries, 1980).
Results
Propagation of the Virus in Allantoic Cavity
The virus was easily detected by HA test and gave a titre of 1024 HAU/0.1 ml of allantoic fluid. The titre of the virus by the use of EID50 method described by Reed and Muench (1938) showed high titre of 1010.5 EID50 / 0.1 ml. Hyper immune serum obtained from rabbits inoculated by H9N2 vaccine strain appeared with high titre of 211 HIU/ 0.1 ml of prepared serum, at the same time it was curly identified the stock virus (H9N2-MH368755.1) used in this test and study.
Challenge of Chicken
Clinical Signs: The challenged groups of broilers with H9N2 AIV local isolates showed different clinical signs.
Group A: Clinical signs appeared at day 4 post infection and continued till the end of observation period in this group. These clinical signs were by moderate to severe nasal discharge, prominent depression, mild oedema of the head and ruffling feather. The mortality rate was recorded as 2 birds with a ratio of 3.3%. Group B: The clinical signs appeared at 12 days post inoculation as mild nasal discharge, mild depression with mild diarrhoea and no mortality was reported. Group C: In control group had no remarkable signs or gross lesions, also no mortality was recorded as zero.
Circulation of IgG anti AIV in the chickens: The mean tit: er of IgG anti AIV as maternal antibodies determined in broiler chickens 3 days old for all the three groups appeared as 6541.66 and significantly (P≤0.05) decreased in group A when compared to the level of maternal antibodies. In group B the level of antibodies appeared higher than that of group A after vaccination of group B with commercial H9N2 vaccine, but significantly decreased in control group C at 14 days of age, which was the date of vaccination with both NDV for all groups and for H9N2 for group B. The mean titer appeared as 1173.16, 2503.77, and 373.14 for group A, B, and C respectively (Table 2). At age of 35 days the mean of AIV IgG titer appeared 337.77, 2303.22, and 174.27 for group A, B, and C respectively (Table 2).
Detection of the H9N2 in infected chickens by Real-Time PCR: In the current study broiler chickens groups (A and B) were challenged by the LPAI H9N2 at day 28. This was done by intranasal dropping of 0.3 ml (10 10.5 EID 50/0.1 ml) the virus of each bird. All the infected groups were watched daily for 15 days. Freshly dead and diseased chickens were subjected to post-mortem, and tissue samples were collected from lungs, trachea, spleen, liver, and kidneys were processed for virus re-isolation and identification on molecular technique by using Real time –PCR by using primers and probe targeting the matrix gene of AIV (M gene 42). All samples from group B give negative result for LPAI H9N2 whereas the group A, the selected samples included processing tissue of (trachea, lung and liver) which selected randomly from AIV infected group and subjected to Real time –PCR, four samples showed positive results with matrix gene(M gene 42). The four samples were named as followed in (Table 3).
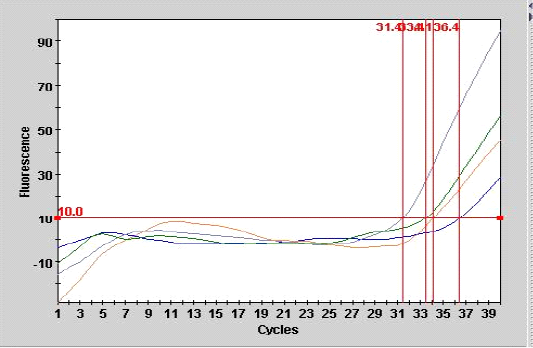
Figure 1: Smart cycle fluorography illustrating the RT-PCR detection of viral nucleic acid for the matrix gene of sample from experimentally infected chickens with LPAIV (H9N2).
As shown in Table 3, the mean number of CT for the sample (A 9) from tracheal tissue that was detected in real- time PCR was 36.4 with end point of 28, compared with the second sample of other tracheal case (A 10) with the CT of 31.4 at endpoint of 95 as showed in Figure 1.
Real-time PCR (one step) are most sensitive assay for detection of minute amount of viral particles as illustrated in Figure 1 for the two samples the CT are 34.1, 33.4 with end point of 45, 56 belonged to lung and liver samples of experimental infected chickens respectively. The result showed that inverse correlation was found between CT value and the end point for the all detection positive samples in the current study which indicated the sensitivity and specificity of the procedure. The efficiency of real time PCR reaction for doubling the M gene at the exponential phase was equal to 100% which indicate a stability of amplification.
Table 2: The mean IgG antibody in study groups at 3, 14 and 35 days of age.
| Groups | Age/Days | No. | IgG | |||
| Mean | Std. Deviation | Std.Error | CV% | |||
|
All Groups against AIV |
3 | 180 | 6541.66 | 2327.64 | 548.63 | 28.99 |
|
Group A |
14 | 60 | 1173.16* | 753.37 | 177.57 |
45.56 |
| 35 | 60 | 337.77* | 661.36 | 155.88 | 118.50 | |
|
Group B |
14 | 60 | 2503.77* | 1689.85 | 388.56 |
50.82 |
| 35 | 60 | 2303.22* | 1689.85 | 398.30 | 51.82 | |
|
Group C |
14 | 60 | 373.94* | 779.50 | 183.73 |
129.71 |
| 35 | 60 | 174.27* | 499.62 | 117.76 |
193.23 |
|
*The result was significant at P≤ 0.05 compared with maternal derived antibodies.
Table 3: Results of real time RT-PCR performed on four randomly selected samples of AIV experimentally infected birds
| Site ID | Sample ID | Assay Result | CT | Endpt |
| A 9 | Trachea 1 | Positive | 36.4 | 28 |
|
A 10 |
Trachea 2 | Positive | 31.4 | 95 |
|
A 11 |
Lung | Positive | 34.1 | 45 |
|
A 12 |
Liver | Positive | 33.4 |
56 |
Histopathological Examination
Histopathological results of trachea: The histopathological results of experimental intranasal dropping of the infected chickens with avian influenza H9N2 indicated an obvious histological changes associated with some areas of haemorrhage, necrosis and mild to moderate inflammation (Fig 2, A). The histological results showed that in some areas there were a clear degeneration and mild desquamation of the pseudostratified columnar epithelium of the trachea accompanied with an obvious haemorrhage in the mucosa and submucosa (Fig 2, B). Edema and congestion of the sub-mucosa were also observed in some areas and these had variable degrees of necrosis, vacuoles filled with blood, and disintegration in basal cells and some separation was observed in submucosa (Fig 2, C). Additionally, histological examination was also found hypertrophy of epithelium associated with mononuclear inflammatory cells.
Histopathological Results of Lung
Histological investigation of lung tissues in chickens revealed that there was a clear pathological changes in bronchial tree and parenchymal cells of the lung (Fig 3, A). These pathological lesions were mainly observed in lung parenchyma and range from moderate to severe inflammation in some cases. The histopathological changes included multifocal necrosis and infiltration with mononuclear inflammatory cells were observed diffused in the parenchymal cells of lung tissues. Alveolar damages were also indicated in lung parenchyma accompanied with infiltration with many inflammatory cells (Fig 3, B). In addition, some lung tissue exhibited thickened in the bronchioles
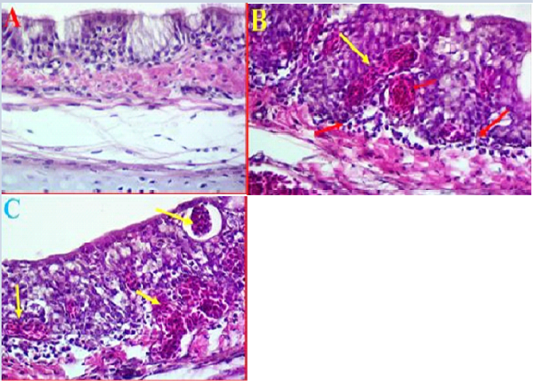
Figure 2: A- Histological changes in trachea of chickens infected with avian influenza (norml trachea). (40X, H&E). B- showed a clear degeneration in the epithelium and mucus gland with sever congestion and haemorrhage in trachea due to infected with LPAIV H9N2 (yellow arrows) (40X, H&E). C- Shows some pathological changes in trachea exhibited haemorrhage extended to sub mucosa and there was also observed vacuoles filled with blood (yellow arrows) (40X, H&E).
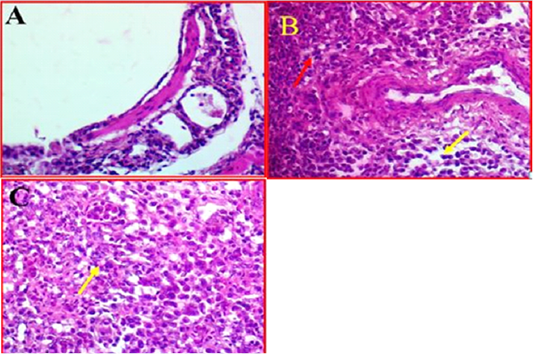
Figure 3: A-Photomicrographs illustrates the control bronchial tree and lung tissue. 40X (40X, H&E). B- Shows the local infiltration of inflammatory cells represented by red arrows, whereas yellow arrows indicated the mononuclear inflammatory cells, while. (40X, H&E). C- shows thickened in bronchioles wall (Yellow arrow). (40X, H&E).
and alveolar walls with alveolar epithelial cells associated with inflammatory cells. Furthermore, investigation revealed that there was some infected area had a clear oedema and hypertrophy associated with moderate congestion, degeneration and multifocal necrosis in lung parenchyma (Figure 3C).
Histopathological Results of Kidney
Kidney showed clear damages to interstitial tissue manifested by tubulointerstatial nephritis (Figure 4, A). There was also observed a clear cortical necrosis had lobular collapse, fibrosis, sever tubular atrophy and tubular dilatation (Figure 4, B). In some areas were also found aggregations of lymphoid follicles, and degeneration of glomeruli, also there was an obvious necrosis and atrophic renal tubules extended from the subcapsular area of cortical tubules toward the medullary cones associated with haemorrhage and sever congestion (Figure 4, C). Furthermore, as most of the pathological changes observed in cortical regions, it was also found that most of the cortical tubules were collapsed and multifocal aggregations of inflammatory cells were observed in this area.
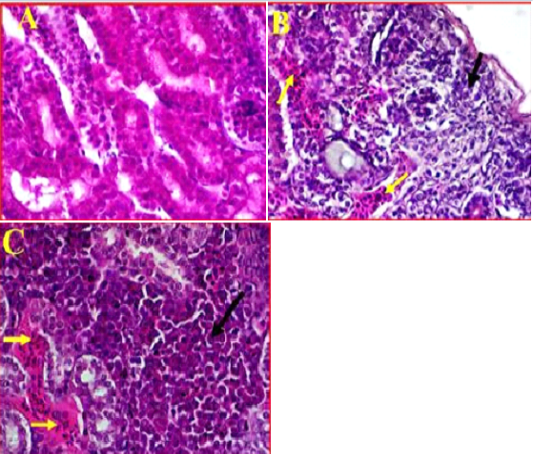
Figure 4: A- Illustrates a cross section of control or normal kidney tissue. (40X, H&E). B- shows the pathological changes exhibited in cortical region accompanied with sever infiltration with the inflammatory cells (black arrow) and sever congestion (Yellow arrows). (40X, H&E). C- shows multifocal necrosis and sever degeneration on the subcapsular area extended towards the medullary cones. (40X, H&E).
Histopathological Results of Liver
Liver showed a moderate to severe inflammation indicated by present multifocal necrosis and degeneration of hepatocytes, accompanied with severe congestion and haemorrhage and infiltration with many mononuclear inflammatory cells (Fig 5, B) as compared to control (Fig 5, A).
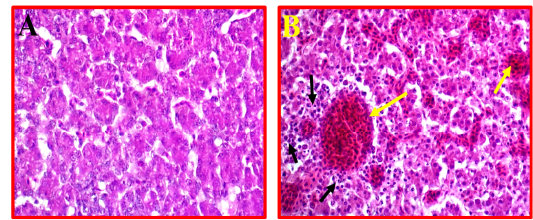
Figure 5: A- Shows control or normal liver. (40X, H&E). B- Illustrates pathological changes appeared as multifocal necrosis accompanied with infiltration with the many inflammatory cells (black arrows) and sever congestion (yellow arrows). (40X, H&E).
Histopathological Results of Spleen
As compared to control (Fig 6, A). The histological changes in the spleen of infected birds showed, a multifocal necrosis in splenic parenchyma with many mononuclear inflammatory cells in white pulp area (Fig 6, B). These inflammatory cells were observed in peri-arteriolar sheaths with the prominent of fibrinoid necrosis of arterioles (Fig 6, C). Additionally, blood capillaries were distended and there was moderate to severe edema and congestion.
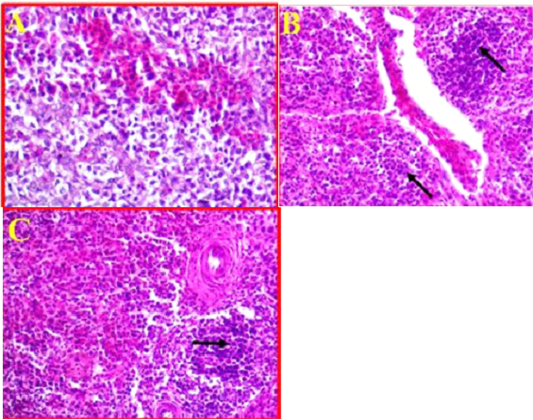
Figure 6: A- Illustrates a cross section of control or normal spleen. (40X, H&E). B- Shows the pathological changes revealed in splenic parenchyma accompanied with sever infiltration with the inflammatory cells (black arrows) and sever congestion. (40X, H&E). C- Shows inflammatory cells observed in peri-arteriolar sheaths with the prominent of fibrinoid necrosis. (40X, H&E).
Histopathological Results of Intestine
The pathological changes in intestine revealed that there was a mild necrotic foci observed in the mucosal layer of intestine and did not show any inflammation in the sub-mucosa (Fig 7, B) comparing to normal intestinal tissue as showed in Fig 7, A.
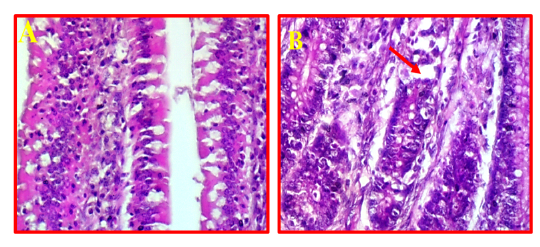
Figure 7: A- Shows normal intestine. (40X, H&E).B- Illustrates mild pathological changes presented in mucosal layer (red arrows). (40X, H&E).
Discussion
Influenza virus type A is the only member of the family Orthomyxoviridae that have been reported to cause a serious threat to the poultry lead to causing respiratory or systemic diseases that vary in severity. Avian influenza viruses are divided into two groups, LPAI and HPAI (highly pathogenic AI) viruses. Both HPAI and LPAI virus infections are present all over the world and associated with high morbidity and low mortality (Aamir et al., 2007; Suarez, 2010).
Virus samples with accession number (MH368755.1) that used in the current study was propagated well in allantoic fluid of embryonated eggs, and produced high titers in both HA and EID50, the method of propagation, detection and titration of propagated virus are the same that be mentioned by may reports (Pansota et al., 2013; Ahmad et al., 2014). The propagated stock virus was confirmed as H9N2 by the use of specific hyper immune serum in HI test. This test was used widely in identification and differentiation AIV depending on the antiserum against AIV, in the same time used to determine the level of immunity for any tested flock. (Dormitorio et al., 2009; EL Zowalaty et al., 2011).
The results of current study revealed that there were obvious clinical signs in group A that was challenge with LPAI H9N2 and not vaccinated against the AIV characterized by severe nasal discharge, prominent depression, mild edema of the head and ruffling feather, also there were mortality of 2 birds with ratio of 3.3% comparing to other group. These findings are consistent with those of previous works (Soliman, 2014; Dabbour, 2015) who found that AIV serotype H9N2 affected many commercial flocks in the Middle East and most of the flocks of broiler chickens that experimentally infected with the LPAI H9N2 showed typical manifestation that characterized by respiratory signs which included rals, sneezing with nasal and ocular discharge, whitish to greenish diarrhea, depression and anorexia with 20% mortality. The low mortality and minimal clinical signs observed in experimentally infected broilers of present study consistent with other published studies of (El Zowalaty et al., 2011; Franca et al., 2014) who reported that LPAI H9N2 induces minimal or no clinical signs in controlled specific pathogen-free (SPF) challenge experiments. Zhang et al. (2008) reported that to control the spread of H9N2 avian influenza, a vaccination program for the H9N2 influenza virus has been widely implemented over the past two decades throughout the world by using inactivated vaccine for H9N2. Vaccination against H9N2 influenza virus will not prevent the shedding of this strain (H9N2) and may play a role in driving the evolution and spread of H9N2 influenza virus. The second scenario under which H9N2 avian influenza virus is transmitted relies on the ability of the H9N2 virus to re-assort with other influenza virus subtypes to generate new influenza viruses. In this manner, an H9N2 virus supplied internal genes to an H5N1 virus that emerge and also to other strain like H7N9 and H10N8 which is considered as highly pathogenic AIV (Ye et al., 2016). The results of the present study reinforce the previous study by (Mosleh et al., 2009) who reported that the predominant infection due to experimental challenge of broiler chickens with intranasally (IN) of 100µl allantoic fluid LPAI H9N2, the clinical signs appeared in the respiratory tract at 3-4 days post inoculation and all the sings represented in the respiratory and gastrointestinal tract.
On the other hand the results of the present study were unparalleled with the other studies (Guo et al., 2000; Khamas, 2008) who reported that AIV H9N2 was more pathogenic to broiler chickens, and it produced more severe gross and histologic changes in the respiratory system compared with SPF chickens on the affected farms. Mixed infections of AIV with other viral diseases were more pathogenic in older birds compared with younger birds, and they had caused more than 70% to 80% mortality in broiler chickens.
The current study exhibited that, in group B from vaccinated group with (available commercial H9N2 inactivated vaccine) the clinical signs were appeared after 12 days post inoculation. The clinical signs characterized by mild nasal discharge, mild depression and no mortality rate. These results were agreed with the findings of (Bano et al., 2003; Banet-Noach et al., 2007) who reported that the pathogenicity of AIV H9N2 is variable and depends on the presence of other pathogens in the field. Bublot et al. (2007) and Capua and Alexander, 2008 found that inactivated AI vaccines (Influenza H9N2) are capable of inducing antibody response, which aids in the protection of the infected birds from mortality, and egg production decline that was compatible with the current study for group B which vaccinated (with influenza H9N2) and challenge with H9N2.
ELISA is the most common serologic assay allows potentially a rapid and specific assay for processing of large numbers of serum samples and to increase the diagnostic sensitivity lead to provide useful information on environmental exposure to pathogens and response to vaccination and also to measure the maternal antibody of new hatching chickens (Sharma, 2003; Zhang et al., 2006).
Maternal antibodies are immunoglobulins that are transferred from vaccinated or naturally infected breeder hens to the progeny through the egg, which provide passive immunity to progeny and protect them against infectious agents that cannot be cleared by the immature immune system (Alizadeh et al., 2016).
Maternal IgG level against AIV in broilers of present study was determined at age of 3 days, and found to be high in all groups. This comes in agreement with many studies as the peak levels of maternal IgG in the circulation of the newly hatched chick reach around 2–3 days of age. Maternally derived antibodies decline after 2–5 weeks (Alizadeh et al., 2016). The same findings were also reported by Sharma, (2003) and Hamal et al. (2006) who found that the high level of IgG in the serum of broiler chickens at 3 days old might be attributed to vertical transmission of maternal antibodies from parent to the progeny against certain pathogens lead to provide critical protection. Such level of IgG ant AIV was reduced on following weeks significantly (P≤0.05) when compared to maternal antibody levels This finding was in agreement with results of Gharaibeh and Mahmoud (2013) and Ming et al. (2019) who displayed that the maternal derived antibodies (MDA) that present in the hen’s plasma declined, subsequently after 2-3 weeks.
In experimentally infected broiler chicks in the current study, group A and B were vaccinated with NDV (La-Sota) and AIV H9N2 (inactivated bivalent vaccine) showed high level of antibodies with the mean titers of 1173± 177.57 and 2503.77±388.56 respectively comparing with group C (control group) which was not vaccinated (373.94 ±183.73) in 14 days old. Group B was significantly (P≤ 0.05) higher than those of group A and C, whereas in 35 days group B showed little drop in mean titer (2303.22±398.30) compared to group A and C (337.77±155.88 and 174.27±117.66) respectively. It seemed that the vaccinated group with ND-AIV H9N2 (inactivated bivalent vaccine) led to increase the antibodies titer against AIV in group B broilers. These results come in consistent with finding of many workers (Song et al., 2010; Lee et al., 2011) who demonstrated that H9N2 inactivated oil-emulsion vaccine have been used successfully against AIV outbreaks and elicited high levels of antibody responses to the virus as shown by hemagglutination inhibition activity.
The current study showed that the one-step RRT-PCR was highly sensitive and reliable technique for detection the matrix gene (M gene) by using forward primer (M25F) and reverse primer (M124R) and specific probe (FAM M64), this finding was in agreement with the with many previous works (Jackwood and Sommer, 2002; Mosleh et al., 2009) who found that because of the high speed and relatively low cost and sensitive methods, there is considerable interest in using RT-PCR-based methods directly targeted to the different subtypes of AIV as primary diagnostic tests. In contrast Mosleh et al., (2009) found that by using real time PCR for detection LPAI H9N2 in chickens experimentally infected with AIV, the presence of the virus is restricted to the trachea and lung with ratio of 40% and 20% respectively. Inconsistent to above mentioned result, Swayne and Halvorson, (2003) stated that the LPAI viruses produce infections that are localized to the respiratory and gastrointestinal (GI) tracts of chickens indicating that H9N2 AI virus is pneumotropic and nephrotropic following IN inoculation.
Avian influenza viruses can induce two different types of pathological changes based on their virulence and pathogenicity (Gharaibeh, 2008; Subtain et al., 2011; Eladl et al., 2019). The present study investigated on the pathophysiological impacts of H9N2 virus on certain tissues. In this study, the common obvious finding to emerge from the histological examinations was that a clear inflammation and necrosis accompanied with infiltration and congestion of trachea, lung and kidney (interstitial tubular nephritis), and to a lesser extent observed in liver and spleen. In accordance with the present results, previous studies worked on H9N2 found that tracheitis, pneumonia and pancreatitis were the most frequent and prominent histological changes (Gharaibeh, 2008; Aslam et al., 2015). In the current findings indicated that infected chickens with H9N2 virus caused sever tubular atrophy and nephritis which broadly supports the work of other studies in this area linking the majority of death of poultry farms to renal damages (Hadipour et al., 2011). It can thus be suggested that these results provide further support for the hypothesis that there is a straight pathogenic and pathophysiologic mechanism in the respiratory system and/or urinary system. In this study, experimental infected chickens with H9N2 were found to cause multifocal necrosis with mild to moderate congestion and edema, in addition to infiltration with mononuclear inflammatory cells in trachea, lung, kidney, spleen and liver simultaneous with histological lesions reveal direct pathologic impacts of H9N2 virus (Hablolvarid et al., 2004; Hassan et al., 2017).
It was concluded from above mentioned findings that H9N2 local isolate of AIV produced mild infections in both vaccinated and unvaccinated broilers with available commercial vaccine, but the vaccine reduced the severity of the infection and the mortality. It also led to high levels of anti H9N2 antibodies that might have the key role of protection. Circulation of other influenza A viruses in the same area, and especially the high pathogenic strain may led to emerging of new strains of AIV due to reassortment or genetic shift and drift. Accordingly, applying of epidemiological molecular and serological studies to screen different Iraqi provinces for the presence of HPAIV and LPAIV is too important.
Acknowledgements
The authors would like to thank the staff of the molecular biology laboratory, College of Veterinary Medicine, University of Diyala. Also the authors would like to extend their appreciations to the Mohammed Abdulkadhim Hussein and his Supervisor Prof. Dr. Emad J. Khammas from College of Veterinary Medicine, University of Baghdad for kindly providing the reference AIV sample with accession No. (MH368755.1) as a courtesy for our study.
conflict of interest
The authors declare that they have no competing interests.
Authors contribution
All authors carried out the study, analyzed the data, drafted the manuscript, read and approved the final manuscript.
References






