Journal of Animal Health and Production
Research Article
Antibiogram Studies of Salmonella Tshiongwe Isolates Obtained from a Broiler Farm in Western Uttar Pradesh, India
Jinu Manoj1*, S. Rawat1, Manoj Kumar Singh2
1Department of Veterinary Public Health & Epidemiology; 2Department ofLivestock Production and Management, College of Veterinary and Animal Sciences, Sardar Vallabhbhai Patel University of Agriculture & Technology, Meerut-250110, U.P., India.
Abstract | Salmonellosis is a common public health threat globally. Poultry have a major role in disseminating salmonellosis to humans. In this article, we report the presence of Salmonella Tshiongwe isolate from a broiler farm in western Uttar Pradesh, India and its antibiogram pattern. In the present study, 180 samples comprising of 160 poultry faeces, 5 feed and 15 drinking water samples were examined for the presence of Salmonella by growth enrichment on culture media. The suspected colonies obtained from selective media were further confirmed by biochemical tests, which consisted of Indole, Methyl Red, Voges Proskauer, Citrate, Triple Sugar Iron test, urease and motility tests. The presumptive Salmonella isolates obtained were serotyped at National Salmonella Centre, Indian Veterinary Research Institute, Bareilly, India according to Kauffmann-White scheme. The antibiotic susceptibility test of isolates was analyzed by Kirby-Bauer disc diffusion method. The biochemical testing of various samples collected from broiler farms showed a presence of 6.87% Salmonella in the samples, while serotyping revealed a prevalence of 1.88%. All the Salmonella isolates were obtained from faecal samples of broilers and none of the feed and water samples showed the presence of pathogen. The antimicrobial susceptibility test of isolates revealed that all the serotypes were resistant to Penicillin, Ampicillin, Gentamicin, Erythromycin, Sulpha-Trimethoprim, Tetracycline and Vancomycin. Most of the isolates were found to be highly sensitive to Amikacin, Azithromycin, Ceftriaxone, Ofloxacin, Ciprofloxacin and Chloramphenicol. The Salmonella isolates obtained were multidrug resistant. The presence of S. Tshiongwe in Indian broiler farms was reported for the first time and this serotype may be transmitted through the contaminated germplasm, spread by global trade.
Keywords | Antibiotic, Poultry, Salmonella, Serotyping, Tshiongwe.
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 20, 2017; Accepted | May 27, 2017; Published | May 28, 2017
*Correspondence | Jinu Manoj, Department of Veterinary Public Health & Epidemiology Meerut-250110, U.P., India; Email: [email protected]
Citation | Manoj J, Rawat S, Singh MK (2017). Antibiogram studies of salmonella tshiongwe isolates obtained from a broiler farm in western Uttar Pradesh, India. J. Anim. Health Prod. 5(2): 74-78.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.2.74.78
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Manoj et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Salmonella infections are an important public health problem worldwide (Manoj et al., 2015). Salmonellosis is a common foodborne illness, which can be transmitted to humans by the ingestion of contaminated foods and drinking water (Ajayi and Egbebi, 2011). The Salmonella serovars especially of the nontyphoidal nature (NTS) are mainly associated with foods of animal origin and are responsible for zoonotic transmission (Fernandez et al., 2012). Salmonella spp. is increasingly associated with poultry production. Poultry products which are contaminated serve as the most important sources for food borne salmonellosis (Braden, 2006). Salmonella can frequently be isolated from most species of poultry, such as broilers, turkeys, ducks and geese (Kabir, 2010; Hoelzer et al., 2011). The incidence of human salmonellosis increased rapidly due to the presence of Salmonella in broilers (Wegener, 2003).In India, salmonellosis is hyperendemic and causes heavy economic losses every year (Rahman, 2002). The presence of Salmonella infection in a flock can be better identified from the faecal samples (Carrque-Mas and Davies, 2008). Faecal cultures are considered to be 100% specific in the detection of Salmonella (Chow, 2004). In order to control salmonellosis, early detection of infected flocks is very crucial. The regular surveillance of flocks is required to prevent reach of contaminated poultry productsupto the consumer (Jinu et al., 2014).
Salmonella Tshiongwe is an unusual serovar, belonging to the serogroup C3and the antigenic formula of S. Tshiongwe is 6,8:enz15 (Edwards and Ewing, 1986) and has never been reported from India. Hoszowski et al. (2000) reported isolation of S. Tshiongwe from European countries like Lativa, Poland and Moldova. S. Tshiongwe isolates emerged in Malaysia were considered as the cause for food poisoning episodes in the country, but the factors responsible for the emergence of this Salmonella serovar was remained unclear (Thong et al., 2004). S. Tshiongwe was also reported from the sewage and veterinary environment (Hoszowski and Wasyl, 2001).
The use of antibiotics in poultry has been a common practice and this creates antibiotic resistant bacteria. Many of the Salmonella species isolates from food products are multidrug resistant serotypes, which can cause serious public health problems (Dallal et al., 2014). In this article, the presence of Salmonella Tshiongwe serovar from a broiler farm in western Uttar Pradesh, India and its antibiogram pattern is reported.
Materials and Methods
Sample Collection
A total of 180 samples including 160 poultry faeces, 05 feed and 15 drinking water samples were collected from broiler farms of western Uttar Pradesh, India. Fresh faecal samples were collected from the broilers randomly at every week upto 42 days of rearing time. The samples were collected into sterile polyethylene bags except the drinking water samples, which were collected into sterile test tubes. After collection, the samples were brought to zoonoses laboratory and immediately processed.
Isolation of Salmonella from Different Samples
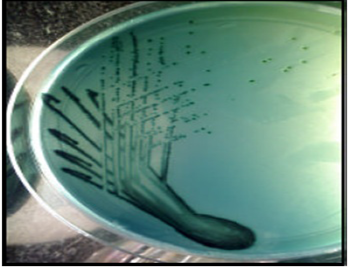
The samples were processed according to standard guidelines (OIE, 2008). Approximately 1 g of each sample is inoculated into 9 ml of Buffered Peptone Water (BPW), pH 7.2±0.2 and incubated for 16 hours at 37ᵒC for pre-enrichment. One ml of pre-enriched culture was transferred into 9 ml of Tetrathionate (TT) broth (HiMedia, India) for enrichment, which was then incubated at 42ᵒC for 24 hours. A loopful of the incubated TT broth was streaked onto the selective media, Hektoen Enteric agar (HEA) (HiMedia, India) and incubated aerobically at 37ᵒC for 24 hours. From each plate, a single colony of typical morphology (typical black coloured colonies surrounded by narrow green margin) were picked and restreaked onto another selective media, bismuth sulphite agar (BSA) for purity checking.
Biochemical Identification of the Isolates
Two to three presumptive Salmonella colonies from selective media were selected and used for the biochemical tests such as Indole (I), Methyl Red (M), Voges Proskauer (V), Citrate (Ci). The colonies showing Salmonella specific IMViC pattern (-+-+) were inoculated on Triple Sugar Iron (TSI) agar (HiMedia, India) slants, by streaking the surface of TSI slants and stabbing the butt and incubated at 37ᵒC. The inoculated TSI slants were examined at intervals of 24 h upto 72 h, for typical Salmonella reactions. The colonies producing alkaline slant (pink) and acidic butt (yellow) with or without H2S production (blackening) were tested for urease production on urea agar slant. All the urease negative isolates were considered as biochemically confirmed for Salmonella spp. The presumptive salmonella colonies were also tested for motility test by inoculating putative isolates.
Serotyping of Salmonella Isolates
Depending upon the results of biochemical reactions, the presumptive Salmonella isolates were stored onto nutrient slant at 4°C and submitted to National Salmonella Centre (NSC), Indian Veterinary Research Institute (IVRI), Bareilly, India for serotyping. Serotyping was performed according to Kauffmann-White scheme.
Antimicrobial Susceptibility Test
The antibiotic susceptibility test of isolates was performed by Kirby-Bauer disc diffusion method (CLSI, 2015). The antimicrobial agents tested include Penicillin-G (10 units), Ampicillin (10μg), Azithromycin (15μg), Amikacin (30μg), Ceftriaxone (30μg), Chloramphenicol (30μg),Ciprofloxacin (5μg), Erythromycin (15μg), Gentamicin (10μg), Ofloxacin (5 μg), Tetracycline (30 μg), Sulpha-Trimethoprim (Co-Trimoxazole) (25μg) and Vancomycin (30 μg) (HiMedia, Mumbai, India). The isolates were grown in Mueller Hinton broths (HiMedia, Mumbai, India) for 18 hrs at 37 °C. About 100 μl of the inoculums, equivalent to a 0.5 MacFarland standard were spread on Mueller Hinton agar using sterile disposable L shaped spreader and antibiotic discs were placed onto the plate using sterile forceps. The plates were incubated at 37ᵒC for 24 hrs aerobically. The diameter of the zone of inhibition surrounding the antimicrobial discs was measured to the nearest mm. The results were interpreted using the criteria of the Clinical and Laboratory Science Institute as Susceptible (S), Intermediate (I), and Resistant (R) based on diameter of zone of growth inhibition. The E. coli ATCC 10536 was used as the control strain for the antibiotic susceptibility tests.
Results
In the present study, 180 samples comprising of 160 poultry faeces, 5 feed and 15 drinking water samples were screened for the presence of Salmonella by growth enrichment on culture media. The suspected colonies obtained from selective media (Figure 1) were further confirmed by biochemical tests, which consisted of Indole (I), Methyl Red (M), Voges Proskauer (V), Citrate (Ci), Triple Sugar Iron (TSI) test, urease and motility tests.
Prevalence of Salmonella
Out of 160 faecal samples, 11 samples (6.87%) were found to be biochemically positive (results not shown) for Salmonella as per IMViC pattern, TSI reaction (Figure 2) and urease tests (Figure 3). All these isolates were motile also. Out of 15 water samples collected from poultry farm, none were found as Salmonella positive. All the feed samples collected were also Salmonella negative.
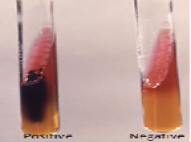
Figure 2: Triple Sugar Iron (TSI) test
Serotyping of Salmonella Isolates
Serotyping of all 11 suspected isolates showed the prevalence of Salmonella in 3 (1.88%) samples. Among these, 2 isolates were confirmed as Salmonella Tshiongwe, with the antigenic formula 6,8: eh: enz15,while 1 isolate was unidentified, eventhough it possessed an antigenic formula of 6,8 and it was belonging to Group 2 of Salmonella, as per Kauffmann-White scheme (Table 1). All the Salmonella isolates were obtained from faecal samples of broilers and none of the feed and water samples showed the presence of pathogen. The positive isolates were found from the same farm at 3rd and 4th week of rearing time of broilers.

Figure 3: Urease test
Antimicrobial Susceptibility Test
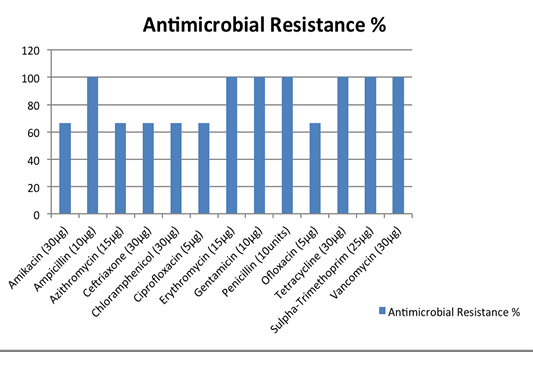
The antimicrobial susceptibility test of isolates revealed that all the serotypes were 100% resistant to Penicillin, Ampicillin, Gentamicin, Erythromycin, Sulpha-Trimethoprim, Tetracycline and Vancomycin, which are commonly used antibiotics. However, the isolates werefound to be sensitive against Amikacin, Azithromycin, Ceftriaxone, Ofloxacin, Ciprofloxacin and Chloramphenicol (Figure 4).
Table 1: Details of Salmonella isolates and their incidence percentage.
|
Serotype |
Antigenic formula* |
Incidence |
Incidence percentage in faeces |
||
|
Faeces |
Feed |
Water |
|||
|
S. Tshiongwe |
6,8: eh: enz15 |
2/160 |
0/5 |
0/15 |
1.25% |
|
Salmonella (Group 2) |
6,8 |
1/160 |
0/5 |
0/15 |
0.63% |
|
Total |
3/160 |
- |
- |
1.88% |
|
* based on Kauffmann-White scheme
DiscussionS
In this study, biochemical testing of various samples collected from broiler farms showed a presence of 6.87% Salmonella in the samples, while serotyping revealed a prevalence of 1.88%. Other researchers also reported that some of the Salmonella isolates can be diagnosed as ‘false negative’ in serotyping (Kaushik et al., 2014). This may be due to the presence of rough mutant strains or some additional abnormalities of the core structure resulting in negative result in serotyping (Topley and Wilson, 1990). Serotyping of the isolates revealed the presence of Salmonella Tshiongwe which has never been reported from India, even though it was isolated in other countries. The source of the Salmonella Tshiongwe serotype obtained in this study remained unknown and it was not clear how this serovar emerged among the most common serovars of Salmonella. Due to the global trade and the easy importation of contaminated germplasm may be responsible for the presence of thisSalmonella serovar in India. In addition, the survival of Salmonellain the environment for months could grave the situation (Wray and Wray, 2000).
The frequent use of antimicrobials as therapy or feed additives in animals causes antimicrobial resistance. The current study reveals that Salmonella isolates obtained were multidrug resistant Salmonella, which substantiate the reports of other researchers (Siemon et al., 2007; Kessel et al., 2013). The use of antibiotics may not be effective because of the presence of resistant bacterium (Kumar et al., 2012). For the successful control of antibiotic resistant Salmonella, there should be adequate programmes for poultry entrepreneurs about the discriminate use of antimicrobials in the poultry industry.
Conclusion
Salmonellosis is a significant public health issue causing food poisoning. The presence of Salmonella in the food chain affects the economic status of the food firm and its country. It can also pose health hazards due to its invasive nature. The presence of Salmonella in faecal samples indicates the infection status of the flock. In our study, both the biochemical testing and serotyping confirmed the presence of Salmonella in the broiler farm and this can act as a source of human infection. The presence of S. Tshiongwe in Indian broiler farms was reported for the first time and this serotype may be transmitted through the contaminated germplasm. The increment of global trade between nations can further makes its easy spreading among countries. The Salmonella isolates obtained in this study were found as multiantibiotic resistant also. The study concluded that the use of antibiotics in broilers should be strictly regulated to minimize the emergence and dissemination of antibiotic resistant bacteria and thus avoiding the risk of infection to humans by them.
Conflict of Interest
There is no conflict of interests among authors regarding the publication of this paper.
Authors Contribution
All the authors collected the samples. Jinu Manoj and S. Rawat screened the samples. Jinu Manoj conducted the antimicrobial susceptibility tests, while Manoj Kumar Singh contributed to critical revisions of the article.
References