Advances in Animal and Veterinary Sciences
Research Article
First Detection of Equine Anaplasmosis and Hemoplasmosis of Horses in Mosul City, Iraq
Basima Abdulfatah Albadrani*, Osamah Muwaffag Al-Iraqi
Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul-Iraq.
Abstract | Equine anaplasmosis (Anaplasma phagocytophilum) and hemoplasmosis (hemotropic Mycoplasma spp.) are two bacterial diseases diagnosed in horse’s blood for the first time in Iraq. Both diseases can be transmitted by different ticks and some reservoir hosts Anaplasmosis and hemoplasmosis also play a role in their transmission. In September 2017 to July 2018, clinical and hematological examinations of 45 cases of suspected equine anaplasmosis and/or hemoplasmosis at veterinary teaching hospital of college of veterinary medicine, University of Mosul, Mosul, Iraq. The clinical examination were revealed Depression, ataxia, fever, palemucousmembranes, limb edema and colic in some cases. The hematological examination showed mild anemia, thrombocytopenia and leukopenia, with inclusions visual in the buffy coat and blood smears. Hemotropic Mycoplasma spp. (in erythrocytes) and A. phagocytophilum morulae (in neutrophils and monocytes) were observed in 33.3 % and 28.9% of samples, respectively with 37.8% of samples had co-infection of hemotropic Mycoplasma spp. and A. phagocytophilum .The tick hemolymph test was successfully applied to naturally infected adult ticks of horses and provides a rapid identification of the elementary bodies of A. phagocytophilium.
Keywords | Anaplasmosis, Hemoplasmosis, Horse, Ticks, Iraq
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 22, 2018; Accepted | November 01, 2018; Published | November 27, 2018
*Correspondence | Basima Abdulfatah Albadani, Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul-Iraq. Email: [email protected]
Citation | Albadrani BA, Al-Iraqi OM (2019). First detection of equine anaplasmosis and hemoplasmosis of horses in mosul city, iraq. Adv. Anim. Vet. Sci. 7(2): 106-111.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.2.106.111
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Albadrani and Al-Iraqi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Equine anaplasmosis (formerly Equine Granulocytic Ehrlichiosis) is an infectious, non contagious, seasonal tick-borne transmitted disease of horses first reported by Gribble in northern California in 1969 (Gribble, 1969). The causal rickettsial agent was initially termed Ehrlichia equi, but based on DNA sequence relationships; the organism is now referred to as Anaplasma phagocytophilum. It also can infect horses, ruminants, dogs and cats (Raoult and Parola, 2007) and human beings (Karasartova et al., 2018). It is not similar or clinically related to anaplasmosis in cattle (Franzen, 2008). Small mammals can be a reservoir of A. phagocytophilum (Greene, 2012). A. phagocytophilum frequently infects horses wherever the tick vector (Ixodes spp) is present (Caruso and Bartol, 2013). It affects horses of all ages, and the symptoms are usually appear suddenly. Unlike many diseases, this condition causes less severe symptoms in horses that are less than three years old. Horses <1 yr old may have a fever only; horses 1–3 yr old develop fever, depression, mild limb edema, and ataxia. Adults exhibit the characteristic signs of fever, partial anorexia, and depression, reluctance to move, limb edema, petechiation, and icterus (Pusterla et al., 1998). Equine ehrlichiosis is seen chiefly in the USA in northern California but now recognized in many states where the tick vector occurs; it is also reported in Europe, Africa, and South America (Korbutiak and Schneiders, 1994; Inokuma et al., 2005; Alberti et al., 2005). The zoonotic risk of infection to people via horses has not been observed, although horses and people appear to be infected with strains of the same disease, it is believed that human exposure occurs through tick bites and not by direct transmission from horses to people (Dieckmann et al., 2010). Blood smear provides the earliest laboratory diagnosis for acute infection; demonstration of the characteristic cytoplasmic inclusion bodies in neutrophils and less often in eosinophils a standard blood smear is diagnostic (Elias, 1991). PCR can detect A. phagocytophilum DNA in unclotted blood or Buffy coat smears (Rassouli and Aghazamani, 2015). An indirect fluorescent antibody test can detect rising antibody titers to A. phagocytophilum. Hemotrophic mycoplasmas (HM) or hemoplasmas (formely, Epirythrozoonosis and haemobartonellosis) are small, cell wall-less Gram-negative bacteria, Mycoplasma spp., class Mollicutes and infections are known for a wide range of animals (Millán et al., 2015). Hemoplasmas may be transmitted by transfer of infected blood (blood transfusion or use of contaminated needles, surgical instruments, herd or flock management equipment) or via arthropod vectors such as flies (Hornok et al., 2011), lice (Song et al., 2013), ticks (Mohd Hasan et al., 2017) and mosquitoes (Reagan et al., 2017). Vertical transmission from mother to offspring has been reported in cats, swine, and camelids (Messick, 2004; Wernery, 2012; Toledo et al., 2016). Direct transmission associated with fighting is suspected in cats and supported by studies reporting presence of hemoplasma DNA in saliva, on gingiva, and on claw beds of infected cats (Reagan et al., 2017). One possible indication of equine hemoplasma infection was given in 1978, when a ‘haemobartonellosis’ outbreak was diagnosed in Nigerian horses by microscopy. However the first molecular proof of HM in horses was not reported until 2010, when a fragment of about 900bp of the 16S rRNA of the equine HM was obtained, Using a HM specific PCR assay and subsequent sequencing the infective agents isolated from the blood of said horses were confirmed as closely related to the HM species Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemobos’ (Dieckmann et al., 2010). Hemotrophic mycoplasma infection is still a neglected zoonotic disease, which poses a threat to public health and the animal industry, especially in China (Hoelzle et al., 2007). The zoonotic potential of the Hemotropic mycoplasmas is evident because the disease is more prevalent in farmers and veterinary doctors, who have frequent close contact with domestic animals, than in other persons (Yang et al., 2000). The role of ticks, the zoonotic potential and the likelihood of domestic animals to be used as sentinels for human infection on hemoplasma transmission remain to be fully established (Tasker et al., 2010).
MATERIALS AND METHODS
Forty five horses aged 3-8 years old, were referred to the Veterinary Teaching Hospital, University of Mosul, Mosul, Iraq, because of loss of performance, abdominal pain, and limb edema. The horses underwent a thorough clinical examination, and 5ml of blood was collected for determination of a complete blood count (hemoglobin, total red blood cell count, total and differential white blood cell and thrombocyte counts were analyzed electronically on an automatic full digital cell counter (Beckman, USA), the results were compared with normal hematological values of other healthy seven horses. Diagnosis can be done by direct identification of inclusion bodies or morulae of Anaplasma phagocytophilia in leucocytes from blood smears (Elias, 1991), or from buffy coat smears (Mylonakis et al., 2003). Blood smears were prepared from EDTA K2 anticoagulated blood within five minutes after blood collection to ensure no hemoplasma detachment from red blood cell surface. The slides were stained using Romanowsky staining (Giemsa). Blood smear preparations were scanned for the presence of hemoplasmas. For each slide, 1000 red blood cells were counted in different randomly chosen, high power (100 X) fields and the number of structures morphologically compatible with hemotropic mycoplasmas noted (Arsenault and Messick, 2005). There is no information of vectors and reservoirs of above pathogens in Iraq. One hundred adult ticks were collected from infested horses for detection and identification the tick species was affected horses in the local area by gently remove actively feeding ticks from an animal and place in 3% topical hydrogen peroxide for 5 minutes and then in 70% ethanol for 10 minutes to surface sterilize. With a pap pen draw a circle onto a saline coated microscope slide and place the tick within the pap pen circle. Saline coated slides are used for better adherence of the hemolymph to the microscope slide. View the tick under a dissecting microscope (1X objective, 10X eyepiece, 3.5 X magnifications). Gently push down on the tick’s dorsum with forceps to splay the tick’s legs and immobilize the tick. Amputate the tick’s leg or legs at the distal joint with a fine point disposable scalpel. To determine infectivity via hemolymph only 1 leg needs to be amputated. For collection of hemolymph on a slide several legs may be amputated. After the leg or legs are cut continue to gently apply pressure to the tick’s dorsum for the hemolymph to secrete out of the legs onto the slide. Gently move the tick around on the slide to spread the hemolymph. The hemolymph is collected on a slide, heat fixed, stained by Giemsa method, and examined microscopically (Patton et al., 2012). Adult ticks were identified to the species level using a published taxonomic key (Al-Moula and Rahemo, 2004).
Statistical Analysis
The newly recorded diseases were analyzed among horses by chi-square test, SPSS software. The breeds and ages were not analyzed because they had mostly not been recorded in the case histories.
RESULTS
Hemotropic Mycoplasma spp. was observed in 15 blood smear samples out of 45 (33.3%) and Anaplasma phagocytophilum morulae were observed in 13 buffy coat smears
Table 1: Hematology profile of Anaplasmosis and Hemoplasmosis infected horses in comparison with healthy horses
|
Hemoplasmosis infected horses (n=15)
|
Anaplasmosis infected horses (n=13) |
Healthy horses (n=7) |
Hematology profile |
|
± 0.02* 4.4 |
2.4± 0.03** |
7.6 ± 0.27 |
Leukocytes (×106 cells/L) |
|
± 0.01** 5.2 |
7.3± 0.02* |
8.2 ± 0.35 |
Erythrocytes (×1012 cells/L) |
|
± 0.23** 19.2 |
23. 6± 0.35* |
40.5± 2.24 |
Hematocrit (L/L) |
|
± 0.06** 9.2 |
11.2± 0.22* |
14.5± 0.45 |
Hemoglobin (g/L) |
|
± 2.20* 90.4 |
70.1± 5.10** |
127.5± 15.2 |
Platelets (×106 cells/L) |
The numbers represent mean ± SD.
*Observed differences were considered to be significant in comparison of healthy horses (p <0.05) **Observed differences were considered to be significant in comparison between both infected horses groups (p <0.05).
Table 2: Differential Leukocytic count (Mean ± SD.) in Anaplasmosis, hemoplasmosis infected and healthy horses.
|
Hemoplasmosis infected horses (n=15)
|
Anaplasmosis infected horses (n=13) |
Healthy horses (n=7) |
Parameters |
| 6.4± 0.03** | 1.6 ± 0.02** | 5.2± 0.01 |
Neutrophils(x109/L) |
|
3.8 ± 0.02 |
7.1± 0.03** | 3.8 ± 0.6 |
Lymphocytes(x109/L) |
| 0.7 ± 0.01 | 1.2 ± 0.01** | 0.8± 0.02 |
Monocytes(x109/L) |
| 0.1 ± 0.01 | 0.1 ± 0.04 | 0.2± 0.08 |
Eosinophil(x109/L) |
*Observed differences were considered to be significant in comparison of healthy horses (p <0.05) **Observed differences were considered to be significant in comparison between both infected horses groups (p <0.05)
out of 45 (28.9%) and 17 blood smears samples out of 45 (37.8%) had co-infection of Mycoplasma spp. and A. phagocytophilum (Figs 1, 2, 3). Thirteen horses with anaplasmosis (Anaplasma phagocytophilum) showed clinical signs included fever (40° to 41.5°C), anorexia, and the mucous membranes were mildly icteric with yellowish gums, the capillary refill time was 2 s, bilateral hind limb edema, somnolence, conjunctivitis, and reluctance to move. Some horses showed the presence of tick with clearly visible skin reactions located on the fore limb in a horse covered with tick nymphs. Microscopic examination of blood and buffy coat smears revealed a raspberry like structure called a morulae (microcolonies of Ehrlichiae) in the cytoplasm of neutrophils and monocytes in 66.7% of cases (Figure 1:A and B). Fifteen horses with hemotrophic Mycoplasma are suffering from poor performance, apathy, weight loss, and anemia. Examination of peripheral blood smears revealed numerous small basophilic staining coccids structures 0.5-1 μm in diameter with a number ranging from 1 to 10 organisms on the surface of the erythrocytes attached to cell membranes singularly or in clusters centered on a single membrane focus which caused different levels of RBC deformation, some erythrocytes were not infected (Figure 2: A and B). The adult ticks (n =100) collected from the horses (n = 30) were classified as Hyalomma spp. (48 males and 12 females) in 60% of ticks, and 30% was Rhipicephalus spp. (14 males and 16 females) and 10% of Boophilus spp. (5 males and 5 females). Only 15 of the 45 (33.3%) of the examined horses were infested by at least one of these tick species.In this study it was found that 51% of Hyalomma spp. hemolymph smears infected with Anaplasma phagocytophilium, 34% of Rhipicephalus spp. hemolymph smears were infected with hemoplasma and 15% Rhipicephalus spp. hemolymph smears co-infected with Anaplasma phagocytophilium and hemoplasmas. (Figure 4: A and B and C). On hematology, anemia, leucopenia and thrombocytopenia are typically found in clinical cases of anaplasmosis and hemoplasmosis of horses. Anemia had indicated by values of hemoglobin concentrations, erythrocytes counts and hematocrit in the clinically infected horses. Hemotropic Mycoplasma spp. infected horses had significantly (p <0.05) lower hemoglobin, erythrocytes and hematocrit levels than those in Anaplasma phagocytophilia infected horses in comparison with healthy control horses. Mild anemia and reactive lymphocytosis with monocytosis were observed in horses infected with Anaplasma phagocytophilia. There was also a leucopenia and thrombocytopenia is typically found in clinical cases of anaplasmosis in all leukopenic samples, only neutrophils could be counted in low numbers. Leukocytosis with mild neutrophilia was detected in horses infected with hemoplasmosis (Table 1, 2).
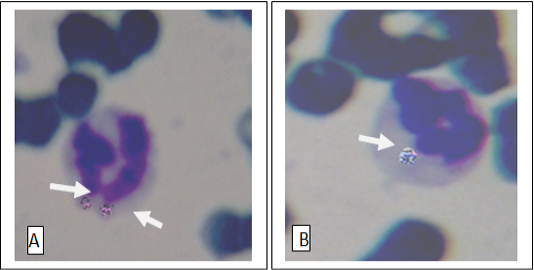
Figure 1: Anaplasma phagocytophilia organisms in a neutrophil (A) and Monocyte (B) Buffy coat smear; Giemsa stain. Magnification, 1,000.
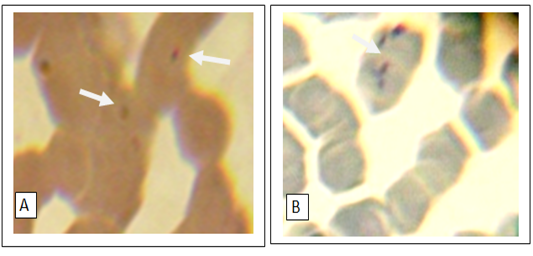
Figure 2: Numerous small basophilic structures of hemotropic Mycoplasma spp. organisms on the surface of the erythrocytes attached to cell membranes singularly(A) or in clustered (B). Peripheral blood smear; Giemsa stain. Magnification, 1,000.
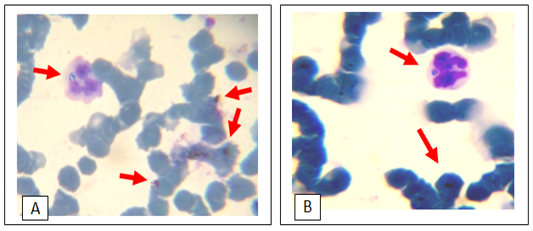
Figure 3: Giemsa stain light micrograph (Magnification, 1,000) of horse blood smear, co-infection of hemotropic Mycoplasma spp. and A. phagocytophila
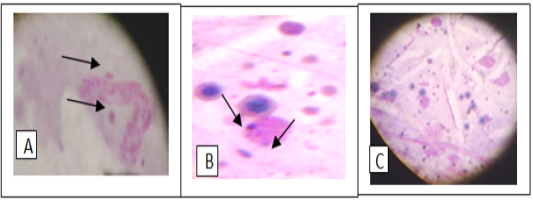
Figure 4: Giemsa stain of tick hemolymph cells infected with Anaplasma phagocytophilium (A), hemotropic Mycoplasma spp. (B) and co- infection of both bacteria (C).
DISCUSSION
Our investigation shows that Anaplasma phagocytophilum and Hemotropic Mycoplasma spp. infect horses in Iraq are relatively new emerging diseases which were first described in cattle of Mosul –Iraq by AL-Badrani and Rhaymah (2012); AL-Badrani (2013); Al-badrani and Al-abadi (2016), but have since become increasingly recognized in horses. In our study the diagnosis of Anaplasmosis was based on clinical signs, blood abnormalities and identification of Anaplasma phagocytophilum organisms (Ehrlichia morula) in blood and buffy coat smears. Visualization of more than one morula in the cytoplasm of granulocytes during examination of blood smears is highly suggestive of a diagnosis. Hemotropic mycoplasma were detected for the first time in the blood of 15 horses suffering from poor performance, apathy, weight loss, and anemia. Observable changes in blood films were recorded in horses infected with Anaplasmosis and hemoplasmosis , the most important abnormality was anemia, leucopenia and thrombocytopenia, but platelet counts are not helpful for diagnosis Mild anemia and reactive lymphocytosis are observed by Gribble (1969), Madigan (1993). Leucopenia occurs due to Anaplasma phagocytophilum organisms from the tick bite tend to be engulfed by white blood cells and this causes the destruction of these infection- fighting white blood cells (Rassouli. and Aghazamani, 2015). Left shift neutrophilia is often associated with inflammatory conditions. Bacterial infections, among other infectious diseases are more important for the left shift occurrence (Stevens et al., 2011). Because of the seasonality of the diseases, vector-associated transmission has long been suspected; recently, the causative agents were identified in three genus of ticks including Hyalomma spp., Rhipicephalus spp., and Boophilus spp., these are important ticks were classified in horses of Mosul city by Al-Moula and Rahemo (2004). A.phagocytophilum can be transmitted by members of the Ixodes spp (Richter et al., 1996). Hyalomma spp.might also play a role in transmitting A. phagocytophilum to cattle (Woldehiwet, 2010, Greene, 2012). Rhipicephalus spp., R. sanguineus serves as a vector and reservoir of M. haemocanis because of transstadial and transovarial transmission of the organism (Greene, 2012). The collection of tick hemolymph is important in the study of tick-borne pathogen transmission (Brossard & Wikel, 2004). Hemolymph collection can be difficult because the ticks are moving and it can be problematic to immobilize them without rupturing the midgut. Immobilizing the tick with double sided tape is an option, but the hemolymph can be lost onto the tape if the tick is not removed. Other studies have collected hemolymph from several ticks by cutting the tick’s legs at the distal joints and using centrifugation to collect the hemolymph (Burgdorfer, 1970). Once hemolymph collection is mastered the hemolymph can be used to determine tick infectivity, tick to pathogen interactions, and the migration of pathogens from the midgut to the salivary glands (Patton et al., 2012). The presence of A. phagocytophilum with the Haemoplasma species further strengthens the case for ticks being involved in the transmission of these organisms (Ayling et al., 2012). There were some reports of co- infection of A. phagocytophilum with Borrelia burgdorferi , Babesia spp., Rickettsia rickettsia in different hosts (Kordick et al., 1999; Nieto and Foley, 2009; Greene, 2012). In this research only co-infection of A. phagocytophilum with hemotropic Mycoplasma spp. was observed. Further researches are needed for accurate diagnosis of the reported infections and complete our findings are necessary. To our knowledge, no information is available regarding the presence of A. phagocytophilum and hemotropic Mycoplasma spp. in horses and ticks in Iraq.
ACKNOWLEDGEMENTS
The authors thank Dean of Veterinary Medicine College for financial support for surveillance work, the horse’s owners for their cooperation and the expert technical assistance from veterinary clinical pathology laboratory of veterinary teaching hospital, Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES






