South Asian Journal of Life Sciences
Research Article
Trophic Ecology of Hemichromis Fasciatus (Pisces: Cichlidae: Perciformes) from Opkara Stream, Oueme River, Northern Benin: Needs for Species Management and Rational Exploitation
Rachad Sidi Imorou, Alphonse Adite*, Stanislas P. Sonon, Hamidou Arame, Nambil K. Adjibade
Laboratoire d’Ecologie et de Management des Ecosystèmes Aquatiques (LEMEA), Département de Zoologie, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, BP 526 Cotonou, Benin.
Abstract |The carnivorous cichlid Hemichromis fasciatus (Peters, 1852) displays a wide distribution in the freshwaters and brackish waters of Tropical Africa and particularly in the West African water bodies. In Okpara stream of Oueme River in Benin, Hemichromis fasciatus is the dominant fish species making numerically 29.20% of the fish community. We investigated on the feeding ecology of this cichlid to evaluate the level of predation and impact on the Okpara stream fish community. Hemichromis fasciatus individuals were sampled monthly from December 2015 to May 2017 using an experimental gill net and a seine. The dietary analysis showed that H. fasciatus foraged mainly on fishes that made 61.212% of the diet, but also ingested aquatic insects (26.8%), zooplankton (2.721%) and detritus (4.09 %). H. fasciatus showed a moderate diet breadth ranging between 1.20 and 13.77 (mean =5.7) and diet overlaps among size categories varied between Øjk=0.21 and Øjk=1, indicating an ontogenetic diet shift. The study revealed an eco-morphological trend of the feeding habit indicated mainly by the increase of fish consumption with SL (r=0.52) and GL (r=0.68), the increase of detritus consumption with GL (r=0.332) and the decrease of zooplankton consumption with SL (r=-0.648) and GL (r=-0.51). Hemichromis fasciatus displayed a trophic flexibility behavior that favored seasonal and spatial diet variations. The piscivorous feeding pattern was also shown by the relative gut length (GL/SL = 0.73) that fall in the carnivorous range. The dominance of H. fasciatus and its high level of predation depicted in this study constitute a threat for the fish community. The implementation of a rational management and exploitation scheme of this carnivorous cichlid is required to guaranty a balanced food web and the survival of the Okpara stream fish community.
Keywords: Eco-morphology, Hemichromis fasciatus, management, Okpara stream, Piscivore.
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 12, 2019 Accepted | May 27, 2019; Published | August 14, 2019
*Correspondence | Alphonse Adite, laboratoire d’ecologie et de management des ecosystèmes aquatiques (lemea), département de zoologie, faculté des sciences et techniques, université d’abomey-calavi, bp 526 cotonou, benin.; Email: [email protected]
Citation | Sidi Imorou R, Adite A, Sonon SP, Arame H, Adjibade NK (2019). Trophic ecology of hemichromis fasciatus (pisces: cichlidae: perciformes) from opkara stream, oueme river, northern benin: needs for species management and rational exploitation. S. Asian J. Life Sci. 7(2): 46-61.
DOI | http://dx.doi.org/10.17582/journal.sajls/2019/7.2.46.61
ISSN | 2311–0589
Copyright © 2019 Sidi Imorou R et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Hemichromis fasciatus (Peters, 1852) is a voracious carnivorous cichlid that displays a wide distribution in the freshwaters and brackish waters of Tropical Africa where the species, though less abundant compared to tilapiine species, shows a great fisheries and commercial importance. Also called the banded jewelfish and five-spot cichlid, H. fasciatus is widely distributed throughout West Africa where the species is known from most hydrographic basins, from Senegal to Angola (Paugy et al., 2003), but also occurred in the Nile Basin, Lake Chad, and the upper Zambezi (Leveque et Paugy, 2006).
Taxonomic classsification indicated that H. fasciatus belongs to Animalia kingdom, Chordata phylum, Actinopterygii class, Perciformes order, Labroidei suborder, Cichlidae family, Hemichromis genus and Hemichromis fasciatus species (http://arctos.database.museum/name/Hemichromis%20fasciatus). Synonimies of H. fasciatus were Hemichromis elongates (Guichenot, 1861), Hemichromis leiguardii (Capello, 1870), Hemichromis desguezii (Rochebrune 1880), Hemichromis frempongi (Loiselle, 1979). Hemichromis fasciatus shows a cylindrical body with four to five large black glossy oval patches on the side of the body along the median line (Paugy et al., 2004). The first one is confluent with the opercular spot and the last one on the caudal fin base. The upper profile of the snout is straight or concave. The pre-maxillary is extremely protrusible and the lower jaw is very prominent. The body height is 32.3 to 37% the standard length and the head length is 32.3 to 40% the standard length. Also, the species shows 28-30 scales at the lateral line and 7 to 9 gill rakers on the lower part of the first gill arch (Paugy et al., 2004).
Hemichromis fasciatus occurs in habitats such as estuaries, lakes and lagoons (Albaret, 1994). The species is also found in running waters such as streams and rivers that exhibit a low current velocity, a high water level of about three (3) meters, a substrate made up of blocks and plants and tolerates a temperature range of 15 °C to 33 °C in the natural environment. H. fasciatus is stenothermal and cannot survive at temperatures that vary greatly from this range (Trewavas, 1983; Pouilly, 1993). This cichlid is a voracious carnivore with diet essentially made up of fishes (Fagade and Olaniyan, 1973; Adebesi 1981; Sene, 1994: Paugy 1994; Ndour 2007). Because of its carnivorous feeding habit, H. fasciatus is used in fish farming to regulate or to control the population of fishes of high reproductive potential such as Oreochromis niloticus. The species is also widely utilized as ornamental fish and raised and maintained in aquarium because of its colorful body.
In Benin, H. fasciatus was found not only in rivers and streams such as Couffo, Mono, Oueme, Zou, Okpara, Sô, Niger, Hlan etc., but also in lakes and lagoons such as Lake Nokoue, Porto-Novo lagoon, Lake Aheme, Lake Hlan, Coastal lagoon, Toho-Todougba lagoon, Lake Cele etc. (Adite and Van Thielen, 1995, Hazoume et al., 2017; Niyonkuru and Laleye, 2010; Montchowui et al., 2007; Laleye et al., 2004). Numerically, in all these ecosystems, H. fasciatus relative abundance has never exceeded 6% of the fish community. Indeed, numerical abundance of H. fasciatus in Southern Benin water bodies such as Lake Toho, Coastal lagoon, Sô river, “Whedo” from Oueme River, mangrove ecosystems and the ecotonal zone of the Mono River ranged between 0.37% and 6% (Jackson et al., 2013, Adite et al., 2013; 2017, Hazoume 2017). In contrast, Sidi Imorou et al. (2019) reported that in the Okpara stream, H. fasciatus was the most dominant fish making numerically 29.49% of the fish community. This abundance indicated a high predation in the Okpara fish community and thus, implies that H. fasciatus could negatively affect the food web and constituted a risk in term of species survival and ecosystem balance. However, in the Okpara stream, nothing is known on the bioecology of this carnivorous cichlid and in particular, data on the feeding ecology and resource utilization are scant and not documented.
The current study aims at studying the trophic ecology of H. fasciatus in order to evaluate the level of predation and provide dietary data that will contribute to design an appropriate management scheme for species survival, species conservation and ecosystem balance.
Materials and methods
Study Area
This study was implemented on the Okpara stream, the longest tributary of the Oueme River. The Okpara stream belongs to the northern hydrographic system and take source at the Southwest of Nikki city at an altitude of 450 m (Laleye et al., 2004). The Okpara stream is located between 8°14’- 9°45’ North and 2°35’-3°25’ East and extended on about 200 Km (Laleye et al., 2004). The climate is tropical with three main seasons, the dry season (November - April), the wet season (May –August) and the flood period (September - October). The annual average temperature was 26.6 °C and lower values were recorded in December and January. The mean rainfall was about 1200 mm with a peak (1300 mm) recorded in July, August or September (Kora, 2006; INSAE, 2004). Most of the soils are tropical ferruginous and alluvial with deposits of sediments left by the stream (Dossou-Yovo, 2009; Ogouwale, 2013). In general, the soil is covered by a wooded savanna characterized by the presence of Parkia biglobosa, Khaya senegalensis and Vitellaria paradoxa. Also, the vegetation included marshy meadows, bamboo and fallow bushes. Multi-species fisheries occur in the Okpara stream that appears to be the main source of fish resources for the surrounding populations and even for most cities of the northern region of Benin (Sidi et al., 2019). The stream provides water for irrigated agriculture and supply the surrounding populations with drinking water from a dam built by SONEB, the Benin water company (Sidi et al., 2019).
Sampling Sites
Five (05) sampling sites were selected (Figure 1). These sites were chosen according to localities, accessibility, fisheries importance and level of site degradations (Sidi et al., 2019). Site 1 is situated in Perere town at Okpara up stream and Site 2 is localized in Parakou town at Gadela village (Okpara up stream) about 2 km from SONEB dam. Site 3 is located at Kpassa village where a dam was built to serve as a source of drinking water for the populations of Tchaourou and Parakou towns and surrounding villages. Site 4 is situated around Okpara downstream at Yarimarou village (Tchaourou town) where the dam withdraws its water and Site 5 is also located around Okpara downstream at Sui village of Tchaourou. At all stations, samplings were done in the “aquatic vegetation habitat” at the edge of the stream and in the “open water habitat” characterized by a high depth and exempt of vegetation.
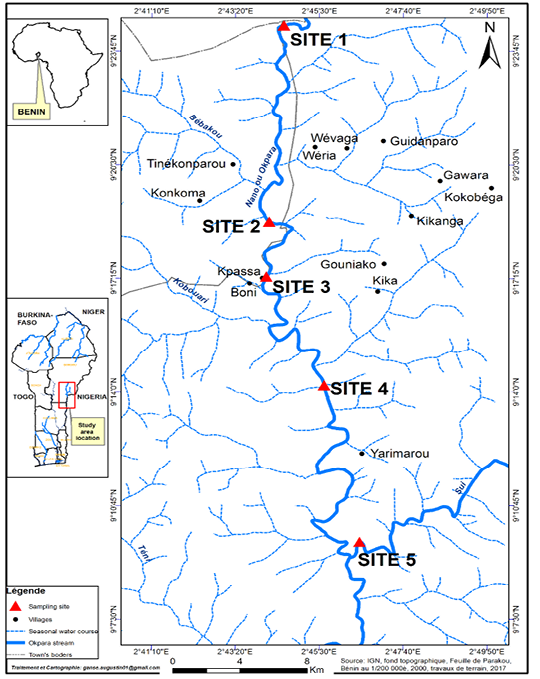
Figure 1: Okpara stream and sampling sites. Site 1= Perere Township, Site 2 = Gadela village (Parakou Township), Site 3= Kpassa village (Tchaourou Township), Site 4= Yarimarou village (Tchaourou Township), Site 5 = Sui village (Tchaourou Township).
Fish Sampling
Hemichromis fasciatus individuals were collected once a month in all habitats from December 2015 to May 2017 at the five sampling sites. Experimental fishings have been done in the open water with an experimental gill net (25 m x 1.30 m, 30 mm-mesh; 25 m x 1.30 m, 15 mm-mesh) and in marginal aquatic vegetation with a seine (4.20 m-length, 2 m – width, 5 mm-mesh) (Adite et al., 2013). In addition, fish samplings were directly made in the fishermen artisanal captures and one third of each fisherman catches was sampled (Okpeicha, 2011). After collection, the fish samples were first identified in situ using fish identification references such as Reed et al., (1967), Van Thielen et al., (1987), Skelton (1993), Paugy et al., (2004), Lévêque et al., (1990-1992). The fish assemblages were preserved in a cooler and then transported to the Laboratory of Ecology and Management of Aquatic Ecosystem (LEMEA) to confirm identifications. In the laboratory, each individual of H. fasciatus was preserved in 10% formalin and latter in 70% ethanol to facilitate stomach content analysis (Murphy and Willis, 1996).
Stomach Content Analysis
In the laboratory, fish names were confirmed and each specimen was measured for total length (TL) and standard length (SL) to the nearest 0.1 cm and weighted to the nearest 0.1g. Hemichromis fasciatus individuals were then dissected, the digestive tract was unrolled and lengths were measured to the nearest 0.1 cm. After opening the gut, the stomach content was extracted and spread on a container for examination first under a binocular (model: Pierron) to identify large preys. Prey identifications were made to the lowest taxonomic level by using Needham (1962) identification key. Sometimes, water was added to facilitate separation of small items. To identify the plankton (phytoplankton and zooplankton), a sub-sample of known volume of the stomach contents was taken and examined under a photonic microscope (Winemiller, 1990; Adite et al., 2005). Then the total gross volume was determined by the method of water displacement using a graduated burette (Winemiller, 1990; Adite, 2007). The volumes of small food resources (<0.002 ml) were estimated by spreading the food item on a glass slide. Then, the volume was visually approximated by comparison with a 0.01 ml drop of water delivered with a pipet onto a glass slide. In addition to the volume, the numerical method was used by counting each preys. To count the preys like insects parts, detritus and unidentified preys, the number 1 was attributed to their presence in the stomach whatever their quantity and weight (Rosecchi and Nouaze, 1987).
Data Analysis
A total of 2,357 specimens of H. fasciatus were dissected. For each individual, the volume of each identified prey was recorded on Excel spreadsheet and the volumetric proportion was computed following the formula (Adité et al., 2005):
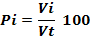
Where Pi is the volumetric proportion of item i, Vi is the volume of food item i and the total volume of food ingested by n stomachs, n is the total number of stomachs examined. The Coefficient of Emptiness (Ec), the ratio between the number of empty stomachs (Ne) and all examined stomachs (Nt) was calculated following the formula:
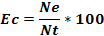
Also, the numeric frequency (%N) of the preys was computed following the formula:
%N = (ni/N)*100
Where ni is the number of individuals of a given prey i, and N the total number of all preys.
The relative importance of the prey in the diet of H. fasciatus was estimated by the occurrence frequency (FO) of prey consumed. This index is calculated according to the formula:
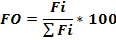
With 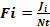
Where Ji = number of stomachs containing prey i, Nt = total number of stomachs examined.
According to Sorbe (1972), prey importance was classified as follows:
FO <10% = accidental prey
10% ≤ FO ≤ 50% = secondary prey
FO> 50% = preferential prey
To express the importance of prey in the stomach contents of a fish, Lauzanne (1975) combined the volumetric percentage (Pi) and the frequency of occurrence (FO) of the prey to generate the dietary index (DI):
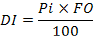
This index varies from 0 to 100:
For values less than 10, the prey has secondary importance.
10 ≤ DI < 25 the prey is important.
25 ≤ DI ≤ 50, the prey is essential in diet.
DI >50, the prey is largely dominant.
The diet breadth (DB) is a measure of food spectrum. DB was determined using Simpson (1949) diet breadth formula:
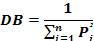
Where Pi is the proportion of food item i in the diet, and n is the total number of food items in the diet. DB ranges from 1, when only one resource is used, to n, when all resources are consumed in equal proportions. The diet overlap (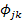 ) between two groups of different classes was calculated following Pianka’s (1976) overlap index:
) between two groups of different classes was calculated following Pianka’s (1976) overlap index:
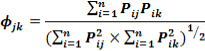
With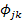 , the dietary overlap between species j and species k, pij the proportion of resource i used by species j, pik the proportion of resource i used by species k, and n is the number of resource categories utilized.
, the dietary overlap between species j and species k, pij the proportion of resource i used by species j, pik the proportion of resource i used by species k, and n is the number of resource categories utilized.
Statistical Analysis
Seasonal (wet, flood, dry) variations in volumetric proportions were depicted with one-way analysis of variances (ANOVA1) using SPSS software (Morgan et al., 2001). The values of diet breadth were submitted to one-way analysis of variance (ANOVA1) using SPSS software. Eco-morphological relationships have been explored through linear regression analysis (LRA) between body weight (W) and gut length (GL), standard length (SL) and gut length (GL). The data of W, SL and GL underwent logarithmic transformations before running the LRA. The ratio of gut length and standard length (GL/SL) was computed and compared to references indices in order to characterize the type of diet (herbivore, omnivore or carnivore).
Results
Internal Anatomy
Hemichromis fasciatus possesses a short tubular esophagus extended from the posterior end of the pharynx to the anterior cardiac region of the stomach. The stomach is divided in three parts, (1) the cardiac, (2) the fundic and (3) the pyloric regions (Figure 2).
Diet Composition
In Okpara stream, the dietary analysis of 2,357 stomach contents of H. fasciatus showed that this cichlid ingested about 61 food items composed of various taxa such as aquatic insects, fishes, frogs, shrimps, mollusks, zooplankton and detritus (Table 1). Dominant prey categories were fishes with a volumetric proportion of 61.212%, followed by insects (26.8%), detritus (4.096%), zooplankton (2.995%) and frogs (1.062%) aggregating about 96.165% of the diet. The most dominant fishes in the diet were cichlids (13.545%) mainly Oreochromis and Tilapia, Alestidae (9.934%), Mormyridae (4.484%), unidentified fishes (16.82%), fish eggs (6.82%) and some minor fish items such as Cyprinidae (Barbus) and Clariidae. Dominant aquatic insects ingested were Odonata (14.988%) with families such as Libellulidae (7.769%) and Coenagrionidae (2.278%), Diptera (4.726%) composed mainly of Chironomidae (3.891%) and Ceratopogonidae (0.431%) (Table 1), Ephemeroptera, Trichoptera, Coleoptera etc. Zooplankton (2.995%) was constituted of Ploima, Copepoda and Cladocera (Table 1). Minor food resources consumed by H.
Table 1: Ingested preys by Hemichromis fasciatus collected in Okpara stream from December 2015 to May 2017
| Prey categories | Orders | Families | Genera |
Volumetric percentage (%) |
Numeric percentage (%) |
Occurrence frequency (%) |
| Zooplankton | Ploima | Gastropodidae | Chromogaster | 0.013 | 0.120 | 0.635 |
| Copepoda | - | Unidentified | 0.001 | 0.04014 | 0.317 | |
| Cladocera | - | Nauplius | 0.528 | 5.921 | 2.540 | |
| Cladocera |
Daphniidae |
Daphnia | 2.179 | 37.90 | 9.524 | |
|
Total Zooplankton |
2.721 | 43.98 | 13.016 | |||
| Insects | Coleoptera | Dytiscidae | Hydrovatus | 0.059 | 0.04014 | 0.317 |
| Elmidae |
Macronychus Larvae |
0.053 | 0.02007 | 0.317 | ||
| Elmidae (unidentified) | 0.080 | 0.10 | 1.587 | |||
| Hdrophilidae |
Hydrobius larvae |
0.136 | 0.0803 |
0.952 |
||
| Hydrocara | 0.071 | 0.020 | 0.317 | |||
| Unidentified Coleoptera | 0.536 | 0.12043 | 1.905 | |||
| Diptera | Chironomidae |
Chironomus Larvae |
0.328 | 0.46166 |
1.905 |
|
|
Chironomus Nymphea |
0.490 | 0.86311 | 3.810 | |||
|
Chaoborus Larvae |
0.572 | 2.52910 | 4.444 | |||
|
Chaoborus Nymphea |
2.501 | 10.478 | 4.762 | |||
| Ceratopogonidae |
Dashyheleinae Larvae |
0.286 | 1.365 | 2.540 | ||
| Ceratopogoninae | 0.145 | 0.622 | 2.222 | |||
| Dixidae |
Dixa Nymphea |
0.168 | 0.18065 | 1.270 | ||
| Culicidae |
Culex Larvea |
0.177 | 0.26094 | 2.222 | ||
|
Culicinae Larvea |
0.059 | 0.2409 | 1.587 | |||
| Odonata | Libellulidae |
Libellula Larvea |
2.088 | 0.723 | 4.762 | |
|
Sympetrum Larvae |
5.681 | 0.923 | 7.302 | |||
| Coenagrionidae |
Coenagrion Larvae |
2.278 | 0.1606 | 2.857 | ||
| Lestidae | Lestes | 0.236 | 0.04014 | 0.635 | ||
| Aeshnidae |
Aeshna Larvea |
0.354 | 0.0201 | 0.317 | ||
| Cordulegastridae | Cordulegaster | 0.177 | 0.0201 | 0.317 | ||
| Unidentified Odonata | 4.165 | 1.064 | 13.651 | |||
| Plecoptera | Perloidae | - | 0.006 | 0.0201 | 0.317 | |
| Ephemeroptera | Baetidae |
Baetis Larvae |
0.121 | 0.281 | 1.905 | |
|
Baatopus Larvae |
0.531 | 3.31 | 0.317 | |||
| Unidentified Baetidea | 1.245 | 4.82 | 7.937 | |||
| Leptophlebiidae | Paraleptophlebia | 0.024 | 0.14051 | 0.317 | ||
| Unidentified Ephemeroptera | 0.378 | 0.18065 | 2.540 | |||
| Heteroptera | Hydrometridae | Hydrometra | 0.189 | 0.04014 | 0.635 | |
| Gerridae | Gerris | 0.28 | 0.14051 | 1.905 | ||
| Corixidae | Corixa | 0.153 | 0.12 | 0.635 | ||
| Pleidae | Plea | 0.218 | 0.56 | 0.952 | ||
| Unidentified Heteroptera | 0.177 | 0.161 | 1.905 | |||
| Trichoptera | Hydroptilidae |
Hydroptila Lavae |
0.071 | 0.04014 | 0.635 | |
|
Hydroptila etui |
0.059 | 0.02007 | 0.317 | |||
|
Tricholeiochiton Larvae |
0.032 | 0.04014 | 0.635 | |||
| Philopotamidae |
Philopotamus Larvare |
0.354 | 0.06022 | 0.635 | ||
| Unidentified Trichoptera | 0.851 | 0.18065 | 2.539 | |||
| Insects cocoon | 0.236 | 0.16058 | 0.317 | |||
| Unidentified Insects | 1.234 | 0.42152 | 5.714 | |||
|
Total Insects
|
26.800 | 28.34 | 62.54 | |||
| Fish | Alestidae | Brycinus | 8.259 | 0.321 | 4.444 | |
| Unidentified Alestidae | 1.675 | 0.08029 | 0.635 | |||
| Cichlidae | Oreochromis | 0.708 | 0.02007 | 0.317 | ||
| Tilapia | 12.837 | 0.522 | 5.714 | |||
| Cyprinidae | Barbus | 1.463 | 0.08029 | 0.635 | ||
| Distichodontidae | 3.009 | 0.14051 | 0.635 | |||
| Mormyridae | Unidentified | 0.708 | 0.02007 | 0.317 | ||
| Marcusenius | 3.776 | 0.02007 | 0.317 | |||
| Clariidae | - | 0.991 | 0.02007 | 0.317 | ||
| Fish Larvae | - | - | 3.430 | 3.111 |
4.762 |
|
| Fish scale | - | - | 0.558 | 0.08029 | 4.762 | |
| Fish eggs | 6.820 | 21.12 | 4.762 | |||
| Unidentified fish | - | 16.978 | 0.56202 |
13.016 |
||
|
Total Fishes
|
61.212 | 26.82052 | 54.603 | |||
| Frog | - | - | - | 1.062 | 0.04014 | 0.635 |
| Shrimp | - | - | - | 0.708 | 0.06022 | 0.952 |
| Mollusca | - | - | - | 0.001 | 0.08029 | 0.635 |
| Detritus | - | - | - | 4.096 | 0.04014 | 13.016 |
| Unidentified | - | - | - | 3.4 | 0.20072 | 16.190 |
| Total | - | - | - | 100 | 100 |
- |
Table 2: Seasonal variations of Volumetric percentage (%) of preys consumed by seasons of Hemichromis fasciatus from Okpara stream of Oueme river (North-Benin)
| Prey categories | Orders | Families | Genera | Dry | Wet | Flood | |
|
Zooplankton
|
Ploima | Gastropodidae | Chromogaster | 0.031 | - |
- |
|
| Copepoda | - | Unidentified | 0.003 | - | - | ||
| Cladocera | - | Nauplius | - | 1.13 | - | ||
| Cladocera | Daphniidae | Daphnia | - | 4.66 | - | ||
| Insect | Coleoptera | Dytiscidae | Hydrovatus | - | 0.13 | - | |
| Elmidae |
Macronychus Larvae |
- | 0.11 | - | |||
| Elmidae (unidentified) | - | 0.17 | - | ||||
| Hydrophilidae |
Hydrobius larvae |
- | 0.29 | - | |||
|
Hydrocara |
- | 0.15 | - | ||||
| Unidentified Coleoptera | 0.30 | 0.88 | - | ||||
| Diptera | Chironomidae |
Chironomus Larvae |
0.53 | 0.23 | - | ||
|
Chironomus Nymphea |
1.01 | 0.15 | - | ||||
|
Chaoborus Larvae |
- | 1.22 | - | ||||
|
Chaoborus Nymphea |
1.08 | 4.39 | - | ||||
| Ceratopogonidae |
Dashyheleinae Larvae |
- | 0.61 | - | |||
| Ceratopogoninae | 0.05 | 0.26 | - | ||||
| Dixidae |
Dixa Nymphea |
- | 0.36 | - | |||
| Culicidae |
Culex Larvea |
- | 0.38 | - | |||
|
Culicinae Larvea |
- | 0.13 |
- |
||||
| Odonata | Libellulidae |
Libellula Larvea |
0.01 | 4 | - | ||
|
Sympetrum Larvae |
- | 12.15 | - | ||||
| Coenagrionidae |
Coenagrion Larvae |
3.69 | 1.59 | - | |||
| Lestidae | Lestes | - | 0.25 | - | |||
| Aeshnidae |
Aeshna Larvea |
- | 0.76 |
- |
|||
| Cordulegastridae | Cordulegaster | - | 0.38 | - | |||
| Unidentified Odonata | 2.84 | 6.64 | - | ||||
| Plecoptera | Perloidae | - | 0.01 | - | - | ||
| Ephemeroptera | Baetidae |
Baetis Larvae |
- | 0.26 | - | ||
|
Baatopus Larvae |
- | 1.14 | - | ||||
|
Unidentified Baetidea |
- | 2.66 | - | ||||
| Leptophlebiidae | Paraleptophlebia | - | 0.05 | - | |||
| Unidentified Ephemeroptera | 0.0003 | 0.81 |
- |
||||
| Heteroptera | Hydrometridae | Hydrometra | - | 0.40 | - | ||
| Gerridae | Gerris | - | 0.56 | 0.18 | |||
| Corixidae | Corixa | 0.01 | 0.30 | - | |||
| Pleidae | Plea | - | 0.47 | - | |||
| Unidentified Heteroptera | 0.43 | - | |||||
| Trichoptera |
Hydroptilidae |
Hydroptila Lavae |
- | 0.15 | - | ||
|
Hydroptila etui |
- | 0.13 | - | ||||
|
Tricholeiochiton Larvae |
- | 0.07 |
- |
||||
| Philopotamidae |
Philopotamus Larvare |
- | 0.76 | - | |||
| Unidentified Trichoptera | 1.48 | 0.26 | 1.05 | ||||
| Insects cocoon | - | 0.5 | |||||
|
Unidentified Insects
|
1.05 | 0.68 | 1.78 | ||||
| Fish | Alestidae | Brycinus | 12.78 | 6.31 | - | ||
| Unidentified Alestidae | - | - | 14.87 | ||||
| Cichlidae | Oreochromis | 1.51 | - | ||||
| Tilapia | 25.55 | 3.23 | 6.28 | ||||
| Cyprinidae | Barbus | 3.52 | - | - | |||
| Distichodontidae | Unidentified | - | - | 26.70 | |||
| Mormyridae | Unidentified | 1.70 | - | - | |||
| Marcusenius | 9.09 | - | - | ||||
| Clariidae | - | 2.39 | |||||
| Fish Larvae | - | - | 0.51 | 6.04 | 3.48 | ||
| Fish scale | - | - | 0.87 | 0.26 | - | ||
| Fish eggs | - | - | 3.24 | 11.71 | - | ||
|
Unidentified fish
|
- | 18.74 | 8.81 |
45.02 |
|||
| Frog | - | - | - | 1.70 | 0.76 | - | |
| Shrimp | - | - | - | 0.57 | 1.01 | - | |
| Mollusca | - | - | - | 0.003 | - | - | |
| Detritus | - | - | - | 5.66 | 3.43 | 1.36 | |
| Unidentified | - | - | - | 1.58 | 5 | - | |
| Total | - | - | - | 100 | 100 |
100 |
|
fasciatus were frog (1.062%), shrimps (0.708%) and mollusks (0.001%).
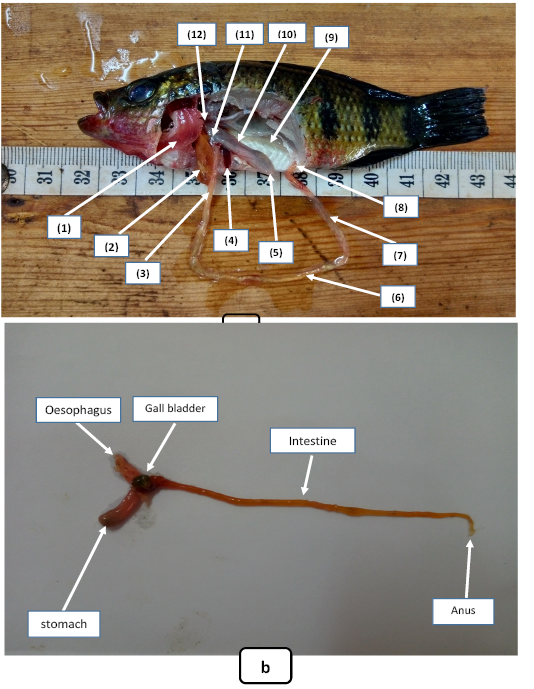
Figure 2: Internal anatomy of Hemichromis fasciatus : a= internal disposition of organs (1= gills; 2= liver; 3= anterior intestine; 4= spleen; 5= pyloric stomach; 6= middle intestine; 7= post intestine; 8= anus; 9= testis immature; 10= fundic stomach; 11= cardiac stomach; 12= oesophagus) b= Structure of digestive tracts.
Seasonal Variations of Diet
Table 2 shows the volumetric proportions of food resource consumed by H. fasciatus during dry, wet and flood seasons in the Okpara stream. One-way analysis of variance (ANOVA 1) on the food items consumed by H. fasciatus, showed significant (p<0.05) seasonal variations for zooplankton (F2, 2355 = 20.136, p = 0.0001), for fish (F2, 2355 = 5.368, p = 0.005), for frogs (F2, 2355 = 5.805, p = 0.003), and for insects (F2, 2355 = 29.581, p = 0.000). Indeed, the results indicated that proportional consumption of zooplankton and aquatic insects were higher during the wet season. Also, proportional consumption of fishes and frogs were higher during the flooding. Nevertheless, there were no significant (p>0.05) seasonal dietary variations for shrimps (F2,2355=0.281, p=0.755), mollusks (F2,2355=0.461, p=0.631), fish eggs (F2,2355=1.849, p=0.158) and detritus (F2,2355=0.115, p=0.892).
Frequency of Occurrence and Dietary Index
The analysis of the occurrence frequencies of food resources revealed that aquatic insects occurred in 197 stomachs corresponding to a frequency of occurrence FO = 62.54%
while fishes occurred in 109 stomachs (FO=54.603%), making these two (2) food items, aquatic insects and fishes, the preferential food resources for H. fasciatus (Table 1). Detritus and zooplankton were found in 51 (FO=16.19%) and 41 (13.016%) stomachs, respectively, and therefore were qualified as secondary preys in the diet of this piscivorous cichlid (Table 1). Accidental food items like shrimps (FO=0.952%), frogs (FO=0.635%) and mollusks (FO=0.635%) occurred only in few stomachs, 3, 2 and 2 respectively. In addition, computed dietary index (DI) for each food category indicated that fishes with DI = 33.42 appeared to be the “essential prey” for H. fasciatus whereas aquatic insects with DI = 16.76 represented the “important prey items”. Detritus, zooplankton, frogs, shrimps and mollusks with DI <10 represented the preys of secondary importance (Table 3).
Table 3: Dietary index (DI) values for different categories of preys ingested by Hemichromis fasciatus from Okpara stream
| Prey categories | Dietary index (DI) |
Food importance |
| Fish | 33.42 | Essential prey |
| Insects | 16.76 | Important prey |
|
Vegetal debris |
0.533 | Secondary prey |
|
Zooplankton |
0.35 |
Secondary prey |
| Frog | 0.0067 | Secondary prey |
| Shrimp | 0.0067 | Secondary prey |
| Mollusk | 0.00636 |
Secondary prey |
|
Unidentified |
0.4848 |
Secondary prey |
Empty Stomachs
Of a total of 2,357 stomachs of H. fasciatus examined, 2042 stomachs corresponding to 86.64% were empty (Table 3). In general, the coefficient of emptiness varied with seasons and life stages. Seasonally, the percentages of empty stomachs were higher during the flood and dry periods and reached 77.39% and 87.58%, respectively, whereas during the wet season, empty stomachs were reduced and did not exceed 57.10%. Ontogenetically, empty stomachs were higher in small juveniles and averaged 90.47, whereas moderate percentages, 87.10% and 86.26%, were recorded among adults and sub-adults, respectively (Table 4).
Table 4: Seasonal variations of emptiness coefficients (Ec) of H. fasciatus from Okpara stream
|
H. fasciatus life stage |
Dry | Wet | Flood | Total | |||||||
| Nt | Ne | Ec (%) | Nt | Ne | Ec (%) | Nt | Ne | Ec (%) | N | Ec (%) | |
| Small Juveniles | 80 | 78 | 97.5 | 25 | 17 | 68.0 | - | - | 105 |
90.47 |
|
| Juveniles | 43 | 38 | 88.37 | 35 | 23 | 65.71 | - | - | 78 | 83.33 | |
| Sub-adults | 20 | 18 | 90 | 16 | 9 | 51.82 | 60 | 50 | 83.33 | 96 | 87.10 |
| Adults | 1164 | 1139 | 97.87 | 859 | 516 | 60.08 | 55 | 39 | 70.91 | 2073 |
86.26 |
| Total | 1307 | 1145 | 87.58 | 935 | 534 | 57.10 | 115 | 89 | 77.39 | 2357 |
86.64 |
Table 5: Variation of diet breadth (DB) by season and length classes of Hemichromis fasciatus from Okpara’s stream
| Seasons | Length Class (TL, cm) | ||||
| Small juveniles (TL<3) | Juveniles (3≤TL<6) | Sub adults (6≤TL<8) | Adults (TL≥8) | Total | |
| Wet | - | 2.3 | 2.10 | 17.83 | 16.40 |
| Flood | 1 | 2.15 | 2.45 | 4.83 | 3.31 |
| Dry | 1.20 | 3.58 | 2.20 | 8.17 | 7.42 |
| Total | 1.20 | 3.58 | 4.25 | 13.77 |
17.41 |
Table 6: Matrix of diet overlaps by size classes of H. fasciatus from Okpara stream
| Length class | [1 ; 3[ | [3 ; 5[ | [5 ; 7[ | [7 ; 9[ | [9 ; 11[ | [11 ; 13[ | [13 ; 15[ | [15 ; 17[ |
| [1 ; 3[ | 1 | 0.21 | 0.99 | 0.47 | 0.65 | 0.99 | 0.99 | 1 |
| [3 ; 5[ | 1 | 0.26 | 0.86 | 0.85 | 0.32 | 0.24 |
0.21 |
|
| [5 ; 7[ | 1 | 0.51 | 0.69 | 0.99 | 1 | 1 | ||
| [7 ; 9[ | 1 | 0.95 | 0.57 | 0.5 | 0.47 | |||
| [9 ; 11[ | 1 | 0.74 | 0.68 | 0.65 | ||||
| [11 ; 13[ | 1 | 0.99 | 0.99 | |||||
| [13 ; 15[ | 1 | 1 | ||||||
| [15 ; 17[ | 1 |
Niche Breadth
In the Okpara stream, the the Diet breadth (DB) computed for the whole population of H. fasciatus was high and reached DB=17.41, indicating that this species consumed a wide range of food resources. Ontogenetically, the DB increased with fish size (Table 5) and ranged from 1.20 for small juveniles (TL<3cm) to 13.77 for adults (TL≥8 cm) (Table 5). Seasonal variations indicated that the DB was higher during the wet season and reached DB=16.40 whereas lower values, 3.31 and 7.42 were recorded during the flood and dry periods, respectively (Table 5).
Pianka’s Diet Overlaps
The diet similarities of H. fasciatus between different size classes were evaluated through Pianka’s diet overlaps index (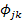 ). The matrix of diet overlaps gave
). The matrix of diet overlaps gave 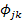 ranging between 0.21 and 1 (Table 6). The lowest value (
ranging between 0.21 and 1 (Table 6). The lowest value (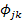 = 0.21) was recorded for the similarity between the subpopulations of size classes “TL: 1-3 cm” and “TL: 3-5 cm”. The highest value (
= 0.21) was recorded for the similarity between the subpopulations of size classes “TL: 1-3 cm” and “TL: 3-5 cm”. The highest value (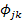 = 1) was recorded for the similarities between the sizes classes “TL: 1-3 cm” and “TL: 15-17 cm”; TL: 5-7 cm” and “TL: 13-15 cm”; TL: 5-7 cm” and “TL: 15-17 cm”; TL: 13-15 cm” and “TL: 15-17 cm
= 1) was recorded for the similarities between the sizes classes “TL: 1-3 cm” and “TL: 15-17 cm”; TL: 5-7 cm” and “TL: 13-15 cm”; TL: 5-7 cm” and “TL: 15-17 cm”; TL: 13-15 cm” and “TL: 15-17 cm
Ecomorphological Correlates
Table 8 shows the matrix of correlation coefficients (r) from the regressions between the different food categories (fishes, insects, zooplankton, detritus, shrimps, frogs, mollusks) and standard length (SL)/ gut length (GL). Overall, the correlation coefficients (r) for the regressions between the food items and standard length (SL) ranged between r = -0.648 and r =0.866 and those with gut length (GL) ranged between r = -0.51 and r =0.866. The food items such as fishes, zooplankton, shrimps and detritus crossed with SL and GL exhibited the higher correlation coefficients (r) whereas aquatic insects displayed an insignificant (p>0.05) correlation when crossed with SL and GL.
Also, the linear regressions between the standard length (SL) and gut length (GL) and between body weight (W) and gut length (GL) were established to evaluate the eco-morphological model of the food habit of H. fasciatus. The resulting regression equations were as follows:
Table 7: Volumetric percentage (%) of preys consumed by size classes (cm,TL) of Hemichromis fasciatus from Okpara stream, Oueme river (North-Benin)
| Prey categories | Orders | Families | Genera | Small juveniles (TL<3) | Juveniles (3≤TL<6) | Sub adults (6≤TL<8) | Adults (TL≥8) | |
| Zooplankton | Ploima | Gastropodidae | Chromogaster | 9.09 | 0.2 | - | - | |
| Copepoda | - | Unidentified | - | 0.2 | - | - | ||
| Cladocera | - | Nauplius | - | - | - | 0.54 | ||
|
Cladocera
|
Daphniidae | Daphnia | - | - | - | 2.21 | ||
| Insect | Coleoptera | Dytiscidae | Hydrovatus | - | - | - |
0.06 |
|
| Elmidae |
Macronychus Larvae |
- | - | - | 0.05 | |||
| Elmidae (unidentified) | - | - | - | 0.08 | ||||
| Hydrophilidae |
Hydrobius larvae |
- | - | - | 0.14 | |||
| Hydrocara | - | - | - | 0.07 | ||||
| Unidentified Coleoptera | - | - | - | 0.54 | ||||
| Diptera | Chironomidae |
Chironomus Larvae |
- | 37.6 | - | 0.11 | ||
|
Chironomus Nymphea |
- | - | - | 0.50 | ||||
|
Chaoborus Larvae |
- | - | - | 0.58 | ||||
|
Chaoborus Nymphea |
- | - | - | 2.54 | ||||
| Ceratopogonidae |
Dashyheleinae Larvae |
- | - | - | 0.29 | |||
| Ceratopogoninae | - | - | - |
0.15 |
||||
| Dixidae |
Dixa Nymphea |
- | - | - | 0.17 | |||
| Culicidae |
Culex Larvea |
- | - | - | 0.18 | |||
|
Culicinae Larvea |
- | - | - | 0.06 | ||||
| Odonata | Libellulidae |
Libellula Larvea |
- | - | - | 2.13 | ||
|
Sympetrum Larvae |
- | - | - |
5.77 |
||||
| Coenagrionidae |
Coenagrion Larvae |
- | - | 28.57 | 2.19 | |||
| Lestidae | Lestes | - | - | - | 0.12 | |||
| Aeshnidae |
Aeshna Larvea |
- | - | - | 0.36 | |||
| Cordulegastridae | Cordulegaster | - | - | - | 0.18 | |||
| Unidentified Odonata | - | - | - | 4.35 | ||||
| Plecoptera | Perloidae | - | - | - | 1.43 | - | ||
| Ephemeroptera | Baetidae |
Baetis Larvae |
- | - | - | 0.12 | ||
|
Baatopus Larvae |
- | - | - | 0.54 | ||||
| Unidentified Baetidea | - | - | - | 1.26 | ||||
| Leptophlebiidae | Paraleptophlebia | - | - | - | 0.024 | |||
| Unidentified Ephemeroptera | - | - | - | 0.38 | ||||
| Heteroptera | Hydrometridae | Hydrometra | - | - | - | 0.19 | ||
| Gerridae | Gerris | - | - | - | 0.29 | |||
| Corixidae | Corixa | - | - | 1.43 | 0.14 | |||
| Pleidae | Plea | - | - | - | 0.22 | |||
| Unidentified Heteroptera | - | - | - |
0.18 |
||||
| Trichoptera | Hydroptilidae |
Hydroptila Lavae |
- | - | - | 0.07 | ||
|
Hydroptila etui |
- | - | - | 0.06 | ||||
|
Tricholeiochiton Larvae |
- | - | - | 0.03 | ||||
| Philopotamidae |
Philopotamus Larvare |
- | - | - | 0.36 | |||
| Unidentified Trichoptera | - | - | - | 0.86 | ||||
| Insects cocoon | - | - | - | 0.24 | ||||
|
Unidentified Insects
|
- | 28 | 25.71 | 0.98 | ||||
| Fish | Alestidae | Brycinus | - | - | - | 8.39 | ||
| Unidentified Alestidae | - | - | - | 1.70 | ||||
| Cichlidae | Oreochromis | - | - | - | 0.72 | |||
| Tilapia | - | - | - | 13.04 | ||||
| Cyprinidae | Barbus | - | - | - | 1.49 | |||
| Distichodontidae | Unidentified | - | - | - | 3.06 | |||
| Mormyridae | Unidentified | - | - | - | 0.72 | |||
| Marcusenius | - | - | - | 3.83 | ||||
| Clariidae | - | - | - | - | 1.01 | |||
| Fish Larvae | - | - | 90.91 | 14.0 | - | 3.28 | ||
| Fish scale | - | - | - | - | - | 0.57 | ||
| Fish eggs | - | - | - | - | - | 6.93 | ||
|
Unidentified fish
|
- | - | 20.0 | 25.71 | 17.01 | |||
| Frog | - | - | - | - | - | - |
1.08 |
|
| Shrimp | - | - | - | - | - | - | 0.72 | |
| Mollusca | - | - | - | - | - | - |
0.0013 |
|
| Vegetal debris | - | - | - | - | - | 2.86 | 4.15 | |
| Unidentified | - | - | - | - | - | 14.29 | 2.98 | |
| Total | - | - | - | 100 | 100 | 100 |
100 |
|
Log (GL) = 0.4223*Log (W) + 0.2311, r = 0.8072 (Figure 3).
Log (GL) = 1.2577*Log(SL) – 0.3721, r = 0.8256 (Figure 4).
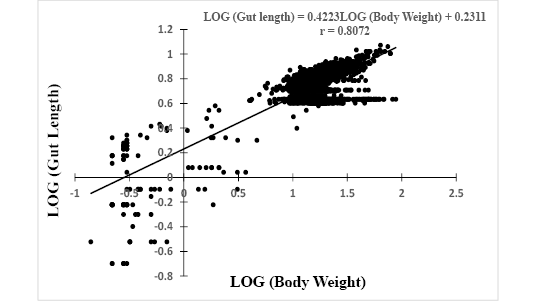
Figure 3: Relationship between Log (gut length) and Log (body weight) of Hemichromis fasciatus (N=2,357) from Okpara stream of Oueme river (North-Benin)
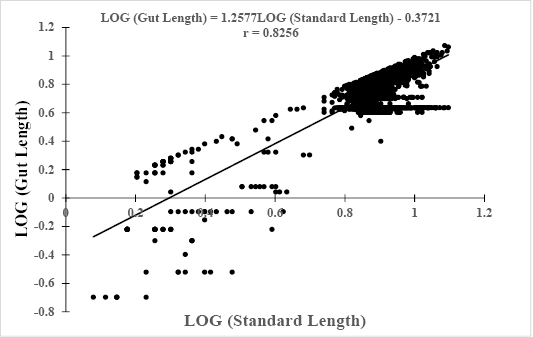
Figure 4: Relationship between Log (gut length) and Log (Standard Length) of Hemichromis fasciatus (N=2,357) from Okpara stream of Oueme river (North-Benin)
The slopes, bsl-gl= 0.4223 and bw-gl= 1.2577 of both equations were positive with significant (p<0.05) correlation coefficients r = 0.81 and r = 0.83, respectively. In addition, the relative gut length (GL/SL) was computed and compared to reference published values. The computed ratio GL/SL ranged between 0.1 (3 cm SL) and 0.97 (12.2 cm SL) with a mean of 0.73 ± 0.17.
Table 8: Spearman correlation coefficient between standard length and volumetric percentages of prey consumed by H. fasciatus
| Prey categories | SL |
GL |
| Zooplankton |
-0.648** |
-0.51* |
| Fish |
0.52** |
0.68** |
| Frog | 1.000 | 1.000 |
| Shrimp | 0.866 | 0.866 |
| Detritus | 0.279 |
0.332* |
| Mollusk | -1.00 | -1.00 |
| Insects | -0.026 |
-0.02 |
**. Correlation is significant at the 0.01 level.
*. Correlation is significant at the 0.05 level.
Discussion
Though foraging on about 59 food items, aggregation by taxa indicated that H. fasciatus consumed only seven (7) categories of food resources numerically dominated by fishes (61.21%), insects (26.8%), detritus (4.096%), zooplankton (2.995%), and frogs (1.062%) cumulating 96.165% of the diet (Table 1). This results suggested that in the Okpara stream, H. fasciatus displayed a piscivorous feeding habit and foraged on a moderate food spectrum. These findings agreed with those reported by Oronsaye (2009) and Adebisi (1981) in Nigeria, respectively in the Ikpoba dam and in the Ogoun River where this cichlid foraged mainly on fishes, insects, detritus and zooplankton. This carnivorous diet pattern was also reported by Ndour (2007) in the Maka anti-salt dam on Casamance in Senegal and by Atindana (2014) in Golinga reservoir of Ghana where H. fasciatus preferentially consumed fishes, fish larvae and fish parts. Likewise, in the hydroelectric dam lake of Ayame in Ivory Coast, fishes were highly ingested by H. fasciatus (Blahoua et al., 2017). Nevertheless, in contrast with the population of Okpara stream, insects were the preferential prey in the Mankessim Reservoir in Central Region of Ghana (Atindana et al., 2014). These spatial changes in the dietary pattern were probably due to the differential availability of each food resource in these habitats, indicating that H. fasciatus displayed a trophic plasticity behavior (Winemiller and Kelso-Winemiller, 2003). As reported by Gbaguidi et al. (2016), this feeding flexibility, even modest, enables this piscivorous cichlid to colonize most inland waters such as freshwater lakes, estuaries, lagoons, streams and rivers. This relative feeding flexibility depicted in H. fasciatus was also shown by the moderate diet breadth (DB) varying between 1.20 for small juveniles (TL<3cm) to 13.77 for adults (TL≥8 cm), and confirmed by the seasonal variations of diet (Table 3). Indeed, the results indicated that proportional consumption of zooplankton and aquatic insects were higher during the wet season where there was a proliferation of vegetation that boosted the colonization of insects. Likewise, proportional consumption of fishes and frogs were higher during the flooding where in general, these food resources were massively recruited (Castillo-Rivera, 2013; Konan et al., 2014; Blahoua et al., 2017).
The high percentage of empty stomachs (86.64%) recorded during this investigation is typical to most carnivorous fishes which digestive enzymes accelerate the digestion of the prey ingested. Also, because the gut length of carnivore is relatively short (GL/SL = 0.73) compared to herbivore, the transit of the digested preys is relatively quick, increasing the percentage of empty stomachs (Koné et al., 2007). In this study, the general trends of the occurrence frequencies (FO) of the food resource ingested by H. fasciatus agreed with those displayed by the volumetric proportion of each food item. Indeed, as shown by the volumetric percentage, aquatic insects with a frequency of occurrence FO = 62.54%, fishes with FO=54.603%, detritus (FO=16.19%) and zooplankton (FO=13.016%) were the main food resources recorded in most stomachs (Table 1). As results, aquatic insects and fishes appeared to be the preferential foods for H. fasciatus whereas detritus and zooplankton could be qualified as secondary preys (N’guessan et al., 2018). Accidentals food items like shrimps (FO=0.952%), frogs (FO=0.635%) and mollusks (FO=0.635%) occurred only in few stomachs, 3, 2 and 2 respectively. These feeding patterns were also confirmed by the greater dietary index DI = 33.42 of fishes qualified as “essential prey” for H. fasciatus, and aquatic insects with DI = 16.76 represented the “important prey items”. Detritus, zooplankton, frogs, shrimps and mollusks with DI <10 represented the preys of secondary importance (Lauzanne, 1975) (Table 2).
The relatively high diet overlaps (mean Øjk: 0.68± 0.29) recorded between size classes indicated that H. fasciatus showed moderate diet similarities between life stage categories. However, the lowest diet similarity (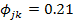 ) recorded between “small juvenile (TL<3 cm)” subpopulations and “large juveniles (3 cm<TL<5 cm)” indicated that H. fasciatus displayed an ontogenetic diet shift (Scharf et al., 2000; Juanes et al., 2002; Sánchez-Hernández et al., 2012; Scharf, 2014). This foraging strategy was also confirmed by the lowest Pianka’s diet overlaps Øjk =0.32, Øjk =0.24, Øjk =0.21 recorded between “large juvenile (3 cm<TL<5 cm)” subpopulations and adults size classes “TL: 11-13”, “TL: 13-15” and “TL: 15-17”, respectively. Indeed, earlier life stages such as larvae, small juveniles and large juveniles of fishes have a relatively less developed digestive tract, and as reported by Gbaguidi et al. (2016) and Adite et al. (2017), only live food resources such as phytoplankton and zooplankton were preferentially ingested. In contrast, large and tuff food items such as fishes, aquatic insects, shrimps, frogs, mollusks and detritus relatively dominated the diet of sub-adults and adults that showed fully developed digestive tracts (Table 7).
) recorded between “small juvenile (TL<3 cm)” subpopulations and “large juveniles (3 cm<TL<5 cm)” indicated that H. fasciatus displayed an ontogenetic diet shift (Scharf et al., 2000; Juanes et al., 2002; Sánchez-Hernández et al., 2012; Scharf, 2014). This foraging strategy was also confirmed by the lowest Pianka’s diet overlaps Øjk =0.32, Øjk =0.24, Øjk =0.21 recorded between “large juvenile (3 cm<TL<5 cm)” subpopulations and adults size classes “TL: 11-13”, “TL: 13-15” and “TL: 15-17”, respectively. Indeed, earlier life stages such as larvae, small juveniles and large juveniles of fishes have a relatively less developed digestive tract, and as reported by Gbaguidi et al. (2016) and Adite et al. (2017), only live food resources such as phytoplankton and zooplankton were preferentially ingested. In contrast, large and tuff food items such as fishes, aquatic insects, shrimps, frogs, mollusks and detritus relatively dominated the diet of sub-adults and adults that showed fully developed digestive tracts (Table 7).
In this study, the increase of fish consumption with SL (r = 0.52) and GL (r = 0.68), the increase of shrimps consumption with SL (r = 0.866) and GL (r = 0.866), the increase of detritus consumption with GL (r = 0.332) and the decrease of zooplankton consumption with SL (r = -0.648) and GL (r = -0.51) suggested an eco-morphological trend of the feeding habit of H. fasciatus. This ecomorphological relationships agreed with that reported by Gbaguidi et al. (2016; 2017) with the cichlid Sarotherodon galilaeus and the claroteid Chrysichthys nigrodigitatus surveyed in man-made lakes of the Southern Benin. Nevertheless, in the current research, and in contrast with S. galilaeus and C. nigrodigitatus, proportional consumptions of aquatic insects were insignificantly (p>0.05) correlated with SL (r = -0.03) and GL (r = -0.02).
With regards to food habit, when considering the general model of food consumed, fishes, making 61.21% of the stomach, dominated the diet of H. fasciatus, suggesting that this cichlid is an ichtyophage specialist. Nevertheless, the 38.79% remaining were mainly shared by insects (26.8%), detritus (4.096%), zooplankton (2.995%), suggesting that, in addition to fishes, this cichlid incorporated invertebrates, mainly aquatic insects in its diet. These feeding habits were also shown and confirmed by the eco-morphological analysis in which the computed ratios (GL/SL) of standard length (SL) to gut length (GL) varying between 0.1 and 0.97 (mean of 0.73 ± 0.17) was consistent with the range 0.5 - 2.4 reported by Al-Hussaini (1947) and Kapoor et al. (1975) for teleost carnivores. This finding agreed with that reported by Paugy (1994) for H. fasciatus from the Baoulé River in Mali (upper Senegal basin) where the relative intestine length (GL/SL) averaged 0.78. Also, the ratio recorded in the present study agreed with those reported by Paugy (1994) for other specialist piscivorous fishes such as Hydrocynus forskalii, Hydrocynus vittatus, Ichthyoborus besse, Lates niloticus showing respectively GL/SL ratios 0.85, 1.06, 1.10 and 0.52 that fall in the range 0.5 - 2.4 of carnivorous species.
Conclusion
The current study provides valuable information on the feeding ecology of Hemichromis fasciatus, the dominant fish species of the Okpara stream in Benin. This cichlid displayed mainly a piscivorous food habit, but also foraged intensively on aquatic insects. The feeding trend depicted was also shown by the relative gut length that fall in the range of carnivorous species. Hemichromis fasciatus showed a trophic plasticity behavior with moderate niche breadth that favored seasonal variations of diet. Niche overlaps between size classes revealed ontogenetic diet shifts. Currently, H. fasciatus constitutes a threat for the Okpara stream fish community for its high abundance coupled with its high level of predation. A rational management and exploitation of this cichlid is required to guaranty the survival of the fish community.
acknowledgements
The authors present their gratitude to the “Laboratoire d’Ecologie et de Management des Ecosystèmes Aquatiques, Département de Zoologie, Faculté des Sciences et techniques, Université d’Abomey-Calavi” for providing us logistic assistances. We express our gratitude to Okpara’s fishermen for their assistance during this fisheries investigation. Also we are grateful to Amoussouga Illary and Mitobaba Aurel for their assistance in laboratory works.
Conflict of interest
There is no conflict of interest.
authors contribution
All mention authors have contributed to this paper during the sampling, data analysing manuscript writing and revision.
References






