Advances in Animal and Veterinary Sciences
Research Article
In vivo -Cellular and Humoral Immune Response for Evaluation of Propolis Effect on Chronic Toxoplasmosis in Rats
Ahmed G. Hegazi1, Nagwa I. Toaleb2*, Hassan A El Fadaly1, Eman H. Abdel-Rahman2, Barakat A.M. A1
1Department of Zoonotic Diseases, National Research Centre, El Buhouth St, Dokki, Giza; 2Department of Parasitology and Animal Diseases, National Research Centre, El Buhouth St, Dokki, Giza, postal code 12622, Egypt.
Abstract | Toxoplasma gondii is a parasite causes latent cysts in the brain; cysts may lead to fatal toxoplasmic encephalitis. Treatment in these cases remains unsuccessful. The present study aimed to assess the effect of propolis as safe apitherapy from nature product of bee compared with drugs Spiramycin and Sulphonamide in eradication of brain cysts and the in vivo reactivation in rats. One hundred forty Sprague-Dawley rats were included in our study; one hundred twenty rats infected orally with 25 brain cysts T. gondii, they divided to six groups and received different treatments for 21 days after 60 days of infection: group (1) infected untreated rats which received saline (n=20), group (2) infected rats treated with Spiramycin 50 mg/kg daily (n=20), group (3) infected rats (n=20) received Sulphonamide 50 mg/kg daily, group (4) infected rats treated with 0.1ml propolis 0.1ml daily (n=20), group (5) infected rats treated with both Spiramycin 50 mg/kg and 0.1ml propolis (n=20) daily, group (6) infected rats treated with both Sulphonamide 50 mg/kg of drug and 0.1ml of propolis daily (n= 20). Twenty healthy non-infected rats received saline only. After treatment, the brain cysts were counted after 7, 14 and 21 days. Propolis increased spiramycin activity, causing a significant highest reduction of T. gondii brain load bradyzoites 112.67 (31.74%) in the rats treated with drug Spiramycin and propolis together (group 5) at 21 days post treatment. While rats treated with propolis only recorded reduction in bradyzoites count 59.33 (16.71%). Rats treatment with Spiramycin plus propolis recorded significant increase in IgG level against T. gondii 21dpt (group 5). While in group (4) that treated with propolis only recorded significant the highest IgG level in infected rats. High decrease in cytokines levels IFN-γ, IL-12 and IL-17 was recorded in rats treated with propolis extract only group (4), while group (5) tread with mix of propolis and Spiramycin recorded significant reduction in cytokines levels at 7, 14 and 21 dpt than the other groups. In conclusion; Spiramycin with propolis causing a significant reduction of T. gondii brain cysts, more than the other treatments with high potentials for chronic toxoplasmosis treatment.
Keywords | Propolis - Brain cysts toxoplasmosis - Anti-Toxoplasma gondii antibodies IgG -Spiramycin - Sulphonamide - Cytokines
Received | March 12, 2021; Accepted | March 17, 2021; Published | June 15, 2021
*Correspondence | Nagwa I Toaleb, Department of Parasitology and Animal Diseases, National Research Centre, El Buhouth St, Dokki, Giza, postal code 12622, Egypt; Email: nagwaibrahim26@yahoo.com
Citation | Hegazi AG, Toaleb NI, El Fadaly HA, Abdel-Rahman EH, Barakat AMA (2021). In vivo -cellular and humoral immune response for evaluation of propolis effect on chronic toxoplasmosis in rats. Adv. Anim. Vet. Sci. 9(7): 1045-1052.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1045.1052
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Toaleb et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Toxoplasma gondii (T. gondii) is a pathogenic protozoan affecting humans and animals causing toxoplasmosis disease (Dubey, 2008). Infection with T. gondii may cause abortion, stillbirth, and neonatal death in human (Innes, 2010) and in animals (Tenter et al., 2000). About one-third of the human populations worldwide are infected with T. gondii (McConkey et al., 2013). Barakat et al. (2018) stated that seropositive aborted women were 50.7% by ELISA versus 37% by PCR in examined sample in Egypt. Whereas, Ibrahim et al. (2017) reported that the percentage of infection was approximately 33.79% and 11.81% in pregnant women using ELISA and RT-PCR respectively. Moreover, Taman and Alhusseiny (2020) recorded toxoplasmosis seroprevalence range from 3% to 42.5% in Egyptian general population and varied percentages between the Egyptian governorates. El-Tantawy et al. (2019) showed that seroprevalence of T. gondii infection was 23.2% and 53.6% for IgM and IgG anti-Toxoplasma among thalassemia children in Egypt. In addition, in animals; Al-Kappany et al. (2018) reported that the T. gondii infection was higher in Egyptian goats (62%) than in sheep which range from 4.1% to 26%, which clarified their important role in the transmission of human toxoplasmosis. Also, the role of sheep as a source of human T. gondii infection showed by Khater et al. (2013).
T. gondii parasite may be appearing as acute via tachyzoites or chronic through bradyzoites (Montoya and Liesenfeld, 2004). T. gondii mostly causes a focal brain lesions in human with acquired immune deficiency (Kovari et al., 2010), and in the brains of warm-blooded animals, T. gondii forms tissue cysts causing changes in the behavior of infected rodents (McConkey et al., 2013). Diagnosis of toxoplasmosis depended mainly on T. gondi tachyzoite antigens (Ghazy et al., 2007; Hassan et al., 2012; Hassanain et al., 2013; Shaapan, et al., 2015). Success in diagnosis is essential for good treatment. Treatment of toxoplasmosis usually includes combinations of two antimicrobials; pyrimethamine-sulfadiazine (pyr-sulf) (Dunay et al., 2018). Treatment of acute human toxoplasmosis was difficult and need higher doses of antibiotics which causing terrible side actions (Robert-Gangneux and Dardé 2012). Therefore, many previous studies have been done on the efficacy of anti-T. gondii drugs in vitro and in vivo animals’ models (Dunay et al., 2018). Rats are considered more resistant to toxoplasmosis and therefore may be more relevant to studying the treatment of human (Innes, 1997). In addition, El Fadaly et al. (2016) proved that rats were holding T. gondii tissue cysts corresponding to the human virulent strains I, II, and III, reflecting their role in toxoplasmosis ecology. Moreover, some researchers have reported that urban rats act as a source of toxoplasmosis infection for domestic cats (Ruffolo et al., 2016). Concerning treatment, propolis proved antimicrobial activities against intracellular and extracellular protozoan parasites (Asfaram et al., 2020) where it suppresses the multiplication of T. gondii tachyzoites (Hegazi et al., 2014, Hegazi et al., 2017).
Therefore, the aim of the present study is to evaluate the immune responses of rats chronically infected with T. gondii and treated with propolis alone or with drugs.
Material and Methods
Ethical approval
The experiment was carried out according to the institutional guidelines of the National Research Centre’s Animal Research Committee number 19/139
Parasite
Isolation of Toxoplasma gondii Strain
Me49 non-virulent strain of T. gondii was maintained by repeated inoculation of Swiss albino mouse with 0.1 ml of brain homogenate of previously infected mice by oral administration via gastric tube containing 1×102 tissue cysts / ml every 8 weeks to establish chronic toxoplasmosis. The mice brains were ground with sterile pestle and mortars then diluted with saline to be at concentration 1×102 cysts / ml by using haemocytometer (Djurković-Djaković et al., 2002).
Collection of fresh viable cysts
Avirulent Me49 Toxoplasma gondii strain was obtained from Zoonotic Department, National Research Centre, Cairo, Egypt. Mice infected with ME49 T. gondii strain were sacrificed; their brains were removed and homogenized with an equal volume of PBS, pH 7.4 to release tissue cysts according to Dubey (1988) in 1mL saline each. 0.1mL of brain homogenate was placed on a glass slide and microscopically counted under a high-power lens (x40) (Abd El-Razik et al., 2018).
Experimental infection
One hundred twenty Sprague -Dawley rats were inoculated by intra-esophageal stomach tube with 25 cysts for sixty days to develop chronic infection.
Propolis extraction and treatment dose
The Egyptian propolis sample (25 grams) was collected from apiary farm near El-Mansoura City, Dakahlia Province, Egypt. The propolis cut into small pieces at room temperature and extracted with 250 ml of 70% ethanol (1:10 w/v) according to Bankova et al. (2019). After 72 h, the extract was filtered and dryness under vacuum at 40°C and stored in desiccator. The resulted 2.5 dry matter was dissolved in 25 ml normal saline 0.1 ml of propolis extract was injected in each rat day after day till the end of the experiment 21days.
Spiramycin and Sulfonamides drugs and Dosage
Spiramycin and Sulphonamides drugs for the treatment of toxoplasmosis as WHO (2008), were purchased from Sigma-Aldrich (USA), stored at 4C°, and protect from light. The treatment of toxoplasmosis is a combination of Sulphonamides Tablet: 500 mg) with propolis and Spiramycin (Tablet: 25 mg) with propolis. Rats were injected with drugs at a dose of 50 mg/kg body Wight/day orally for 21 days.
Rats grouping and experimental design
One hundred forty Sprague-Dawley rats were pathogen free, obtained from the Animal House, National Research Centre, Egypt. They were housed in individual cages and fed with standard diet (El-Fakhry et al., 1998). Examination of rats’ faeces was done to exclude any parasitic infection (Giarcia and Bruckner, 1977); twenty rats of them are healthy non-infected received saline only. And the other one hundred twenty rats are chronic toxoplasmosis infected by T. gondii cysts, then it was divided to six equal groups; 20 rats each. group 1; infected control group, subgroup 2; infected group treated with 50 mg/kg/day Spiramycin drug, group 3; infected group treated with 50 mg/kg/day Sulphonamide drug, group 4; infected group treated with 0.1ml propolis extract/day, group 5; infected group treated with 50 mg/kg/day spiramycin drug plus 0.1ml propolis extract, group 6; infected group treated with 50 mg/kg/day Sulphonamide drug plus 0.1ml propolis extract, treatment began on the sixty-day post infection. Rats were treated by injected orally for 21 days.
Blood Samples
Blood samples were collected from all groups via medial canthus of eyes of rats three times along the experimental period at 7 days post treatment (dpt), 14dpt and 21 dpt. Also, blood samples were taken at these times from control negative group uninfected untreated and from control positive group infected untreated.
Estimation of the parasitic load count
Brain harvesting bradyzoites (chronic) were counted directly in average 10 mg brain tissue by haemocytometer. The removed brain was homogenized with an equal volume of PBS, pH 7.4 to release tissue cysts according to Dubey (1988). The Average Parasite Load (APL/10 mg/tissue) was detected in brain samples of infected treated and infected untreated rats. The evaluation of parasite load was done by counting the total number of cyst-free bradyzoites and cysts bradyzoites/mg/brain in all groups according to Barakat (2007). 25 μL of the brain homogenate was spread on a slide and microscopically counted (Grujic et al., 2005). Average count in every 10 mg brain tissue of infected treated and infected untreated rats was performed using hemocytometer. The reductions % in the brain cyst count were calculated according to (Penido et al., 1994) by the following equation:
% Reduction = [ (Initial ABPL/60 dpi – Treated ABPL/21 dpt) / (Initial ABPL/60 dpi)] X100
Antigen preparation
T. gondii tachyzoites antigen was prepared according to Waltman et al. (1984). Briefly, tachyzoites were repeatedly freeze and thawed to rupture the parasite wall, sonicated and centrifuged at 18,000 rpm for 45 min at 4°C. The supernatant was collected and assayed for protein content by Lowry method (Lowry et al.,1951). Then aliquot and kept at -20°C until use in ELISA assay.
T. gondii specific antibodies detection
To evaluate specific T. gondii IgG titer in chronic infected rat serum after treatment at different intervals of treatment was monitored by indirect ELISA using prepared T. gondii tachyzoites antigen. ELISA was performed according to Lind et al. (1997). Optical density of developed color was recorded at 450 nm with automated ELISA reader (BIO-TEK, INC. ELx, 800UV USA). concentration of antigen, serum and conjugates dilutions were estimated by checkerboard titration. Cutoff value was calculated by negative mean OD values +2SD (Pishkari et al., 2017).
Determination of cytokines level in rat sera
Sandwich ELISA was adapted to measure cytokines concentration IFN-γ, IL-12 and IL-14in rat serum with commercial ELISA kits purchased from Biovision Co., China following the manufacturer’s instructions.
Statistical analysis
The data of the present study were represented as means ± SD and analyzed by using analysis of variance (ANOVA). Samples were compared using the unpaired Student t-test (two-tailed), with equal variance and unpaired samples. The significance of the difference between means at P ≤ 0.05 was calculated using Duncan Multiple Range Test (Steel and Torrie, 1980).
Results
Affection of treatment on brain parasitic load
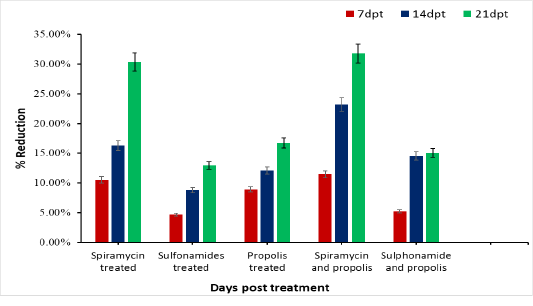
Initial average brain parasite load (bradyzoites) of chronically infected rat is 355. As shown in Table (1) the initial average brain parasite load (IABPL/60 dpt) decreases gradually in group 2 treated with Spiramycin from 355 bradyzoites to 247.33 bradyzoites at 21dpt, and recorded reduction percent (30.33%) (Figure 1). Rats treated with propolis only (group 4) showed decrease in IABPL from 323.33 at 7 dpt to reach 295.67 at 21 dpt with reduction percentage 16.71%. Whereas, group 3 in which rats treated with Sulphonamide drug was recorded the lowest reduction 46 (12.96 %). While the rats treated with drug Spiramycin plus propolis together group 5 record the highest reduction 112.67 (31.74%), and Sulphonamide drug with propolis together group 6 has 53.44 reduced in number of ABPL and with percentage 15.05% reduction at 21 dpt. Compare to the control group infected untreated rats’ group 1 in which the count of ABPL increased from 359
Table 1: Average brain cysts in treated rats by Propolis compared with Spiramycin and Sulphonamide treated groups and untreated rats at (7, 15 and 21DPT).
|
Initial Average brain cyst burden /60 dpi355 /100 mg brain |
|||||||
| brain cyst count in infected untreated and infected treated rats | |||||||
| Groups |
Control infected untreated
|
Spiramycin
|
Sulphonamide
|
Propolis extract | Spiramycin plus propolis | Sulphonamide plus propolis | P-value |
| Days post treatment | |||||||
| 7 |
359.33±1.45cx |
317.67±1.45az |
338.33±1.76bz |
323.33±2.40aby |
314.33±2.33az |
336.33±11.46by |
0.017 |
| 14 |
393.67±5.23ey |
297.22±5.11aby |
323.67±2.40dy |
312.00±3.46cdx |
272.67±1.86ay |
303.33±4.37bcx |
<0.01 |
| 21 |
418.33±8.41dz |
247.33±11.20ax |
309.00±3.46cx |
295.67±6.17bx |
242.33±2.40ax |
301.56±5.13bx |
<0.01 |
| P-value | <0.01 | <0.01 | <0.01 | <0.05 | <0.01 |
<0.05 |
|
Means with different superscripts (a, b, c, d) within row and (x, y, z) within column are
dpt: Day post treatment.
dpi: Day post infection.
reach to 390.44 at 21 days post infection as represent in Table (1). There is difference significance between each group and the other was P≤ 0.0001, also we found different significance between each interval time post treatment for each group individual P ≤ 0.0001in groups 2, 5 and 4 treated with Spiramycin, Spiramycin plus propolis and propolis only. While in group 3 treated with Sulphonamide was lower significance P ≤ 0.001 (Table 1).
Humoral immune response in treated rats
OD values of specific T. gondii antibodies IgG elevated in treated chronic infected rat significantly (P<0.05) at all days post treatment (7, 14 and 21dpt) than control infected untreated rats. IgG level increased gradually from 7dpt until become higher significant at 21dpt in rats’ group 5 treated by Spiramycin with propolis together than the other groups 2 and 3 treated with Spiramycin and Sulphonamide drugs only separately, and group 6 that treated by Sulfonamides plus propolis. At the same time group 4 that treated with propolis only recorded the highest IgG level in infected rats’ sera at 7, 14 and 21dpt as shown in Figure 2.
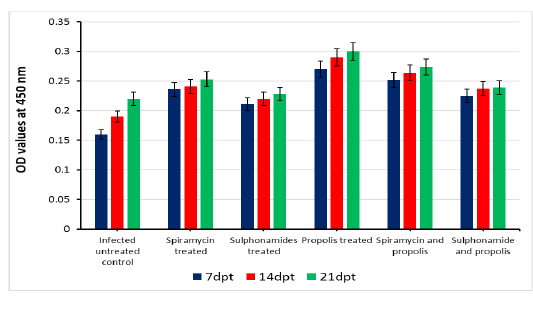
Figure 2: Serum IgG level of infected rats treated and untreated groups along the experimental period at 7,14 and 21days post treatment.
Cellular immune response in treated rats
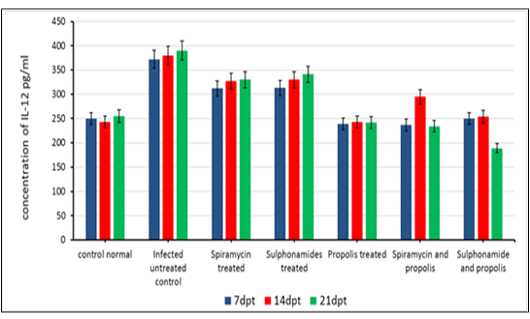
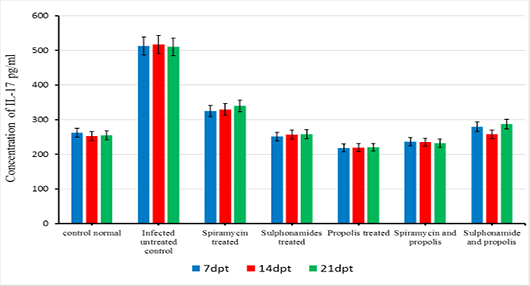
The beneficial effects of propolis extract on the regulation of Cytokines in chronic rats post treatment showed in Figures 3, 4 and 5. There was high decrease in cytokines levels IFN-γ, IL-12 and IL-17 in the group rats treated with propolis extract only (group 4) and group (5) tread with mix of Spiramycin and propolis together at 7, 14 and 21 dpt, but group (4) was recorded the highest decrease with high significance (0.001) than the other groups 3,5 and 6 which treated with Sulphonamides, Spiramycin and propolis and Sulphonamides plus propolis, respectively, compared with control infected untreated ones (Figures 3, 4 and 5).
Discussion
Development of anti- antiparasitic drugs with activity against cysts during the latent stage of infection is very desirable to reduce cyst burden in the host. Where the most antiparasitic drugs checked out allowed control of the acute infection but are not able to clear T. gondii bradyzoites in the chronic infection to set up sterile immunity (Dunay et al., 2018). Therefore, in current research we trail use propolis with drugs Sulphonamide and Spiramycin for treatment of rats’ chronic toxoplasmosis. In the current research we began with 355 bradyzoites initial count in each rat in each group. The results recorded significant decrease in brain cysts (P≤ o.oo1) in group 3 rats treated with Sulphonamide drug alone. Bradyzoites number decreased from, from 355 bradyzoites to reach 309.00±3 .46 days post treatment with reduction percentage 12.96%. While rats treated with propolis plus Sulphonamide drug in group 6 achieved high significant (P≤ 0.0001) reduction in bradyzoites count reached 53.44 (15.05%) 21 dpt. These results demonstrated that the treatment with Sulphonamide drug alone can be useful for treating the chronic phase of toxoplasmosis infection and its efficacy increased at a low dose of 50 mg/kg/day when combined with propolis to achieve higher reduction of bradyzoites burden than Sulphonamide alone. Where, the efficacy of sulfonamides against toxoplasmosis was proved in animal models since 1942 (Biocca and Pasqualin,1942). Moreover, the treatment with Sulfamethoxazole in combination with Trimethoprim led to reduce the level of cysts and to inhibit anti- T. gondii antibodies production (Nguyen and Stadtsbaeder, 1983; Alvarado-Esquivel et al., 2011).
In the present study we recorded significant reduction in average brain parasite load; 107.67 (30.33%) in group 2 rats treated with Spiramycin drug only. These results confirmed by Chew et al. (2012) who demonstrated the activity of spiramycin which reduced the number of brain cysts 3-fold 58% in a mouse model of chronic toxoplasmosis, when the mice were treated with 400 mg of spiramycin /kg/daily. Whereas, Gruji´c et al. (2005) showed 3-fold decrease in brain cysts burden in chronically infected mice with toxoplasmosis treated with spiramycin 200 mg /kg/day for 3 weeks. These results demonstrated that Spiramycin has been marked antiparasitic activity and elevated protection by a reduced brain cyst burden months after infection in a murine model of per oral infection (Grujic et al., 2005). The difference in the reduced number of brain parasite load was attributed to species of animal’s models, treatment protocol, and drug dose.
In the current research, the infected rats in group 5 which treated with spiramycin drug plus propolis was recorded the best bradyzoites reduction 112.67 (31.74 %) and considered a powerful treatment. These results demonstrated the ability of propolis to increase spiramycin activity in vivo. Propolis should be used for treatment of toxoplasmosis with synergetic action with Spiramycin than Sulphonamide. And our treatment advice not used propolis alone, because propolis without antibiotic recorded 59.33(16.71%). So, the significant treatment in this research is to reduce the dose of antibiotics to overcome the side action of the chemical drugs and replace propolis as active apitherapy. Some previous studies demonstrated that propolis can suppress the multiplication of T. gondii tachyzoites (Hegazi et al., 2014; Hegazi et al., 2017).
In the present research, specific T. gondii IgG titer elevated in treated chronic infected rats significantly (P<0.05) at all intervals post treatment (7, 14 and 21dpt) than control infected untreated and control normal rats. The IgG level increased gradually starting from 7dpt to reach its highest value 21dpt in group (5) rats treated by Spiramycin with propolis than the other groups (2and3) treated with Spiramycin and Sulphonamides separately, and group (6) that treated by Sulphonamides and propolis together. At the same time group (4) rats that treated with propolis only recorded the highest serum IgG level 7, 14 and 21DPT.
In the present research serum cytokine levels of chronically infected rats with T. gondii showed significantly elevated levels of cytokines IFN-γ, IL-12 and IL-17. This increase is due to infection with T. gondii characterized by hyper inflammation in acute phase (Gaddi and Yap, 2007), which considered the first function of the innate immune system (Iwasaki and Medzhitov, 2004; Yarovinsky and Sher, 2006). In the current research treatment with propolis (0.1 ml) day after day till the end of the experiment showed significant reduction in IFN-γ, IL-12 and IL-17 levels in rats’ group 4. While group 5 which treated with spiramycin plus propolis recorded the highest decrease with high significance (P≤0.05) than the other groups. These results were attributed to the propolis has biological characteristics depends upon its chemical composition, where it has more than 300 compounds such as, phenolic compounds, aromatic acids, essential oils, waxes and amino acids (Anjum et al., 2019). Also, propolis contains natural flavonoid, chrysin (5,7-dihydroxyflavone), it has beneficial effects including anti-tumor and anti-oxidant activities, the effect of chrysin is reduced the serum levels of pro-inflammatory cytokines such as, tumor necrosis factor-α, IL 1β, IL-4, and IL-6 in mast cells (Bae et al., 2011; Ahad et al., 2014). Moreover, Ansorge et al. (2003) demonstrated that propolis has a direct regulatory effect on basic functional of immune cells where cytokines produced by monocytes/ macrophages (IL-1β, IL-12), by Th1 (IL-2) as well as Th2 (IL-4) lymphocytes were found to be also suppressed. In addition, Mossalayi et al. (2014) reported that propolis decreased both monokines and interferon γ (IFNγ) production and induced potent anti-inflammatory activity due to their complementary immune cell modulation. Moreover, propolis inhibitory effects on lymphocytes proliferation due to its anti-inflammatory property. In immunological assays, the best results were observed when propolis was administered over a short-term to animals (Sforcin, 2007). Masek and Hunter (2013) demonstrated that macrophages can be infected by T. gondii, and able to limit parasite replication and produce cytokines that contribute to resistance, making them important regulatory and effector cells during toxoplasmosis. Neutrophils influence the T-cell response by enhancing the functions of dendritic cells (van Gisbergen et al., 2005) or inflammatory monocytes (Soehnlein et al., 2008).
Finally, Treatment of chronic toxoplasmosis with propolis through the experiment showed increase in IgG level and decrease in serum cytokines (IFN-γ, IL-12 and IL-17) levels but propolis should be used with Spiramycin drug, where Spiramycin with propolis recorded the highest reduction of brain load parasite (31.74 %).
Acknowledgments
This work was financially supported by the project number 12010135, which is funding by a grant from National Research Centre (NRC), Egypt.
Conflict of Interest
The authors declare that they have no competing interests.
authors contribution
Barakat AM A and El Fadaly H A prepared the research plan and the design of experiments. Hegazi A G, Barakat AM A, and El Fadaly H A carried out propolis extract preparation and the selected doses of treatment. Barakat AM A and El Fadaly H A propagated the infection and tested the treatments. Barakat AM A examined the brains of rats and count brain cysts by microscopy. Nagwa I Toaleb prepared antigen. Nagwa I Toaleb and Eman H Abdel-Rahman measured cytokines and IgG levels in rats’ sera and carried out an ELISA assay. All authors analyzed interpreted the data and prepared the manuscript. All authors have critically read and approved the final manuscript.
References