Yucca schidigera Liquid Extract Enhances Growth Performance, Nutrient Utilization, Liver Antioxidative Function, and Welfare Indices of Broilers
Yucca schidigera Liquid Extract Enhances Growth Performance, Nutrient Utilization, Liver Antioxidative Function, and Welfare Indices of Broilers
Asad Sultan1, Ziaul Islam2*, Faiza Shahzadi3, Sarzamin Khan1, Rafiullah Khan1 and Ihsan Ali4
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture Peshawar, Pakistan
2Department of Animal Sciences, Shaheed Benazir Bhutto University Sheringal Dir Upper Khyber Pkhtunkawa Pakistan
3Veterinary Research Institute, Peshawar, Livestock and Dairy Development Department Khyber Pkhtunkhawa Pakistan
4College of Veterinary Sciences, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan
ABSTRACT
This study was planned to evaluate the impacts of Yucca schidigera supplementation in drinking water on the growth performance, nutrient utilization, liver anti-oxidative function, and welfare indices of commercial broilers. A total of 320 one-day-old Cobb 500 chicks were divided into four treatment groups (80 chicks/group). The first control group (G1) was fed with the basal diet without supplementation of Y. schidigera extract. The second, third and fourth groups (G2, G3, and G4) were fed with Y. schidigera extract supplementation rate of 5, 10, and 15ml/200L to drinking water, respectively. The chicks that received Y. schidigera demonstrated the best production performances as compared to the control group. The chicks that received yucca showed a significant decrease in litter nitrogen content when compared to the non-supplemented group. The chicks that received liquid Y. schidigera had reduced total bacterial counts (p<0.05), Escherichia coli, and a non-significant increase in the number of lactic acid-producing bacteria. They also showed increased activity of antioxidant enzymes and decreased levels of lipid peroxidation biomarkers, without a harmful effect on liver and kidney function. In conclusion, the use of natural additives is necessary to improve growth performance, and nutrient digestibility, decrease nitrogen losses, feed cost, and environmental pollution.
Article Information
Received 12 October 2022
Revised 18 January 2023
Accepted 25 February 2023
Available online 08 May 2023
(early access)
Published 22 May 2024
Authors’ Contribution
AS study design and idea, laboratory experiment, feed formulation, data evaluation, manuscript review. FS animal trial, laboratory experiment, statistical analysis, study design and writing. ZI data evaluation, manuscript review. SZK data evaluation, manuscript review. RK improving main body text, discussion, manuscript review. IA improving main body text, discussion, manuscript review.
Key words
Yucca Schidigera, Broiler, Antioxidant, Growth performance, Bacterial count
DOI: https://dx.doi.org/10.17582/journal.pjz/20221012161058
* Corresponding author: ziaulislam43@yahoo.com
0030-9923/2024/0004-1661 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Antibiotics are used in the poultry industry to enhance growth performance, gut health, and nutrient digestibility. The excessive use of antibiotics at a sub-therapeutic level as a growth promoter in the poultry production cycle has developed antimicrobial resistance (AMR) which has proven to be the greatest threat to human health (Carrique-Mas et al., 2017). An increase in drug resistance to frequently used antimicrobial agents in human and animal production is a public health challenge globally (Pourmand et al., 2017). For that reason, many countries restricted the use of antibiotics in feed as a growth promoter due to the overwhelming situation of AMR (Diarra and Malouin, 2014). In the present situation, it is important to find out an alternative to AGPs, which provides similar results and ensures better production and food safety for human consumption (Yadav et al., 2016). The use of probiotics as an alternative to antibiotic growth promoters has increased in the last few decades (Dhama et al., 2015). Among the phyto-biotics Y. shidigera, can be widely used as an alternative feed additive to replace antibiotics in poultry feed. Y. schidigera is a small tree prevalent in the deserts of the southwestern United States and northern Mexico, considered highly for its pharmaceutical values (Patel, 2012). Yucca schidigera contains active ingredients steroidal, saponins that contribute to the emulsification of oil fats, the promotion of their digestion, and the absorption of vitamins and minerals leading to positive effects in poultry (Su et al., 2016). Other main active components, resveratrol and yuccaols, which possess biological functions, were identified in Y. schidigera besides steroidal saponins (Patel, 2012). Resveratrol is well known to be an effective scavenger of hydroxyl, superoxide radicals, as well as inhibiting reactive oxygen species (ROS) formation in cells. It also protects cells from lipid peroxidation in membranes and DNA damage caused by ROS (Leonard et al., 2003). Phenolic constituents such as yuccaols in Y. schidigera, which are structurally related to resveratrol, also possess radical scavenging activity (Piacente et al., 2004; Patel, 2012). Alagawany et al. (2016), reported that Y. schidigera improved superoxide dismutase (SOD) and reduced glutathione (GSH) level, and reduced malondialdehyde (MDA) concentration in the serum in laying hens. Glutathione peroxidase (GPx) and catalase (CAT) activities were increased with Y. schidigera supplementation in rabbits (Ashour et al., 2014). Y. schidigera can also recompense the toxic effects of lead induced oxidative stress in quails (Alagawany et al., 2018; Farag et al., 2018). Many reports have also shown that dietary Y. schidigera incorporation could produce positive effects on the economic traits, performance, carcass characteristics, and health of broilers (Wang and Kim, 2011). In another study, blood and tissue MDA concentrations were decreased, and the GSH activity in blood and tissues was increased when rats were treated with Y. schidigera. However, total antioxidant capacity (T-AOC) was not affected (Cigerci et al., 2009). Therefore, the purpose of this study was to determine the effect of different concentrations of Y. schidigera supplementation on the growth performance, gut microbiota, nutrient utilization and liver anti-oxidative function, and welfare indices of commercial broilers.
MATERIALs AND METHODS
Bird’s husbandry
A total of 320-days old broiler chicks (Cobb 500) purchased from a local hatchery were weighed and randomly allocated into four treatment groups consisting of four replications for each treatment, with 20 broilers in each replicate in a completely randomized design (CRD) experimental model. The broilers were reared in the open-sided house for 35 days. All chicks were vaccinated as per recommended schedule for broilers. Feed and fresh water were available ad libitum.
Diets and treatments
The broilers were fed with commercial diets containing corn and soybean meals as the basal diet. The starter diet was used from day 1 until day 21, while the finisher diet was used from day 22 to day 35. The first control group (G1) was fed on the basal diet without any Y. schidigera liquid extract (DK YUCCA manufactured by Desert king pharma USA) supplementation in water, while the 2nd, 3rd, and 4th groups (G2, G3, and G4) were fed on basal diets with Y. schidigera liquid extract at the rate of 5mL, 10mL and 15mL to drinking water, respectively. The nutrient content of both starter and finisher diets supplemented are presented in Table I.
Table I. Composition and nutrient content of the basal experimental diet.
|
Ingredients (%) |
Starter diet (1-21) |
Finisher diet (22-35) |
|
Maize |
66.5 |
67.0 |
|
Sesame cake |
5.00 |
7.00 |
|
Fish meal |
9.00 |
6.50 |
|
Wheat bran |
15.8 |
15.0 |
|
Methionine |
0.10 |
0.02 |
|
Lysine |
0.60 |
0.05 |
|
Sesame Oil |
2.20 |
3.13 |
|
Salt |
0.40 |
0.40 |
|
Lime stone |
0.40 |
0.90 |
|
Calculated % |
||
|
Dry matter |
92.2 |
93.0 |
|
Ash |
8.40 |
8.44 |
|
Crude fibre |
14.3 |
14.3 |
|
Crude protein |
21.2 |
18.2 |
|
Ether extract |
4.72 |
4.74 |
Growth performance parameters
Throughout the five weeks study period, body weight (BW) and feed intake (FI) were weekly recorded for each replicate using a digital weighing scale with a measurement accuracy of two decimal points. Data recorded on weekly BW and FI were used to calculate the feed conversion ratio (FCR).
Litter sampling and nitrogen analysis
On days, 21 and 35 of the production period litter samples from each poultry pen were randomly collected from 12 different locations. The collected samples were thoroughly mixed in a plastic bag, and 250 g was weighed and shifted to the laboratory for further analysis. The dry matter (DM) of the litter was determined by oven drying at 105 oC for 48 h, and calculating the differences in weight. Nitrogen in the litter samples was determined by using the Kjeldahl method, according (AOAC, 2000).
Nutrient digestibility
To calculate the apparent ileal digestibility on day 35 of the experiment, representative chicken from each treatment was transferred to metabolic cages for digestibility. All the chickens were fed with a diet containing 0.2% Cr2O3 for three days as an indigestible marker before slaughtering. After slaughtering on day 42, the ileal content was collected and stored at −20 oC for further analyses of nutrient content (Islam et al., 2022). Chromium concentrations were determined with a UV absorption spectrophotometer (Shimadzu, UV-1201, Shimadzu, Kyoto, Japan) using the method of (Williams et al., 1962). The following formulas were used to calculate the apparent ileal digestibility and ileal digestible energy (Stefanello et al., 2020).
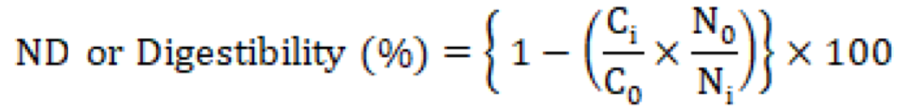
Ci and Co are concentration of chromium in the diet and digesta (%); Ni and No are concentration of nutrient in the diet and digesta (%), respectively.
Evaluation of ileal microbial count
At 21 and 35 days of age, thirty-six birds were randomly selected and slaughtered. In order to evaluate the effect of Y. schidigera supplementation at different concentrations in drinking water on the colonization of pathogenic and beneficial bacteria in comparison to the control group, a total of 20 cecal samples were collected from all chickens, throughout the experimental period (5 samples/group). Samples were transferred to test tubes and stored at -80°C until further analysis. One gram (1 g) of excreta was diluted in 9 mL of 1% peptone broth, homogenized, and then added to the selective media for growth. The bacterial counts were performed by serial 10-fold dilutions (10 g/l peptone solution) onto Lactobacillus MRS Agar plates and MacConkey to isolate the Lactobacillus, and Escherichia coli, respectively. The bacteria colonies were counted using a colony counter (Gao et al., 2019).
Blood hematological and serum biochemical parameters analysis
During the third and fifth weeks of the experimental period, four birds from each group at day 35 were randomly selected and fasted overnight. Blood samples were collected in replicates in sterile vacutainer tubes containing EDTA (Ethylene diamine tetra acetic acid) for hematology analysis. While for serum biochemical, analysis blood samples were collected in non-heparinized chilled tubes and centrifuged at 3500 rpm for 15 min. The separated sera were kept at -20 oC for biochemical investigation. LPO, GSH, CAT activity, SOD, and ALT, were measured were measured using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and automated spectrophotometric analyzer (Cao et al., 2015; Liao et al., 2015). Serum proteins, globulin, albumin, and creatinine were spectrophotometrically determined using industrially available kits.
Statistical analysis
The analysis of all data was done using one-way analysis of variance (ANOVA) based on the completely randomized design model, using Statistical Analysis System (SAS, 2012), and the Tukey posthoc test was used to estimate the significant difference among treatment groups. Results were considered significant at p < 0.05.
Results
Growth performance measurements
Table II presents the overall growth performance results of broilers on day 21 and 35. A significant difference was observed in growth performance in the starter and overall production period of the broiler chickens. Significant differences (p< 0.05) were noted in body weight gain and cumulative FCR during the starter and overall production period. The highest final body weight gain as well as the lowest FCR was demonstrated in G4 supplemented with Y. schidigera 15 mL /200L of water as compared to the other treatments.
Litter moisture, nitrogen and ash content
The results on the litter nitrogen and moisture % on day 21 and 35 are shown in Table III. Significant differences (p<0.05) in these parameters during both the starter and finisher phases were recorded. Both at the starter and at finisher phases the supplementation of Y. schidigera in groups G2, G3, and G4 significantly decrease the litter nitrogen content as compared to G1 at day 21. Concerning the effect of Y. schidigera supplementation on litter moisture content, days 21 and 35 showed significant decreases in moisture content, compared to the G1.
Nutrient digestibility
The results on the apparent ileal digestibility of broilers are shown in Table IV. There were significant differences (p<0.05) in all the digestibility parameters. Significantly, higher DM, crude CP, and EE digestibility were demonstrated in G4 broilers compared to other
Table II. Effect of different levels of Yucca schidigera extract on growth performance parameters.
|
Production parameters |
Treatment groups |
P value |
|||
|
G1 |
G2 |
G3 |
G4 |
||
|
Starter phase (day 1-21) |
|||||
|
BWG, g |
728.43± 0.97b |
780.34±0.37 a |
787.18± 0.85a |
795.92±0.96a |
0.003 |
|
FI, g |
1128.62±0.20 |
1129.31± 0.50 |
1130.92±0.14 |
1128.52±0.71 |
0.986 |
|
FCR |
1.54±0.25a |
1.44±0.04b |
1.42±0.30b |
1.40± 0.01b |
0.020 |
|
Finisher phase (day 22-35) |
|||||
|
BWG, g |
1061.82±0.67 |
1080.51± 0.60 |
1087.25± 0.15 |
1099.01±0.08 |
0.000 |
|
FI, g |
2264.72±0.65 |
2263.81±0.63 |
2255.53± 0.46 |
2258.71±0.52 |
0.154 |
|
FCR |
2.13±0.04 |
2.09± 0.01 |
2.07±0.05 |
2.04± 0.08c |
0.229 |
|
Overall period (day 1 to 35) |
|||||
|
BWG, g |
1790.25±0.91c |
1860.36± 0.20b |
1874.43±0.68 ab |
1894.93±0.02a |
0.000 |
|
FI, g |
3393.11± 0.48 |
3393.01±0.99 |
3386.71± 0.75 |
3438.01± 0.48 |
0.444 |
|
FCR |
1.89±0.04a |
1.82±0.07ab |
1.80a±0.50bc |
1.81± 0.013c |
0.000 |
Different superscripts along the row indicate significant difference (p <0.05). G1, basal diet; G2, basal diet + 5ml of Yucca schidigera liquid extract; G3, basal diet + 10 ml of Yucca schidigera liquid extract; G4, basal diet + 10 ml of Yucca schidigera liquid extract.
Table III. Effect of liquid Yucca schidigera supplementation on litter content of nitrogen and moisture.
|
Parameter |
Treatment groups |
P value |
|||
|
G1 |
G2 |
G3 |
G4 |
||
|
Day 21 |
|||||
|
Nitrogen % |
0.913 ±0.02 a |
0.82 ±0.013 b |
0.62 ±0.03c |
0.52 ±0.02 c |
0.001 |
|
Moisture % |
34.51 ± 0.12 a |
32.97 ±0.16 b |
30.90 ±0.06 c |
30.60 ±0.06 c |
0.001 |
|
Day 35 |
|||||
|
Nitrogen % |
1.24 ±0.03 a c |
0.92±0.05 b |
0.86c ±0.02 |
0.65 ±0.02c |
0.001 |
|
Moisture % |
31.98 ±0.15 a |
30.87 ±0.15 b |
29.77c ±0.20 |
29.67 ±0.20 c |
0.001 |
Different superscripts along the row indicate significant difference (p <0.05). For composition of feed for different group, see Table II.
Table IV. Effect of Yucca schidigera supplementation on the apparent ileal nutrient digestibility of broilers on day 42.
|
Parameters |
G1 |
G2 |
G3 |
G4 |
P-value |
|
Dry matter |
69.51±0.01c |
70.33±0.21c |
72.32±0.00c |
74.74±0.00b |
0.000 |
|
Crude protein |
70.50±0.00d |
73.66±0.31d |
75.60±0.00c |
76.70±0.00b |
0.001 |
|
Ether extract |
71.40±0.00b |
73.68±0.35b |
74.36±0.26b |
76.70±0.00a |
0.000 |
|
AME |
2650.21±0.47c |
2655.20±0.13c |
2755.23±0.48b |
2826.71±0.35a |
0.000 |
Means within the same row that carry different superscripts are significantly different at p < 0.05.
treatment groups and G1. Likewise, similar findings were exhibited for apparent metabolizable energy in-group G4 broilers supplemented with the highest concentration of Y. schidigera extract.
Ceacal microbial count evaluation
The results in Table V show that cecal samples taken at 21 days of age had a significant decrease in total colony counts (p < 0.05) in the group supplemented with yucca as compared to the control group. In addition, a significant decrease in the count of E. coli (p<0.05) in Y. schidigera-supplemented groups was detected, compared to the control group. No significant change in the count of lactic acid-producing bacteria (p>0.05) in either Y. schidigera-supplemented group was recorded. At 35 days of age, a numerical decrease in the total colony count (p > 0.05) in the Y. schidigera-supplemented groups was found.
Table V. Effect of dietary yucca supplementation on the on the cecal microbial counts (log10 cfu g-1) of broilers on days 21 and 35.
|
Parameter |
Treatment groups |
P-value |
||||
|
G1 |
G2 |
G3 |
G4 |
|||
|
Day 21 |
||||||
|
Total count (log10 cfu/g) |
7.2 ± 0.12a |
6.4 ± 0.29ab |
6.2 ± 0.30b |
6.1 ± 0.01b |
0.013 |
|
|
Escherichia coli (log10 cfu/g) |
8.4 ± 0.13a |
6.3 ± 0.32b |
6.0 ± 0.27b |
5.99 ± 0.38b |
0.001 |
|
|
Lactobacillus (log10 cfu/g) |
4.2 ± 0.17 |
5.3 ± 0.18 |
4.3 ± 0.15 |
4.2 ± 0.15 |
0.743 |
|
|
Day 35 |
||||||
|
Total count (log10 cfu/g) |
6.1 ± 0.36a |
4.0 ± 0.23b |
6.0 ± 0.22a |
6.0 ± 0.21a |
0.011 |
|
|
Escherichia coli (log10 cfu/g) |
4.3± 0.19 |
3.2 ± 0.20 |
4.0 ± 0.12 |
4.0 ± 0.20 |
0.623 |
|
|
Lactobacillus .(log10 cfu/g) |
4.0 ± 0.22 |
4.6 ± 0.64 |
4. 5± 0.47 |
4.5 ± 0.17 |
0.743 |
|
Means within the same row that carry different superscripts are significantly different at p < 0.05. For details of groups, see Table II.
Table VI. Effect of dietary Yucca schidigera supplementation on some blood hematological parameters of broiler chickens.
|
Parameter |
Treatment groups |
P-value |
|||
|
G1 |
G2 |
G3 |
G4 |
||
|
RBCs (106ul) |
2.8±0.21 |
2.9±0.62 |
2.9±0.70 |
2.9±0.4 0 |
0.071 |
|
Hemoglobin (g/dl) |
9.9±0.71 |
9.9±0.71 |
9.9±0.60 |
10±0.61 |
0.081 |
|
WBCs (103ul) |
3.10±0.01 |
3.06±0.01 |
3.05±0.03 |
3.07±0.01 |
0.001 |
|
Neutrophils (%) |
39.8±0.9 c |
41.9±0.8 c |
47±0.41 b |
55.8±0.4 a |
0.001 |
|
Eosinophils (%) |
1.8±0.1 a |
2.1±0.8a |
2±0.8a |
2±0.8a |
0.91 |
|
Basophils (%) |
0.7±0.2 |
0.8±0.2 |
0.7±0.2 |
0.9±0.6 |
0.71 |
|
Lymphocytes (%) |
35.6±0.5 d |
44.3±1.5c |
49.9±0.4 b |
52.2±0.5 a |
0.001 |
|
Monocytes (%) |
5.5±1.3 |
5.5±1.3 |
4.6±1.3 |
4.5±0.6 |
0.511 |
Different superscripts along the row indicate significant difference (p <0.05). For details of groups, see Table II.
Table VII. Effect of dietary Yucca schidigera supplementation on some serum Biochemical Parameters of broiler chickens.
|
Parameter |
Treatment groups |
P-value |
|||
|
G1 |
G2 |
G3 |
G4 |
||
|
Day 21 |
|||||
|
ALT |
36.07 ±1.49 |
35.84 ± 2.11 |
37.69±1.21 |
36.69±1.21 |
0.941 |
|
SOD |
37.84±0.38b |
40.16±1.5b |
56.35±0.88 a |
56.35±0.78a |
0.001 |
|
MDA |
62.34± 0.27 |
57.89± 0.33 |
57.930±0.03 |
57.747±0.59 |
0.567 |
|
CAT |
23.88±0.74c |
26.91± 0.08b |
32.20±0.84 a |
32.20±0.84a |
0.016 |
|
Creatinine |
0.48 ±0.04 |
0.47± 0.04 |
0.48±0.01 |
0.48±0.01 |
0.771 |
|
Day 35 |
|||||
|
ALT |
43.76 ±0.04 |
43.41 ±0.06 |
42.96±0.69 |
42.97±0.69 |
0.350 |
|
SOD |
49.02 ±0.84 |
50.19 ±0.18 |
50.52±0.68 |
50.42± 0.78 |
0.373 |
|
MDA |
81.54 ±0.79a |
68.04 ±0.2b |
60.66±0.42c |
60.86±0.05c |
0.005 |
|
CAT |
39.35 ±0.81c |
53.62 ±0.58b |
64.45±0.08a |
64.95 ±0.07a |
0.001 |
|
Creatinine |
0.87 ±0.03 |
0.84 ±0.03 |
0.83±0.05 |
0.83 ±0.05 |
0.836 |
Means within the same row that carry different superscripts are significantly different at p < 0.05. SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; ALT, alanine amino transferase. For details of groups, see Table II.
Blood hematology and serum biochemical parameters
No significant differences were observed in RBC count and Hb level among the treatment groups. Groups supplemented with Y. schidigera liquid extract had higher WBC, counts as compared to the control group Table VI. The results revealed that the addition of Y. schidigera significantly (p<0.05) increased the activity of antioxidant enzymes (SOD) and decreased the level of malondialdehyde (MDA) (a lipid peroxidation biomarker), compared to the G1 at the end of the study. On the other hand, kidney and liver function biomarkers were not affected Table VII.
Discussion
In broiler production, growth performance is considered an important parameter, which can be affected by many factors such as the environment and nutrition (Chung et al., 2020). In the present study, the growth performance parameters were improved by the supplementation of Y. schidigera at different concentrations. The result of this study is in line with previous studies that reported improved performance of broilers with the supplementation of Y. schidigera (Sahoo et al., 2015; Mousa et al., 2019). The growth-promoting effects of Y. schidigera were attributed to the presence of steroidal saponins, which have a positive effect on the digestive tract through the activation of digestive enzymes and enhancement of gut morphology (Wang and Kim, 2011). Similarly, Y. schidigera also contains polyphenols, which have anti-inflammatory, antimicrobial, and antioxidant activity, as well as free-radical hunting characteristics and immune enhancement, which all improve the growth performance of broilers (Su et al., 2016). Phytogenic feed additives like Y. schidigera contain steroidal saponins that have the ability to enhance intestinal health, improve the gut microbiota and stabilize bowel health while preventing intestinal disorder, which could lead to improved nutrient digestibility and absorption (Begum et al., 2015). In the current study, the highest CP, CF, and EE digestibility and apparent metabolizable energy were demonstrated in the groups supplemented with Y. schidigera liquid extract. The supplementation of Y. schidigera extract can affect energy metabolism by modulating hormone secretions and depressing energy compounds in the organism, which may increase nutrient digestibility. Similarly, previous work has documented significantly higher energy and protein values indicating better energy utilization and protein digestibility in broiler chicks supplemented with Y. schidigera (Alghirani et al., 2021). Additionally, the saponin content in Y. schidigera extracts also decreases urea in the blood, ammonia production, and odors from poultry excreta. In the present study, the supplementation of Y. schidigera significantly reduced the nitrogen and moisture content in the excreta. These results confirm that Y. schidigera liquid extract added to drinking water can improve the immersion of nitrogen and reduce its excretion, and ultimately the level of ammonia in the digestive tract and excreta (Alghirani et al., 2019). The impact of Y. schidigera extract supplementation is manifested in mitigating levels of ammonia in the caecum of animals (Mousa et al., 2019; Patoary et al., 2020). The results indicated that the addition of Y. schidigera to the broiler drinking water is of value in reducing total bacterial count and the number of Escherichia coli in different ages, especially at a young age. Wang and Kim (2011) found that Escherichia coli counts were linearly inhibited by Y. schidigera extract treatments, compared with the non-treated group at both five and eight weeks, and no difference was observed in the Lactobacillus population throughout the experimental period. The level of ALT and creatinine were not affected by the supplementation of Y. schidigera liquid extract in drinking water. This revealed that yucca had no adverse effect on liver and kidney functions. Similar results were reported by (Mousa et al., 2019) who observed a non-significant effect of Y. schidigera liquid extract on liver and kidney functions. Regarding anti-oxidative biomarkers, the addition of yucca improved the activity of antioxidant enzymes including SOD, and CAT, and decreased lipid peroxidation biomarkers. SOD is an important substance that exists in various tissues and organisms, and is believed to protect cells from damage caused by superoxide radicals (O2) (Kurutas, 2016). Similarly, Mousa et al. (2019); Su et al. (2016) demonstrated that broiler chickens fed a liquid extract of Y. schidigera showed a significant improvement in SOD activity and exhibited a strong ant-oxidative effect. Y. schidigera contains resveratrol, which has an inflammatory, and antioxidant effect (Farag et al., 2016; Alagawany et al., 2015). The level of MDA in the liver is proven a sensitive indicator of lipid oxidative tendency (Shafey et al., 2015). In the present study, the level of MDA decreased with the increasing level of Y. schidigera supplementation. Dengsheng et al. (2017) observed that MDA concentration was higher finisher period as compared to the starter period. In the current study, the decreases in MDA concentrations and the increases in SOD concentrations might be attributed to the Y. schidigera ability in terms of scavenging secondary reactive radicals or preventing the formation of superoxide and hydrogen peroxide (Enginar et al., 2006).
Conclusion
Y. schidigera liquid extract supplementation at a rate of 15ml/200 liter of drinking water showed an improved growth performance and ileal nutrient digestibility. Y. schidigera appeared to decrease nitrogen excretion, thus improving litter quality and bird welfare, and consequently, improving the gut health, and oxidative status of broiler chickens. Based on the results obtained Y. schidigera supplementation can be recommended as an alternative to antibiotic growth promoters in post-antibiotic era.
Acknowledgement
The authors would like to thank the staff of the Department of Poultry Science for their technical assistant.
Funding
The study receive no external funding.
IRB approval
The experimental protocols were approved by the Departmental Board of Studies (Approval number: 215/PS/UAP
Ethical approval
The experiment was carried out in a poultry research unit at the University of Agriculture Peshawar. All experimental procedures adopted were approved by the departmental board of studies and the Animal Research and Ethics Board of the University of Agriculture Peshawar.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Alagawany, M., Abd El-Hack, Farag, M.R., Elnesr, S.S., El-Kholy, M.S., Saadeldin, I.M., and Swelum, A.A., 2018. Dietary supplementation of Y. schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci., 97: 3126–3137. https://doi.org/10.3382/ps/pey186
Alagawany, M., Abd El-Hack, M.E., and El-Kholy, M.S., 2016. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Y. schidigera extract. Environ. Sci. Pollut. Res., 23: 6774–6782. https://doi.org/10.1007/s11356-015-5919-z
Alagawany, M., Farag, M.R., Dhama, K., Abd El-Hack, M.E., Tiwari, R., and Alam, G.M., 2015. Mechanisms and beneficial applications of resveratrol as feed additive in animal and poultry nutrition: A review. Int. J. Pharmacol., 11: 213–221. https://doi.org/10.3923/ijp.2015.213.221
Alghirani, M.M., Chung, E.L.T., Sabri, D.S.M., Tahir, M.N.J.M., Kassim, N.A., Kamalludin, M.H., Nayan, N., Jesse, F.F.A., Sazili, F.A.Q., and Loh, T.C., 2021. Can Y. schidigera be used to enhance the growth performance, nutrient digestibility, gut histomorphology, cecal microflora, carcass characteristic, and meat quality of commercial broilers raised under tropical conditions. Animals, 11: 2276. https://doi.org/10.3390/ani11082276
AOAC, 2000. Offcial method of analysis, association of official analytical chemist. Washington, DC, USA.
Ashour, E.A., Alagawany, M., Reda, F.M. and Abd El-Hac, M.E., 2014. Effect of supplementation of Y. schidigera extract to growing rabbit diets on growth performance, carcass characteristics, serum biochemistry and liver oxidative status. Asian J. Anim. Vet. Adv., 9: 732–742. https://doi.org/10.3923/ajava.2014.732.742
Ashour, E.A., Farsi, R.M., Alaidaroos, B.A., Abdel-Moneim, A.M.E., El-Saadony, M.T., Osman, A.O., Abou Sayed-Ahmed, E.T., Albaqami, N.M., Shafi, M.E., Taha, A.E., and Abd El-Hack, M.E., 2021. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci., 20: 1357–1372. https://doi.org/10.1080/1828051X.2021.1924087
Begum, M., Hossain, M.M., and Kim, I.H., 2015. Effects of caprylic acid and Y. schidigera extract on growth performance, relative organ weight, breast meat quality, haematological characteristics and caecal microbial shedding in mixed sex Ross 308 broiler chickens. Vet. Med. Czech, 60: 11. https://doi.org/10.17221/8532-VETMED
Cao, L.P., Ding, W.D., Du, J.L., Jia, R., Liu, Y.J., Zhao, C.Y., Shen, Y.J., and Yin, G.J., 2015. Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinus carpio var. Jian) with CCl4-induced liver damage. Fish Shellfish Immun., 43: 150–157. https://doi.org/10.1016/j.fsi.2014.12.025
Carrique-Mas, J.J., and Rushton, J., 2017. Integrated interventions to tackle antimicrobial usage in animal production systems: The ViParc project in Vietnam. Front. Microbial., 8: 1062. https://doi.org/10.3389/fmicb.2017.01062
Chung, E.L.T., Nayan, N., Kamalludin, M.H., Alghirani, M.M., Jesse, F.F.A., Kassim, N.A., Azizi, A., Reduan, M.F.H., and Loh, T.C., 2020. The effects of alkaline and rainwater on the production and health performance of commercial broilers under tropical conditions. Thai J. Vet. Med., 50: 53–61.
Cigerci, I.H., Fidan, A.F., Konuk, M., Yuksel, H., Kucukkurt, I., Eryavuz, A., and Sozbilir, N.B., 2009. The protective potential of Yucca schidigera (Sarsaponin 30®) against nitrite-induced oxidative stress in rats. J. Natl. Med., 63: 311–317. https://doi.org/10.1007/s11418-009-0338-4
Dengsheng, S., Jin, X., Shi, B., Su, J., Tong, M. and Yan, S., 2017. Dietary Yucca schidigera extract improved growth performance and liver antioxidative function in broilers. Ital. J. Anim. Sci., 16: 677-684. https://doi.org/10.1080/1828051X.2017.1302826
Dhama, K., Latheef, S.K., Mani, S., Samad, H.A., Karthik, K., Tiwari, R. and Laudadio, V., 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production. A review. Int. J. Pharmacol., 11: 152–176. https://doi.org/10.3923/ijp.2015.152.176
Diarra, M.S., and Malouin, F., 2014. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Micro., 5: 282. https://doi.org/10.3389/fmicb.2014.00282
Enginar, H., Avcu, G., Eryavuz, A., Kaya, E., Kucukkurt, I., and Fidan, A.F., 2006. Effect of Yucca schidigera extract on lipid peroxidation and antioxidant activity in rabbits exposed to c-radiation. Rev. Med. Vet., 157: 415–419.
Farag, M.R., Alagawany, M., and Tufarelli, V., 2016. In vitro antioxidant activities of resveratrol, cinnamaldehyde and their synergistic effect against cyadox-induced cytotoxicity in rabbit erythrocytes. Drug Chem. Toxicol., 40: 196–205. https://doi.org/10.1080/01480545.2016.1193866
Farag, R.M., Alagawany, M., Abd El-Hack, M.E., El-Sayed, S., Ahmed, S.Y.A., and Samak, D.H., 2018. Y. schidigera extract modulates the lead-induced oxidative damage, nephropathy and altered inflammatory response and glucose homeostasis in Japanese quails. Ecotoxicol. environ. Safe., 156: 311–321. https://doi.org/10.1016/j.ecoenv.2018.03.010
Gao, Y.Y., Zhang, X.L., Xu, L.H., Peng, H., Wang, C.K. and Bi, Y.Z., 2019. Encapsulated blends of essential oils and organic acids improved performance, intestinal morphology, cecal microflora, and jejunal enzyme activity of broilers. Czech. J. Anim. Sci., 64: 189-198. https://doi.org/10.17221/172/2018- CJAS
Islam, Z., Sultan, A., Khan, S.Z., and Khan, S.B., 2022. Effect of organic acids blend, Microencapsulated phyto-essential oils individually or in combination on growth performance, gut health and nutrients utilization of broilers. Pakistan J. Zool., 54: 2391-2399. https://doi.org/10.17582/journal.pjz/20210714190714
Kurutas, E.B., 2016. The importance of antioxidants, which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J., 15: 71. https://doi.org/10.1186/s12937-016-0186-5
Leonard, S.S., Xia, C., Jiang, B.H., Stinefelt, B., Klandorf, H., Harris, G.K., and Shi, X., 2003. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. J. biol. biophys. Res. Comm., 309: 1017–1026. https://doi.org/10.1016/j.bbrc.2003.08.105
Liao, X.D., Ma, G., Cai, J., Fu, Y., Yan, X.Y., Wei, X.B. and Zhang, R.J., 2015. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci., 94: 662–667. https://doi.org/10.3382/ps/pev038
Mousa, M., Ahmed, H.A., Sadek, K.M., Alagawany, M., El-Hack, M.A., Allam, I.A.A., and Latif, M.A., 2019. Effects of liquid yucca supplementation on nitrogen excretion, intestinal bacteria, biochemical and performance parameters in broilers. Animals, 9: 1097-1107. https://doi.org/10.3390/ani9121097
Patel, S., 2012. Yucca: A medicinally significant genus with manifold therapeutic attributes. Natl. Prod. Bioprospect., 2: 231–234. https://doi.org/10.1007/s13659-012-0090-4
Patoary, M.U., Hossain, M., Mofassara, A., and Rube, Z.U., 2020. Effect of supplementation of Y. schidigera extract on ammonia gas emission and performance of broiler chickens. J. World Poult. Res., 10: 57-62. https://doi.org/10.36380/jwpr.2020.8
Piacente, S., Montoro, P., Oleszek, W., and Pizza, C., 2004. Y. schidigera bark: Phenolic constituents and antioxidant activity. J. natl. Prod., 67: 882–885. https://doi.org/10.1021/np030369c
Pourmand, A., Amirshahi, M.M., Jasani, G., and May, L., 2017. Emerging trends in antibiotic resistance: Implications. Emerg. Med., 35: 1172-1176. https://doi.org/10.1016/j.ajem.2017.03.010
Sahoo, S.P., Kaur, D., Sethi, A.P.S., Sharma, A., and Chandra, M., 2015. Evaluation of Yucca schidigera extract as feed additive on performance of broiler chicks in winter season. Vet. World, 8: 556-560. https://doi.org/10.14202/vetworld.2015.556-560
SAS, Institute., 2002. SAS user's guide: statistics. Cary (NC): SAS Institute
Shafey, TM., Al-Batshan, HA., and Farhan, AMS., 2015. The effect of dietary flaxseed meal on liver and egg yolk fatty acid profiles, immune response and antioxidant status of laying hens. Ital. J. Anim Sci., 14: 428–435. https://doi.org/10.4081/ijas.2015.3939
Stefanello, C., Rosa, D.P., Dalmoro, Y.K., Segatto, A.L., and Vieira, M.S., 2020. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci., 6: 491. https://doi.org/10.3389/fvets.2019.00491
Su, J.L., Shi, B.L., Zhang, P.F., Sun, D.S., Li, T.Y., and Yan, S.M., 2016. Effects of yucca extract on feed effciency, immune and antioxidative functions in broilers. Braz. Arch. Biol. Technol., 59: e16150035. https://doi.org/10.1590/1678-4324-2016150035
Wang, J.P., and Kim, I.H., 2011. Effect of caprylic acid and Y. schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br. Poult. Sci., 52: 711–717. https://doi.org/10.1080/00071668.2011.635638
Williams, C.H., David, D.J., and Lismaa, O., 1962. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. agric. Sci., 59: 381-385. https://doi.org/10.1017/S002185960001546X
Yadav, A.S., Kolluri, G., Gopi, M., Karthik, K., and Singh, Y., 2016. Exploring alternatives to antibiotics as health promoting agents in poultry. A review. Exp. Biol. agric. Sci., 4: 368–383. https://doi.org/10.18006/2016.4(3S).368.383
To share on other social networks, click on any share button. What are these?










