Use of Phytoplankton to Assess Water Quality of Eco-Aquaculture System in Super-Intensive Whiteleg Shrimp (Litopenaeus vannamei) Pond
Research Article
Use of Phytoplankton to Assess Water Quality of Eco-Aquaculture System in Super-Intensive Whiteleg Shrimp (Litopenaeus vannamei) Pond
Mohammad Mahmudi1,2*, Muhammad Musa1,2, Sulastri Arsad1,2, Evellin Dewi Lusiana1,2, Alamanda Bunga1, Nur Azlina Wati1
1Aquatic Resource Management Study Program, Faculty of Fisheries and Marine Science, Universitas Brawijaya, Indonesia; 2AquaRES Research Group, Universitas Brawijaya, Indonesia.
Abstract | Rapid development of whiteleg shrimp farming industries has destroyed coastal environments due to land conversion, particularly mangroves. Recently, the concept of eco-green aquaculture by integrating mangroves and shrimp ponds has been discussed as an attempt to preserve mangrove forests as well as using them as wastewater treatment for aquaculture sewage. The aim of this study was to analyze the impact of integrated super-intensive shrimp aquaculture with mangroves in terms of water quality characteristics and phytoplankton as a bioindicator. The study was conducted at coastal area of Probolinggo region, Indonesia. Our research showed that the cultured ponds had high abundance of phytoplankton but low species diversity. Meanwhile, the water inlet and outlet of the shrimp farming which served at mangrove area had higher phytoplankton diversity. The composition of phytoplankton in this work was ideal for shrimp farming because it was dominated by the Chlorophyta and Chrysophyta divisions, which favour shrimp growth. On the other hand, water quality parameters across studied sites were quite similar, except for temperature and dissolved oxygen. Therefore, the study indicated that the existence of mangrove was supporting to increase the quality of water supply for the cultivation as well as the aquaculture water sewage in terms of phytoplankton bioindicator.
Keywords | Bioindicator; Coastal ecosystem; Integrated mangrove aquaculture; Aquaculture sustainability; Shrimp farming
Received | November 02, 2021; Accepted | November 26, 2021; Published | March 25, 2022
*Correspondence | Mohammad Mahmudi, Universitas Brawijaya, Indonesia; Email: mudi@ub.ac.id
Citation | Mahmudi M, Musa M, Arsad S, Lusiana ED, Bunga A, Wati NA (2022). Use of Phytoplankton to Assess Water Quality of Eco-Aquaculture System in Super-Intensive Whiteleg Shrimp (Litopenaeus vannamei) Pond. Adv. Anim. Vet. Sci. 10(5): 971-979.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.5.971.979
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Whiteleg shrimp (Litopenaeus vannamei) aquaculture has grown substantially in the previous decade, with a global output of approximately 4.16 million tonnes in 2016, accounting for 53 percent of total shrimp and prawn supply (FAO, 2018). The majority of this production is located in tropical areas of the world, primarily in Asian regions such as China, Thailand, Vietnam, and Indonesia (Ahmed et al., 2018). The main advantages of L. vannamei include high density tolerance, adaptability to diverse environmental pressures and speedier development even during the lesser culture periods (Suwoyo & Hendrajat, 2021). As a result of these characteristics, this organism has become the most popular shrimp species for large-scale production (Naylor et al., 2021).
The rapid development of shrimp farming industries has led to the destruction of coastal environments as a result of land conversion (Hamilton, 2013). Many mangrove forests in coastal areas have been transformed into shrimp ponds (Bournazel et al., 2015), and this change has negative impacts because mangroves have many environmental benefits, such as defence against tidal abrasion, extreme storms and sediment/nutrient trapping (Peng et al., 2009). As culture systems progress to become super intensive, the water quality in shrimp ponds tends to degrade due to increased shrimp mass and organic matter build-up from leftover feed, excrement and metabolism (Barraza-Guardado et al., 2013). The impairment of water quality caused by nutrient accumulation has been recognized as a possible cause of disease outbreaks in shrimp ponds (Alfiansah et al., 2018). Furthermore, in combination with municipal and agricultural effluents, shrimp farming wastewater discharges could contribute to algal blooms in the surrounding coastal environment. Hence, the shrimp farming industry is undergoing pressure to enhance its ecological sustainability (Briggs et al., 2004).
Recently, the concept of eco-green aquaculture by integrating mangroves and shrimp ponds has been increasingly discussed (Sampantamit et al., 2020). Such a strategy aims to preserve mangrove forests and allows them to function as wastewater treatment for aquaculture sewage (Ahmed et al., 2018). From a physico–chemical characteristics perspective, the system has been found to improve the water quality of extensive ponds’ aquaculture wastewater (Peng et al., 2009); a similar study analyzed super-intensive ponds, but the results were not significant (Musa et al., 2020). Water physico–chemical characteristics, such as nutrients (nitrate and phosphate), dissolved oxygen (DO) and ammonia are routinely monitored either directly by water sampling and empirical studies or indirectly using remote sensing devices (Rahmanian et al., 2015). However, to identify water quality fluctuations, this approach requires a long series of data collection. Therefore, numerous studies have recommended that living organisms be used to measure the water quality of aquatic habitats (McQuatters-Gollop et al., 2009).
One aquatic organism commonly employed as a bioindicator is phytoplankton (Parmar et al., 2016). These organisms have been proposed as promising bioindicators for assessing long-term changes in aquatic habitats, particularly those linked to algal blooms, climate change and water resource management (Gökçe, 2016). In this regard, this organism can swiftly adapt to environmental changes, resulting in a more rapid assessment of water quality (Parmar et al., 2016). Pollutants discharge into water, and the deterioration of aquatic ecosystems can change the phytoplankton community composition. For instance, sewage flows can elevate nutrients, which might stimulate a bottom-up process (Davis et al., 2010) and cause the aquatic ecosystem’s community structure to be dominated by harmful algae (Berdalet et al., in press). In aquaculture ponds, this issue results in disease outbreaks of the cultured organism (Qiao et al., 2020).
Based on physico–chemical water quality monitoring and phytoplankton community structure, this study intended to analyze the impact of integrated super-intensive shrimp aquaculture on mangroves. Such an analysis is critical because this form of aquaculture has both significant economic interest and an increased risk of posing damage to the coastal environment. Coastal ecological degradation is predicted to be avoided by combining aquaculture with mangroves.
MATERIALS AND METHODS
Study Area
The research was carried out at Brawijaya University’s Brackish and Marine Water Laboratory. This facility is located on the coast of Indonesia’s Probolinggo region ( Figure 1a), where it conducts super-intensive L. vannamei cultivation. The study took place between February and March 2021 with weekly sampling.
As shown in Figure 1b, we used six sampling locations. The river water near the entrance channel is utilized as the primary supply of water for the intensive pond (Site 1). Site 2 is a reservoir pond that serves as a water source for ponds as well as a water isolation system to interrupt the disease cycle. The intensive ponds are located at sites 3 and 4. The sewage disposal pond from these ponds is Site 5. The mangrove area is the last site (Site 6).
Water Quality and Phytoplankton Observation
We assessed several water quality characteristics, such as temperature (oC), transparency (m), pH, dissolved oxygen (DO, mg/L), salinity (%o), nitrate (mg/L), nitrite (mg/L), ammonia (mg/L), orthophosphate (mg/L) and total organic matter (TOM, mg/L) at the study sites by observed the water samples. A DO meter type Lutron PDO-520 was utilized to measure temperature, DO and pH, and a secchi disc and refractometer were utilized for transparency and salinity, respectively. Furthermore, nitrate, nitrite and ammonia were quantified by using test kits. Lastly, orthophosphate and TOM were analyzed ex-situ by utilizing colorimetric and titrimetric techniques.
Plankton nets with a pore size of 25 m were used to concentrate 25 L of water samples to identify phytoplankton. The samples were preserved with 4% formalin and stored in 30 mL vial bottle. After that, they analyzed in the laboratory. Phytoplankton identification process was carried out by using light microscope type Olympus CX-21LED with 400x magnification and the morphological characteristics was determined in accordance to Prescott’s book algal identification (Prescott, 1978). In addition, we used the Sedgwick Rafter counting cell and the following Lackey drop formula to determine phytoplankton density (APHA, 1989).
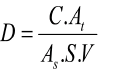
where
D = phytoplankton desnity (cell/ml)
C = number of organisms counted;
At = area of cover slip (mm²)
As = area of one strip (mm²)
S = number of strips counted
V = volume of sample under the cover slip (ml).
Data Analysis
We employed three data analysis methods in this study. The water quality parameters were initially identified using box plots with an ANOVA/Tukey test. We then measured the diversity of phytoplankton composition using a Shannon–Wiener diversity index (Miao et al., 2019) and subsequently used canonical correspondence analysis (CCA) to investigate the connection between observed water quality indicators and phytoplankton abundance (Greenacre, 2010). The analysis was performed using both R and PAST software.
RESULTS
The results of temperature, nitrite and DO, as depicted in Figure 2, indicated that the sample waters differed between sites, as denoted by the unequal notation of the box-plot. Meanwhile, transparency, salinity, pH nitrate, ammonia, orthophosphate and TOM did not vary significantly between locations because these parameters shared similar letter notations. Site 5 had a higher temperature, whereas Sites 3 and 4 had the lowest values. In contrast, nitrite levels in these sites were far higher than in the other sites. A similar pattern occurred for the nitrate and ammonia measurement results, but the values of these indicators were not significantly different between sites. On the other hand, the highest and lowest DO levels were found at Sites 1 and 5, respectively. Furthermore, at all sampling sites, the transparency and salinity were 10-40 cm and 20–30 0/00, respectively. Relatively steady values across sites were shown for the orthophosphate and TOM measurement results.
The composition of phytoplankton genera in the study site is shown in Table 1. A total of 21 genera were recognized, with the Chlorophyta and Chrysophyta divisions mostly dominating (Figure 3). Peridinium is a Dinophyta genus that was exclusively found at Site 4. Sites 1, 2 and 3 had the
Table 1: Phytoplankton community structure and diversity index (H’)
| Division | Genus | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 |
| Chlorophyta | Chlorella | - | - | + | + | + | + |
| Oocystis | - | - | + | + | + | + | |
| Scenedesmus | - | - | - | - | + | - | |
| Cosmarium | + | - | + | + | - | - | |
| Ulothrix | - | + | - | - | - | - | |
| Chlamydomonas | - | - | + | - | - | - | |
| Chrysophyta | Nitzschia | + |
- |
- | + | + | - |
| Melosira | - | - | - | - | + | ||
| Amphora | - | + | + | - | + | + | |
| Cyclotella | + | + | + | - | + | + | |
| Navicula | + | + | + | - | + | - | |
| Gyrosigma | - | - | - | - | + | - | |
| Amphiprora | - | - | - | + | + | + | |
| Stephanodiscus | + | + | + | + | - | - | |
| Cyanophyta | Microcystis | - | + | + | + | + | + |
| Oscillatoria | - | - | - | - | + | - | |
| Anabaena | - | - | - | - | - | + | |
| Nostoc | + | - | - | - | - | - | |
| Chroococcus | - | - | + | + |
- |
- | |
| Spirulina | - | - | - | + | - | - | |
| Dinophyta | Peridinium | - | - | - | + | - | - |
| Shannon-Wiener Diversity Index (H’) | 1.080 | 0.817 | 0.774 | 1.099 | 0.798 |
1.059 |
|
lowest biomass and number of genera. On the other hand, sites 3 and 4, where the shrimp farming pond was located, as well as Site 5, exhibited a reasonably high quantity of phytoplankton. The diversity index (H’) fluctuated across sites. A high diversity index was mainly found at Sites 1 and 6, whereas lower index values were observed at Sites 2, 3 and 5.
We performed a CCA to analyze the water quality factors that were associated with phytoplankton division presence. The relationship was obtained by taking the shortest projection of division point to the water quality parameter vector from the CCA triplot. According to Figure 4, Chlorophyta species were likely to occur in high concentrations of TOM, transparency, pH and nitrate, and low concentrations of temperature, DO, salinity and orthophosphate. The relationship between Dinophyta presence and water
quality characteristics was found to be quite similar to that of Chlorophyta. Meanwhile, a contrasting pattern to Chlorophyta appearance was noted for the Chrysophyta division. On the other hand, the appearance of Cyanophyta corresponded with high temperature, ammonia, nitrite, orthophosphate and salinity; moderate nitrate and pH; low transparency and TOM.
DISCUSSION
Temperature is one of the important indicators for successful L. vannamei cultivation. This parameter can affect the growth, physiological performance and survival of this biota (He et al., 2018; Wang et al., 2019; Zhang et al., 2019). Previous research has shown that the optimum temperature for L. vannamei varied with body size—30 °C and 27 °C for small and large shrimp, respectively (Wyban et al., 1995). Similarly, transparency, as an essential characteristic of aquaculture ponds, is strongly related to photosynthetic activity (Abdel-Raouf et al., 2012). Despite there being no significant variation in transparency among sites, higher levels of transparency were found at Sites 1 and 6, indicating that the peculiarity of the mangrove root system can retain contaminants and silt (Kida & Fujitake, 2020).
The accumulation of aquaculture by-products in water might result in it becoming alkaline or acidic (Boyd, 2016). The pH levels in this study were lower than 6, which can be fatal to aquatic organisms (Velma et al., 2009). Meanwhile, the salinity was less than the recommended value for aquaculture (or 27 0/00) (Ministry of Environment, 2001). Low salinity, on the other hand, is unlikely to impair osmotic regulation to such a point that L. vannamei survival and growth levels would be affected (Gao et al., 2016). This biota has significant potential in coastal saline waters at salinities as low as 1 0/00 if the acclimatization technique is performed appropriately (Allen, 2004). Furthermore, in aquaculture, DO values of 4 to 5 mg/L or higher are considered optimum (Boyd, 2003). Low levels of DO (below 2.0 mg/L) are associated with reduced growth and a higher risk of organism death (Ferreira et al., 2011).
Commercial feed has been commonly utilized in intensive shrimp cultivation systems because farmers were compelled to feed their shrimp on a specified growth plan due to the reduction of the harvest period (Chaikaew et al., 2019). A high abundance of feed waste produces significant amounts of organic matter, which raises water’s TOM concentration. As the amount of organic matter in the environment increases, so will the quantity of nutrients in the water (Lusiana et al., 2019a). Nutrients, such as nitrate and phosphate, are extensively used as indicators for eutrophication and have been linked to phytoplankton biomass (Lv et al., 2011). Phosphate is found in a variety of compounds, but only orthophosphate can be directly used by aquatic microorganisms (Wan et al., 2020). On the other hand, nitrite and ammonia, two of the three types of nitrogen investigated in this study, were found to surpass the quality threshold for fisheries (Ministry of Environment, 2001). High amounts of ammonia in water can harm the gills, reduce shrimp growth and moulting rate, as well as decrease the blood’s ability to transport oxygen (Shaari et al., 2011). When any conversion of ammonia to nitrate is prevented, nitrite will be accumulated to significant levels, lowering the shrimp’s immunity and making them prone to disease caused by the vibrio virus (Widanarni et al., 2020).
In contrast to the other sites, the Chlorophyta proportion at Site 2 was relatively low. Based on the CCA results, this finding might have been due to the high salinity level in this site contributing to the disappearance of Chlorophyta species. It has been reported that a high concentration of salinity can reduce the nutrient uptake rate of Chlorophyta organisms (Choi et al., 2010). On the other hand, specific factors that may enhance Chlorophyta assemblages in Sites 3, 4 and 5 were low temperature, DO, salinity as well as higher TOM levels. In this research, the common Chlorophyta genera in the studied sites were Chlorella and Oocystis. Various investigations have shown that Chlorella species can enhance nutrition, immunity, aquatic environmental remediation, stress relief, fish disease resistance and bacterial quorum sensing (Ahmad et al., 2020; Mtaki et al., 2021). Meanwhile, in subtropical aquaculture farms, Oocystis is a common-dominator genus with a steady population size and a high capacity to meet various environmental circumstances (Huang et al., 2012). The genus is also capable of reducing urea nitrogen levels in ponds (Liu et al., 2018).
There were 8 genera from Chrysophyta or diatoms observed in this study, with most being Cyclotella and Navicula. Diatoms are advantageous algae that play an essential role as a food supply for aquatic invertebrates (Boyd, 2016). Most shrimp aquaculture managers favour a high percentage of diatoms in a phytoplankton community because diatoms are advantageous algae that boost shrimp growth more than cyanophytes (Yusoff et al., 2002). Based on the CCA results, the assemblage of diatoms in this study was associated with high concentrations of DO, salinity and orthophosphate and low concentrations of TOM, transparency, pH and nitrate. This result contradicts previous research, which implied that high nitrate concentration enhances diatom growth (Cremen et al., 2007). From Figure 2 and Table 1, the diversity decreased as the DO level declined from Sites 2 to 3. Hence, DO level is a positive predictor for diatom species richness (Shaari et al., 2011).
Cyanophyta comprise 6 genera. The biomass of Cyanophyta was relatively high at Sites 3 and 4, which coincided with a high level of orthophosphate. Cremen et al. (2007) also noted that high phosphate concentrations typically encourage the growth of Cyanophyta. Depressed diatom growth is most frequently followed by increased Cyanophyta growth (Yusoff et al., 2002). However, this study showed that since the beginning of the culture process, Cyanophyta were less dominating than diatoms, indicating that nitrate variation during the study did not hold diatom growth, whereas an insignificant increase of orthophosphate raised Cyanophyta growth. Finally, Peridinium was the only Dinophyta genus found at Site 4, with very little abundance. This organism is classified into the family Peridiniaceae—a bloom-forming microalgae (Gárate-Lizárraga & Muñetón-Gómez, 2008).
Both Cyanophyta and Dinophyta species are considered to be harmful algae (Baek et al., 2008; Mahmudi et al., 2020). Because of its ability to create toxins, such as microcystin, the presence of poisonous Cyanophyta in aquaculture systems is a reason for concern (Kimambo et al., 2019). Microcystin is the most common cyanotoxin found in freshwater ecosystems (Preece et al., 2017). Microcystis is an example of a Cyanophyta genus that can produce microcystin (Reichwaldt et al., 2013) and was the most common Cyanophyta genus found in this research, representing an alert for the sustainability of shrimp farming. Microcystin reaches the fish body through the gills, feed and food chain, causing liver tissue damage of aquatic organisms (Lehman et al., 2010). Microcystin can also assemble in fish tissues, posing a health risk to humans who consume them (Peng et al., 2010). Meanwhile, the blooming of Dinophyta, such as Peridinium, leads to water discoloration and reduced transparency, which affect the water system’s primary production (Zohary et al., 2014; Rodríguez-Gómez et al., 2019). Algal blooming is generally caused by nutrient enrichment (Lusiana et al., 2019b) and water temperature increases up to 32 °C (Shaari et al., 2011). This phenomenon also causes a shift in the phytoplankton community and diminishes species richness/diversity (Wabnitz et al., 2018), as visible at Sites 3 and 5. In this study, we identified a serious threat to shrimp culture sustainability—nutrient enrichment—as a result of enhanced levels of orthophosphate that increased Cyanophyta biomass. Therefore, appropriate feed management during shrimp culture is required to control the accumulation of organic waste as well as nutrient enrichment (Lusiana et al., 2019a).
Sites 1 (water supply) and 6 (the mangrove area) were found to be the most diverse. In Site 6, the organic matter was absorbed by mangroves. Sedimentation in sewage ponds and organic matter build-up by mangrove plants diminish excessive organic matter content (Bao et al., 2013; Hossain & Nuruddin, 2016). The water supply for the studied sites’ aquaculture systems was obtained from treated wastewater (Site 6), tidal and estuarine water. Due to dilution from sea-, estuary- and wastewater, these three sources result in good water quality for aquaculture practice.
CONCLUSION
The intensification of shrimp culture has resulted in a slew of environmental issues, as well as concerns about the activity’s long-term sustainability. An integrated shrimp farming and mangrove system might be a solution to this issue. This research revealed that significant variation of temperature and DO level was found among sites. These affect the domination of Cholorophyta and Chrysophyta division in the studied ponds, which favour shrimp growth. Furthermore, the diversity index for the cultured ponds, which contain high amounts of organic waste and nutrients, was lower than the mangrove area site, which served as water inlet and outlet, demonstrating that mangroves can absorb organic matter and, therefore, improve water quality and increase phytoplankton structure diversity of the both water supply for the shrimp farming and aquaculture sewage.
ACKNOWLEDGEMENTS
This research was financially supported by Doctor Lektor Kepala Grant 2021 with contract number: 2105/UN10.F06/PP/2021, Faculty of Fisheries and Marine Science, Universitas Brawijaya.
conflict of interest
The authors have declared no conflict of interest.
novelty statement
This research reports the investigation of the use of phytoplankton as a tool for water quality monitoring of eco-aquaculture system in super-intensive whiteleg shrimp pond. This is significant as an attempt to maintain the coastal ecosystem as well as the sustainability of shrimp farming.
authors contribution
Mohammad Mahmudi designed, coordinated, and supervised the research. Muhammad Musa and Sulastri Arsad supervised and revised the manuscript. Evellin Dewi Lusiana analyzed the data and wrote the manuscript. Alamanda Bunga and Nur Azlina Wati collected the data. All authors read and approved the final version of the manuscript.
REFERENCES
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012). Microalgae and wastewater treatment. Saudi J. Biolog. Sci. 19: 257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Ahmad M.T, Shariff M, Md. Yusoff F, Goh YM, Banerjee S (2020). Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquacult. 12: 328–346. https://doi.org/10.1111/raq.12320
Ahmed N, Thompson S, Glaser M (2018). Integrated mangrove-shrimp cultivation: Potential for blue carbon sequestration. Ambio. 47: 441–452. https://doi.org/10.1007/s13280-017-0946-2
Alfiansah Y.R, Hassenrück C, Kunzmann A, Taslihan A, Harder J, Gärdes A (2018). Bacterial Abundance and Community Composition in Pond Water From Shrimp Aquaculture Systems With Different Stocking Densities. Front. Microbiol. 9: 2457. https://doi.org/10.3389/fmicb.2018.02457
APHA (1989). Standard Methods for the Examination of Water and Wastewater. 17th edition American Public Health Association , Washington DC.
Baek S.H, Shimode S, Han M-S, Kikuchi T (2008). Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of nutrients. Harmful Algae. 7: 729–739. https://doi.org/10.1016/j.hal.2008.02.007
Bao H, Wu Y, Unger D, Du J, Herbeck LS, Zhang J (2013). Impact of the conversion of mangroves into aquaculture ponds on the sedimentary organic matter composition in a tidal flat estuary (Hainan Island, China). Continental Shelf Research 57: 82–91. https://doi.org/10.1016/j.csr.2012.06.016
Barraza-Guardado R.H, Wu Y, Unger D, Du J, Herbeck LS, Zhang J (2013). Effluents of Shrimp Farms and Its Influence on the Coastal Ecosystems of Bahía de Kino, Mexico. Scient. World J. 2013: 306370. https://doi.org/10.1155/2013/306370
Berdalet E, Fleming LE, Gowen R, Davidson K, Hess P, Backer LC, Moore SK, Hoagland P, Enevoldsen H (in press). Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. J. Marine Biolog. Assoc. United Kingdom. https://doi.org/10.1017/S0025315415001733
Bournazel J, Kumara MP, Jayatissa LP, Viergever K, Morel V, Huxham M(2015). The impacts of shrimp farming on land-use and carbon storage around Puttalam lagoon, Sri Lanka. Ocean Coastal Manag. 113: 18–28. https://doi.org/10.1016/j.ocecoaman.2015.05.009
Boyd C.E. (2003). Guidelines for aquaculture effluent management at the farm-level. Aquaculture. 226: 101–112. https://doi.org/10.1016/S0044-8486(03)00471-X
Boyd C.E. (2016). Phytoplankton a crucial component of aquaculture pond ecosystems Available at https://www.aquaculturealliance.org/advocate/phytoplankton-a-crucial-component-of-aquaculture-pond-ecosystems/. https://doi.org/10.1016/S0044-8486(03)00471-X
Chaikaew P, Rugkarn N, Pongpipatwattana V, Kanokkantapong V (2019). Enhancing ecological-economic efficiency of intensive shrimp farm through in-out nutrient budget and feed conversion ratio. Sustainable Environ. Res. 1: 1–11. https://doi.org/10.1186/s42834-019-0029-0
Choi T.-S, Kim E-J, Kim J, Kim K-Y (2010). Effect of salinity on growth and nutrient uptake of Ulva pertusa (Chlorophyta) from an eelgrass bed. Algae. 25: 17–26. https://doi.org/10.4490/algae.2010.25.1.017
Cremen M.C.M, Martinez-Goss MR, Corre VL, Azanza R V (2007). Phytoplankton bloom in commercial shrimp ponds using green-water technology. J. Appl. Phycol. 19: 615–624. https://doi.org/10.1007/s10811-007-9210-7
Davis J.MRosemond AD, Eggert SL, Cross WF, Wallace JB (2010). Long-term nutrient enrichment decouples predator and prey production. Proceedings of the National Academy of Sciences of the United States of America 107: 121–126. https://doi.org/10.1073/pnas.0908497107
FAO (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals Rome.
Ferreira N.C, Bonetti C, Seiffert WQ (2011). Hydrological and Water Quality Indices as management tools in marine shrimp culture. Aquaculture. 318: 425–433. https://doi.org/10.1016/j.aquaculture.2011.05.045
Gao W, Tian L, Huang T, Yao M, Hu W, Xu Q (2016). Effect of salinity on the growth performance, osmolarity and metabolism-related gene expression in white shrimp Litopenaeus vannamei. Aquaculture Reports 4: 125–129. https://doi.org/10.1016/j.aqrep.2016.09.001
Gárate-Lizárraga I., Muñetón-Gómez M. del S. (2008). Bloom of peridinium quinquecorne abé in la ensenada de la paz, gulf of california (July 2003). Acta Botan. Mexicana. 83: 33–47. https://doi.org/10.21829/abm83.2008.1059
Gökçe D. (2016). Algae as an Indicator of Water Quality. InTech Open. https://doi.org/10.5772/62916
Greenacre M (2010). Canonical correspondence analysis in social science research. Studies in Classification, Data Analysis, and Knowledge Organization. 279–286. https://doi.org/10.1007/978-3-642-10745-0_30
Hamilton S (2013). Assessing the role of commercial aquaculture in displacing mangrove forest. Bullet. Marine Sci. 89: 585–601. https://doi.org/10.5343/bms.2012.1069
He P, Wei P, Zhang B, Zhao Y, Li Q, Chen X, Zeng D, Peng M, Yang C, Peng J, Chen X (2018). Identification of microRNAs involved in cold adaptation of Litopenaeus vannamei by high-throughput sequencing. Gene. 677: 24–31. https://doi.org/10.1016/j.gene.2018.07.042
Hossain MD, Nuruddin AA (2016). Soil and mangrove: A review. J. Environ. Sci. Technol. 9: 198–207. https://doi.org/10.3923/jest.2016.198.207
Huang X, Li X, Wang Y, Zhou M (2012). Effects of Environmental Factors on the Uptake Rates of Dissolved Nitrogen by a Salt-water Green Alga (Oocystis borgei Snow). Bullet. Environ. Contamin. Toxicol. 89: 905–909. https://doi.org/10.1007/s00128-012-0767-8
Kida M, Fujitake N (2020). Organic carbon stabilization mechanisms in mangrove soils: A review. Forests. 11: 1–15. https://doi.org/10.3390/f11090981
Kimambo ON, Gumbo JR, Chikoore H (2019). The occurrence of cyanobacteria blooms in freshwater ecosystems and their link with hydro-meteorological and environmental variations in Tanzania. Heliyon. 5: e01312–e01312. https://doi.org/10.1016/j.heliyon.2019.e01312
Lehman PW, Teh SJ, Boyer GL, Nobriga ML, Bass E, Hogle C (2010). Initial impacts of Microcystisaeruginosa blooms on the aquatic food web in the San Francisco Estuary. Hydrobiologia. 637: 229–248. https://doi.org/10.1007/s10750-009-9999-y
Liu M, Huang X, Zhang R, Li C, Gu B (2018). Uptake of Urea Nitrogen by Oocystis borgei in Prawn (Litopenaeus vannamei) Aquaculture Ponds. Bullet. Environ. Contaminat. Toxicol. 101: 586–591. https://doi.org/10.1007/s00128-018-2450-1
Lusiana E.D, Arsad S, Kusriani, Buwono NR, Putri IR (2019a). Performance of Bayesian quantile regression and its application to eutrophication modelling in Sutami Reservoir, East Java, Indonesia. Ecolog. Questions. 30: 69–77. https://doi.org/10.12775/EQ.2019.010
Lusiana E.D, Arsad S, Kusriani, Buwono NR, Putri IR (2019b). The application of Bayesian quantile regression to analyse the relationship between nutrients content and phytoplankton abundance in Sutami reservoir. In: IOP Conf. Ser. Earth Environ. Sci. Vol. 230. https://doi.org/10.1088/1755-1315/230/1/012082
Lv J, Wu H, Chen M (2011). Effects of nitrogen and phosphorus on phytoplankton compositionand biomass in 15 subtropical,urban shallow lakes in Wuhan, China. Limnologica. 41: 48–56. https://doi.org/10.1016/j.limno.2010.03.003
Mahmudi M, Serihollo LG, Herawati EY, Lusiana ED, Buwono NR (2020). A count model approach on the occurrences of harmful algal blooms (HABs) in Ambon Bay. Egyptian J. Aquat. Res. 46: 347–353. https://doi.org/10.1016/j.ejar.2020.08.002
McQuatters-Gollop A, Gilbert AJ, Mee LD, Vermaat JE, Artioli Y, Humborg C, Wulff F (2009). How well do ecosystem indicators communicate the effects of anthropogenic eutrophication? Estuarine, Coastal Shelf Sci. 82: 583–596. https://doi.org/10.1016/j.ecss.2009.02.017
Miao X, Wang S, Liu M, Ma J, Hu J, Li T, Chen L (2019). Changes in the phytoplankton community structure of the Backshore Wetland of Expo Garden, Shanghai from 2009 to 2010. Aquacult. Fisheries. 4: 198–204. https://doi.org/10.1016/j.aaf.2019.02.004
Mtaki K, Kyewalyanga MS, Mtolera MSP (2021). Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Ann. Microbiol. 71: 7. https://doi.org/10.1186/s13213-020-01618-0
Musa M, Lusiana ED, Buwono NR, Arsad S, Mahmudi M (2020). The effectiveness of silvofishery system in water treatment in intensive whiteleg shrimp (Litopenaeus vannamei) ponds, probolinggo district, East Java, Indonesia. Biodiversitas. 21: 4695–4701. https://doi.org/10.13057/biodiv/d211031
Naylor R.L, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M(2021). A 20-year retrospective review of global aquaculture. Nature 591: 551–563. https://doi.org/10.1038/s41586-021-03308-6
Parmar T.K, Rawtani D, Agrawal YK (2016). Bioindicators: the natural indicator of environmental pollution. Front. Life Sci. 9: 110–118. https://doi.org/10.1080/21553769.2016.1162753
Peng L, Liu Y, Chen W, Liu L, Kent M, Song L (2010). Health risks associated with consumption of microcystin-contaminated fish and shellfish in three Chinese lakes: Significance for freshwater aquacultures. Ecotoxicol. Environ. Safety. 73: 1804–1811. https://doi.org/10.1016/j.ecoenv.2010.07.043
Peng Y, Li X, Wu K, Peng Y, Chen G (2009). Effect of an integrated mangrove-aquaculture system on aquacultural health. Front. Biol. China. 4: 579. https://doi.org/10.1007/s11515-009-0056-z
Preece E.P, Hardy FJ, Moore BC, Bryan M (2017). A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae. 61: 31–45. https://doi.org/10.1016/j.hal.2016.11.006
Prescott GW (1978). The Freshwater Algae. WM. C. Brown Company Publishers., Iowa.
Qiao L, Chang Z, Li J, Chen Z (2020). Phytoplankton community succession in relation to water quality changes in the indoor industrial aquaculture system for Litopenaeus vannamei. Aquaculture. 527: 735441. https://doi.org/10.1016/j.aquaculture.2020.735441
Rahmanian N, Ali SHB, Homayoonfard M, Ali NJ, Rehan M, Sadef Y, Nizami AS (2015). Analysis of Physiochemical Parameters to Evaluate the Drinking Water Quality in the State of Perak, Malaysia. J. Chem. 2015: 716125. https://doi.org/10.1155/2015/716125
Reichwaldt E.S, Song H, Ghadouani A (2013). Effects of the Distribution of a Toxic Microcystis Bloom on the Small Scale Patchiness of Zooplankton. PLOS ONE. 8: e66674. https://doi.org/10.1371/journal.pone.0066674
Rodríguez-Gómez C.F, Vázquez G, Aké-Castillo JA, Band-Schmidt CJ, Moreno-Casasola P (2019). Physicochemical factors related to Peridinium quadridentatum (F. Stein) Hansen (Dinophyceae) blooms and their effect on phytoplankton in Veracruz, Mexico. Estuarine, Coastal Shelf Sci. 230: 106412. https://doi.org/10.1016/j.ecss.2019.106412
Sampantamit T, Ho L, Lachat C, Sutummawong N, Sorgeloos P, Goethals P (2020). Aquaculture production and its environmental sustainability in Thailand: Challenges and potential solutions. Sustainability (Switzerland). 12: 1–17. https://doi.org/10.3390/su12052010
Suwoyo HS, Hendrajat EA (2021). High density aquaculture of white shrimp (Litopenaeus vannamei) in controlled tank. IOP Conference Series: Earth Environ. Sci. 777. https://doi.org/10.1088/1755-1315/777/1/012022
Velma V, Vutukuru SS, Tchounwou PB (2009). Ecotoxicology of hexavalent chromium in freshwater fish: a critical review. Rev. Environ. Health. 24: 129–145. https://doi.org/10.1515/REVEH.2009.24.2.129
Wabnitz CCC, Lam VWY, Reygondeau G, Teh LCL, Al-Abdulrazzak D, Khalfallah M, Pauly D, Palomares MLD, Zeller D, Cheung WW (2018). Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLOS ONE. 13: e0194537. https://doi.org/10.1371/journal.pone.0194537
Wan J, Yuan X, Han L, Ye H, Yang X (2020). Characteristics and distribution of organic phosphorus fractions in the surface sediments of the inflow rivers around hongze lake, China. Int. J. Environ. Res. Pub. Health. 17: 1–16. https://doi.org/10.3390/ijerph17020648
Wang Z, Qu Y, Yan M, Li J, Zou J, Fan L (2019). Physiological Responses of Pacific White Shrimp Litopenaeus vannamei to Temperature Fluctuation in Low-Salinity Water. Front. Physiol. 10: 1025. https://doi.org/10.3389/fphys.2019.01025
Widanarni W, Rahmi D, Gustilatov M, Sukenda S, Utami DAS (2020). Immune responses and resistance of white shrimp (Litopenaeus vannamei) fed Probiotic Bacillus sp NP5 and prebiotic honey against White Spot Syndrome Virus infection. J. Akuakultur Indonesia. 19: 118–130. https://doi.org/10.19027/jai.19.2.118-130
Wyban J, Walsh WA, Godin DM (1995). Temperature effects on growth, feeding rate and feed conversion of the Pacific white shrimp (Penaeus vannamei). Aquaculture. 138: 267–279. https://doi.org/10.1016/0044-8486(95)00032-1
Yusoff F.M, Zubaidah MS, Matias HB, Kwan TS (2002). Phytoplankton succession in intensive marine shrimp culture ponds treated with a commercial bacterial product. Aquacult. Res. 33: 269–278. https://doi.org/10.1046/j.1355-557x.2002.00671.x
Zhang W, Chen B, Niu C, Yuan L, Jia H, Storey KB (2019). Response of the Chinese Soft-Shelled Turtle to Acute Heat Stress: Insights From the Systematic Antioxidant Defense. Frontiers in Physiology 10: 710. https://doi.org/10.3389/fphys.2019.00710
Zohary T, Sukenik A, Berman T (2014). Lake Kinneret. In: Lake Kinneret. https://doi.org/10.1007/978-94-017-8944-8
To share on other social networks, click on any share button. What are these?






