Unraveling the Bioherbicidal Potential, Elemental Analysis and Nutritional Evaluation of Crataeva adansonii Dc Leaf and Bark
Syeda Farzana Bibi* and Siraj ud Din
Department of Botany, University of Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | There is an urgent need to explore and utilize naturally occurring products for combating harmful agricultural and public health issues. Plant-derived substances can exhibit significant results with lesser side effects, and they are easily accessible and more economical than their synthetic alternatives. This study aims to conduct a phytotoxic study of ethanolic extract of Crataeva adansonii DC. leaf and stem bark for assessment of its bioherbicidal potential. Elemental and nutritional analysis of the plant parts was also done to accentuate this plant’s use in the nutritional and agricultural industry. Elemental analysis of C. adansonii stem bark and leaf was assessed through atomic absorption spectrophotometry to detect ten essential macro and microelements, including Na, Ca, Co, Cd, Cu, Mn, Zn, Pb, Ni, and Fe. In the bark part, the highest percentage of relative standard deviation was observed for Co, Cd, and Na, which is 175.6, 22.86, and 5.58%, respectively. The leaf part’s elemental analysis revealed the highest percentage of R.S.D. for Co, Mn, and Pb, which is 20, 9, and 4.44%, respectively. Imperative nutritional aspects of the proposed plant extracts were determined, including percentages of carbohydrates, proteins, fats, fiber, ash, and moisture. The bioherbicidal potential of the proposed plant was evaluated through the Lemna minor model of phytotoxicity. C. adansonii is a source of several essential macro and micronutrients. A nutritional value analysis of this plant offers its use as a food source. Bioherbicidal/phytotoxic activity revealed significant results in the form of dose-dependent inhibition of frond growth. Ethanolic extract of C. adansonii exhibited 30% inhibition at 1000 μl concentration, and at the same concentration, the bark extract inhibited the frond growth by 40%. The pharmacognostic evaluation of this medicinal drug will be supportive in the detection of adulteration and quality control. Due to the herbicidal potential, it can be used in the agricultural industry. For future studies, it is suggested to understand the mode of action of its active compounds through future studies.
Received | February 06, 2020; Accepted | November 12, 2020; Published | December 19, 2020
*Correspondence | Syeda Farzana Bibi, Department of Botany, University of Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Bibi, S.F. and S. Din. 2020. Unraveling the bioherbicidal potential, elemental analysis and nutritional evaluation of Crataeva adansonii Dc. leaf and bark. Sarhad Journal of Agriculture, 36(4): 1316-1324.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.4.1316.1324
Keywords | Crataeva adansonii, Phytotoxic activity, Elemental analysis, Nutritional evaluation, Herbicidal potential
Introduction
Crataeva adansonii, commonly known as Varun, temple plant, and sacred garlic pear, belongs to the family Capparaceae (Ryan and Ray, 2004). It is a moderate-sized deciduous tree bearing trifoliate compound leaves. The flowers are showy, bearing long stamens. Due to the elongated appearance of stamens, the plant is often called a spider tree. Flowers of this plant are white, and stamens are purplish. In traditional medicine, C. adansonii is astringent, antipyretic, anti-inflammatory, appetizer, liver stimulant, contraceptive, stomachic, diuretic, tonic, and demulcent. The use of this plant against bronchitis, calculi, asthma, cough, renal and urinary troubles, dyspepsia, and uterine infections is also well known in traditional medicine (Burkill, 1985; Gitte et al., 2012).
Elements like Cu, Zn, Cr, and many other trace elements can be preventive and curative in many aspects. They can play a vital and therapeutic role in combating various ailments (Kaneez et al., 2001). The role of mineral elements is incredible in physiology and pharmacology despite their small proportion and body weight (Bamiro et al., 1995).
Nutritional components of plants, including fats, minerals, carbohydrates, proteins, water, vitamins, and oils, are considered necessary for the development and growth of plants and animals. On the other hand, some plant chemicals are known as antioxidants; they protect humans from several diseases (Agte et al., 2000). Caloric needs and metabolic requirements of humans are fulfilled by essential nutrients (Underwood, 1977). The quality and purity of crude drugs can be determined through ash values. It shows the presence of impurities like oxalate, silicate, and carbonate. Analytical techniques are applied for detecting quality, identity, potency, purity, efficacy, and safety of herbal drugs, pure active compounds, and standardized extracts (Patil et al., 2013).
Synthetic herbicides destroy seed bank in soil and reduce their production capability. Several secondary metabolites produced by plants can work as allelochemicals to other plants growing in the vicinity (Morimoto et al., 2009). Nowadays, bio-herbicides are preferred over synthetically produced herbicides (Duke et al., 2000; Dudai et al., 1999). Phytotoxins showing significant agricultural potential can lead to the discovery of novel herbicides showing better activity as compared to synthetic products, due to their unexplored action sites (Westwood et al., 2018; Teixeira et al., 2019).
Lemna plant belongs to the family Lemnaceae; it is used in various scientific experiments to observe various phytoextracts’ effects on several physiological processes. It is an aquatic angiosperm consisting of a filamentous root. They have no stems or true leaves and usually consist of a single or a few flat, oval-shaped, and small “fronds.” New fronds are budded from pre-existing fronds (Atta-ur-Rahman and Thomsen, 2001). It is observed that the application of typical antitumor compounds results in its growth inhibition.
Similarly, some chemical substances may stimulate frond proliferation, thus acting as growth stimulants. Crop productivity is reduced because of weeds as they compete for water, sunlight, minerals. Synthetic drugs for weed control cause water pollution, soil pollution, and several diseases in humans and plants (Ibrar and Muhammad, 2011). Efforts are being made in the field of phytotoxicity to search for weedicides/herbicides from natural sources.
Materials and Methods
Extract preparation
The collected material of C. adansonii bark and leaf parts was cleaned thoroughly from dust by gently washing with water. The material was shade dried and pulverized in an electric grinder to fine powder of 60 mesh diameter. 200 gram of each sample was soaked in 1 litre of 70% ethanol for 72 h. The mixture was vigorously shaken several times each day. It was filtered through Whatman filter paper No.1823, and the same procedure was repeated three times. Each filtrate was concentrated under a vacuum by using a rotary evaporator. Each extract’s concentrates were stored at 4 °C for future use (Hussain et al., 2010).
Elemental analysis
Elemental analysis was carried out by following the recommended methods (Sucman et al., 2008; AOAC, 2000). Dry powder of C. adansonii leaf and bark were subjected to elemental analysis through atomic absorption spectrophotometry for the detection of macro and microelements including Manganese (Mn), Zinc (Zn), Cobalt (Co), Lead (Pb), Calcium (Ca), Copper (Cu), Iron (Fe), Cadmium(Cd), Sodium (Na) and Nickel (Ni).
Wet digestion
The wet digestion method was used to prepare the samples. Powder drug (1 gm) and 67% concentrated HNO3 (10 ml) were mixed in a conical flask and kept at room temperature up to 24 hours. 67% HClO4 (4 ml) was added to the mixture, and after 30 minutes flask was heated on a hotplate until only 1 ml clear solution was left (Hseu, 2004).
The evaporated solution was cooled down and diluted with double distilled water until its volume was raised to 100 ml. After filtration through a Whatman # 42 filter paper, this stock solution was analyzed with the help of atomic absorption spectrophotometer (Eslami et al., 2007). The stock solution was diluted to prepare each metal’s calibration standard (Saeed et al., 2010).
Procedure
Each element condition of the instrument was adjusted according to the conditions mentioned in Table 1. Acetylene flame was ignited after warming the cathode lamp. The stock solution of different elements was diluted for calibration of the instrument, and standardization was verified, for each element ppm was calculated (Tuzen, 2003) through atomic absorption spectrometry. The samples were analyzed for quantitative detection of various elements (AOAC, 2000).
Statistical analysis
The elemental data were analyzed statistically using arithmetic mean and standard deviation (Saeed et al., 2010).
Nutritional analysis
Plants contain nutrients and other necessary substances for our growth and development, including fats, carbohydrates, and proteins (Aruoma, 2003). The methodology of Hussain et al. (2011) was followed to determine a few essential nutritional aspects of the proposed plant.
Determination of ash
Ash analysis of C. adansonii powder drugs and ethanolic extracts of leaf and bark parts were conducted during the present research. Ash is the inorganic substance which is obtained through the burning of organic matter. During ignition, some of the compounds are lost due to their volatile nature or chemical interaction; therefore, ash is quite different in composition, as compared to the plant material (Jarald and Jarald, 2007). Exhausted drugs can be detected through an ash test. Similarly, the detection of adulteration in the drug can also be achieved through ash determination (Wallis, 1985).
Procedure
A thoroughly washed flat bottom silica crucible was dried in an oven (70 °C) for 30 minutes; it was ignited and tarred. After cooling it in desiccators and weighing (W1), plant powder drug (4 gm) was spread evenly into it and heated with a Bunsen burner. It was then shifted to an ignited muffle furnace. The temperature was gradually increased to 550 °C; the process was continued until the carbon content was burnt out, and a white appearance was observed. It was transferred to desiccators and weighed (W2) after cooling. Percent of ash and the total ash values were calculated (Wallis, 1985).
Weight of the empty crucible = W1
Weight of the empty crucible + ash = W2
Total ash (mg/g) = W2 – W1
% Ash = W2-W1/ Wt of sample ×100
Determination of moisture
A Petri dish was weighed (W1), and 2 gm plant powder drug was put into it. The Petri dish was covered and placed inside the oven (105 °C) for 4-6 hours, cooled in desiccators, and weighed again (W2). Percent moisture content was calculated using the formula (AOAC, 2000).
% Moisture= X / Wt of sample ×100
X= Weight of the sample (after heating) = W2 - W1
W2= Weight of the empty Petri dish+sample (after heating)
W1 = Weight of the empty Petri dish
Table 1: Conditions applied for detection of various elements.
|
Elements |
Flame type |
Slit width (nm) |
Wavelengthh (nm) |
Cathode lamp current (mA) |
Air oxide flow (L/min) |
Acetylene flow (L/min) |
|
Pb |
Air/ acetylene |
0.7H |
283.3 |
10 |
17 |
2.0 |
|
Zn |
Air/ acetylene |
0.7H |
213.9 |
15 |
17 |
2.0 |
|
Mn |
Air/ acetylene |
0.2H |
279.5 |
20 |
17 |
2.0 |
|
Co |
Air/ acetylene |
0.2H |
240.7 |
30 |
17 |
2.0 |
|
Cd |
Air/ acetylene |
0.7H |
357.9 |
25 |
17 |
2.5 |
|
Cu |
Air/ acetylene |
0.7H |
324.8 |
15 |
17 |
2.0 |
|
Fe |
Air/ acetylene |
0.2H |
248.3 |
30 |
17 |
2.3 |
|
Ca |
Air/ acetylene |
0.7H |
766.5 |
12 |
17 |
2.0 |
|
Na |
Air/ acetylene |
0.2H |
589 |
0.8 |
17 |
2.0 |
|
Ni |
Air/ acetylene |
0.2H |
232 |
25 |
17 |
2.0 |
Determination of proteins
Macro Kjeldahl method was used for protein determination. A digestion mixture was prepared by mixing ferrous sulfate, potassium sulfate, and copper sulfate in the ratio of 1, 94, and 5, respectively. 25 ml of concentrated H2SO4 was mixed into it and digested for 6 hours. After cooling, the material was transferred to a 250 ml flask. Distilled water was added to increase its volume up to 50 ml, and a strong alkali (10 ml) was added.
About 50 ml of 4% boric acid solution was transferred to the distillation flask, and 3-5 drops of the mixed indicator (prepared by dissolving 0.03 g of bromocresol green and 0.016 g of methyl red into 100 ml of alcohol) were also added. Water (50 ml) and 32% NaOH solution (60 ml) were then added. After distillation, titration was done by taking HCl (0.1 N) in a burette. The percentage of proteins was calculated (AOAC, 2000).
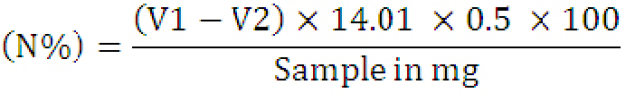
V1= Titration reading of the sample; V2= Titration reading of blank; 14.01= Atomic weight of nitrogen (N).
Crude percent protein contents were calculated for all the samples by multiplying the nitrogen content of the sample by 6.25.
Protein (%) = % Nitrogen × 6.25
Determination of fats
The extraction of crude fats was done with a Soxhlet apparatus (Zarnowski and Suzuki, 2004). In cellulose extraction thimble made of filter paper, two gms of plant powder drug was packed and placed inside the extraction chamber. A weighed 250 ml round bottom flask was filled with petroleum ether and connected to the extraction tube containing thimble. The apparatus was kept for 5-6 hours. A water bath was used, and the extract in a round bottom flask was evaporated and weighed (W2). Fats percentage was calculated using the following equation (AOAC, 2000).
% fats (ether extract)= X/ Wt of the sample ×100
X = Weight of the fats = W2 - W1
W1 =Weight of the empty flask
W2 = Weight of empty flask + Sample after evaporation
Determination of fiber
Procedure: The powder drug (3 g) was heated and oven-dried until a constant weight was attained. 2 g of this material was extracted with petroleum ether to remove crude fats. A digestion flask asbestos (0.5 g) was mixed with the residue material, and 200 ml boiling H2SO4 (0.255 N) was also added to it. Flask was boiled for 30 minutes. I was filtered through a linen cloth; acids were removed by washing the residue with distilled water. It was then transferred to the digestion flask, and boiling NaOH (0.313 N) was added until its volume increased exactly up to 200 ml; after boiling for 30 minutes, it was filtered, initially washed with boiling water, followed by ethyl alcohol (15 ml). The contents were put into a crucible and dried at 110 °C in a hot air oven till constant weight was achieved (W1). The crucible was then transferred to the muffle furnace, ignited till the color turned white, and weighed (W2). The weight of crude fibers was calculated (AOAC, 2000).
% Crude fibers= W2-W1/ Wt of sample ×100
W2 – W1 = Crude fiber
Determination of carbohydrates
To calculate the sample’s carbohydrate contents, the sum of ash, proteins, moisture content, fats, and crude fibers percentage was subtracted from 100 (Merrill and Watt, 1973; Muller and Tobin, 1980).
Carbohydrates (%) = 100– [Moisture content (%) + Total Ash (%) + Crude fiber (%) + Fats (%) + Protein (%)]
Phytotoxic Activity
Lemna minor model was used to determine the phytotoxic potential of Crataeva adansonii D.C. leaf and bark (Atta-ur-Rhman et al., 2001). Nutrients like potassium dihydrogen phosphate, potassium nitrate, calcium nitrate, magnesium sulfate, boric acid, manganous chloride are used in specific amounts to prepare E-medium for this assay. Required amounts of chemicals are weighed and dissolved in distilled water until the total volume is made up to 1000 ml. (Table 2). K.O.H. is added to adjust the pH between the ranges of 5.5 - 6.
During this assay, a solution of 15 mg of plant extract and 1.5 ml respective solvent (ethanol) was prepared. 5, 50 and 500 ml portions from this solution are transferred to flasks (3 replicates), solvents were evaporated under laminar flow. 20 ml of E. medium is poured into each flask. In this way, three replicates were prepared to have 10, 100, and 1000 µg/mL concentrations. E. medium and standard drug (Atrazine) were used as a negative and positive control, respectively. To each flask, ten plants were transferred, and they were kept in 12 h day length conditions. For this purpose, plants having 2-3 fronds were selected. The number of fronds was counted again, after six days. Through the following formula, percent growth inhibition is recorded:
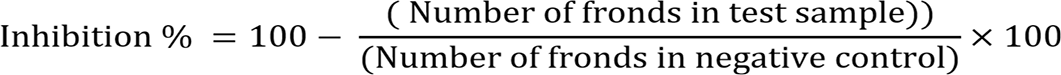
Table 2: Composition of E-medium.
|
S. No |
Chemical name |
g/L |
|
1. |
Potassium dihydrogen phosphate (KH2PO4) |
0.68 |
|
2. |
Potassium nitrate(KNO3) |
1.515 |
|
3. |
Calcium nitrate (Ca(NO2)2.4H2O) |
1.180 |
|
4. |
Magnesium sulfate (MgSO4.7H2O) |
0.492 |
|
5. |
Boric acid (H3BO3) |
0.00286 |
|
6. |
Manganous chloride (MnCl2.4H2O) |
0.00362 |
|
7. |
Ferric chloride (FeCl2.4H2O) |
0.00540 |
|
8. |
Zinc sulfate (ZnSO4.5H2O) |
0.00022 |
|
9. |
Copper sulfate (CuSO4.5H2O) |
0.00022 |
|
10. |
Sodium molybdate (Na2MO4.2H2O) |
0.00012 |
|
11. |
Ethylene diamino tetra acetic acid (EDTA) |
0.01120 |
The plant has been collected, and under different growth conditions (Yang and Jiang, 2009).
Results and Discussion
Elemental analysis
Powdered plant material was used to conduct atomic absorption spectroscopy, and the various trace and heavy metals were detected. It is reported that the plant species dissent markedly in the composition of elements depending upon species, the land from where the plant has been collected, and under different growth conditions (Yang et al., 2009).
Different elemental constituents in plants have a tremendous role in developing medicinal products despite their existence at trace levels (Zafar et al., 2010). C. adansonii leaf and bark’s elemental composition was determined using Atomic Absorption Spectrophotometer (A.A.S.). A total of 10 elements Na, Ca, Co, Cd, Cu, Mn, Zn, Pb, Ni, and Fe have been determined. Various elements were found in the leaf and bark in specific concentrations. The presence of various minerals like Ca, Mg, P, Na, and K was revealed. In the bark part, the highest percentage of relative standard deviation was observed for Co, Cd and Na that is 175.6, 22.86 and 5.58%, respectively. Elemental analysis of the leaf part revealed the highest percentage of R.S.D. for Co, Mn and Pb that is 20, 9 and 4.44%, respectively. The usefulness of these components in the body is considerable, which adds to the results (Hussain et al., 2011). The elemental analysis showed considerable variation amongst different elements (Tables 3 and 4). The medicinal plant species showed considerable variation amongst different parts during elemental analysis (Loutit et al., 1988).
Table 3: Elemental composition of leaf extract of C. adansonii.
|
Sample |
Analyte |
Mean |
SD |
% RSD |
|
Crataeva adansonii Leaf extract |
Cd 228 |
0.033 mg/L |
0.0014 |
4.13 |
|
Pb 283.3 |
0.462 mg/L |
0.0205 |
4.44 |
|
|
Zn 213.9 |
0.173 mg/L |
0.0012 |
0.69 |
|
|
Fe 248.3 |
1.558 mg/L |
0.0100 |
0.64 |
|
|
Cu 324.8 |
0.094 mg/L |
0.0028 |
2.96 |
|
|
Ca 422.7 |
45.21 mg/L |
0.476 |
1.05 |
|
|
Mn 279.5 |
0.170 mg/L |
0.0158 |
9.25 |
|
|
Na 589.0 |
2.025 mg/L |
0.0130 |
0.64 |
|
|
Co 240.7 |
0.007 mg/L |
0.0014 |
20.51 |
|
|
Ni 232.0 |
0.180 mg/L |
0.0028 |
1.56 |
SD: Standard deviation; RSD: Relative standard deviation; Cd: cadmium; Pb: lead; Zn: Zinc; Fe: Iron; Cu: Copper; Ca: Calcium; Mn: Manganese; Na: Sodium; Co: Cobalt; Ni: Nickel.
Table 4: Elemental composition of bark extract of C. adansonii.
|
Sample |
Analyte |
Mean |
SD |
% RSD |
|
Crataeva adansonii Bark extract |
Cd 228 |
0.038 mg/L |
0.0087 |
22.86 |
|
Pb 283.3 |
1.796 mg/L |
0.0121 |
0.67 |
|
|
Zn 213.9 |
0.228 mg/L |
0.0028 |
1.23 |
|
|
Fe 248.3 |
2.631 mg/L |
.0167 |
0.63 |
|
|
Cu 324.8 |
0.106 mg/L |
0.0011 |
1.03 |
|
|
Ca 422.7 |
48.24 mg/L |
0.535 |
1.11 |
|
|
Mn 279.5 |
0.172 mg/L |
0.0058 |
3.39 |
|
|
Na 589.0 |
3.081 mg/L |
0.0176 |
0.57 |
|
|
Co 240.7 |
0.007 mg/L |
0.0130 |
175.6 |
|
|
Ni 232.0 |
0.183 mg/L |
0.0102 |
5.58 |
SD: Standard deviation; RSD: Relative standard deviation; Cd: cadmium; Pb: lead; Zn: Zinc; Fe: Iron; Cu: Copper; Ca: Calcium; Mn: Manganese; Na: Sodium; Co: Cobalt; Ni: Nickel.
In this regard, all the plant’s studied parts showed decreased Cr levels compared to the recommended toxic levels (Broyer et al., 1972). Plants can accumulate heavy metals that are toxic and injurious for health (Foy et al., 1978). However, Pb’s concentration in this plant is far lower than the suggested concentrations (2 to 6 mg/l), which clarify its use as a medicinal plant. Atomic absorption spectrophotometry was carried out for the elemental analysis of powder drug and detecting the various trace and heavy elements under specific conditions (Tables 3 and 4). In humans, trace elements acquire an essential role against diseases (Lobo et al., 2010).
Table 5: A nutritional value analysis of C. adansonii leaf and stem bark.
|
Sample |
Carbohydrates % |
Proteins % |
Fats % |
Fiber % |
Ash % |
Moisture % |
|
CLE |
72.09 |
3.12 |
7 |
0.39 |
10 |
7.4 |
|
CBE |
72.82 |
3.12 |
6 |
0.46 |
10 |
7.6 |
CLE: C. adansonii leaf extract; CBE: C. adansonii bark extract.
Table 6: Phytotoxic activity of C. adansonii leaf and bark.
|
Treatment |
Conc.(µl ) |
Total no of Fronds |
No of fronds in test |
No of frond in –ve control (E medium) |
% inhibition inhibition |
|
CLE |
10 |
30 |
28 |
30 |
6.7 |
|
100 |
30 |
25 |
16.67 |
||
|
1000 |
30 |
21 |
30 |
||
|
CBE |
10 |
30 |
24 |
30 |
20 |
|
100 |
30 |
21 |
30 |
||
|
1000 |
30 |
18 |
40 |
CLE: C. adansonii leaf extract; CBE: C. adansonii bark extract.
Nutritional analysis
Plants are one of the primary sources of nutritional components and serve as the fundamental source of proteins, carbohydrates and fats required for humans and other organisms (Nesamvuni et al., 2001). Dry powder of C. adansonii bark and leaf were tested to confirm the presence of carbohydrates, proteins, fats, fibers, ash, and moisture content (Table 5). Both leaf and bark parts were rich in carbohydrates (72.09 and 72.82%, respectively) and they were having an equal amount of proteins (3.12%).
There is mounting awareness about wild plants’ prospective benefits (Nesamvuni et al., 2001). Many of these plants offer people their basic needs in terms of food, shelter, medicine, and as a basis of income (Allemann et al., 1995). A nutritional value analysis of this plant offers its use as a food source.
Phytotoxic study
The phytotoxicity of a given plant inhibits the growth of weeds. However, it does not impart any harmful effects on crop growth, resulting in an overall improvement of the production (Batish et al., 2007). The problems associated with species showing resistance to various chemical herbicides have escalated with the introduction of genetically modified crops (Duke, 2018). Several researchers have proven that weed diversity, species evenness and richness can be reduced by long-term herbicides strategies (Zhang et al., 2019; Mncube and Mloza Banda, 2018). Some other genera of family Capparaceae have been studied to evaluate their phytotoxic potential viz; Ladhari et al. (2013) worked on phytotoxicity of Capparis spinosa L. The study revealed that C. spinosa leaves were potent phytotoxic. Further, three flavonoids were isolated through bioassay-guided fractionation of leaf extracts, including kaempferol-3-O-β-D-glucopyranoside, quercetin and Quercetin-3-O-β-Dglucopyranoside with potent phytotoxic activity. Phytotoxic assay of C. adansonii leaf and bark extract using Lemna minor model showed that 40 % of inhibition was observed at C.B.E. concentration of 1000 µl. Similarly, C.L.E. manifested 30 % inhibition when used at a concentration of 1000 µl. Overall a dose-dependent inhibition of the frond growth (Table 6) was shown in terms of percent inhibition.
Conclusions and Recommendations
Nutritional analysis of the plant reported the occurrence of proteins, carbohydrates, and other nutritional constituents. Elemental analysis showed the presence of several macro and microelements as Cd, Mg, Ca etc in the bark and leaf parts. In-vitro pharmacological screening of the plant drug showed potential phytotoxic activity, which indicates that it can be used as a herbicides source. Isolation of bioactive compounds and their identification is needed in order to proceed with targeted studies. Nutritional and trace micro and macro elements analysis will be helpful during future research work on the plant. The current study is limited to the leaf and stem bark portion of C. adonsonii. Further studies on the other parts of the plants are required to explore any potential constituents’ existence.
Novelty Statement
Syeda Farzana Bibi: Conducted research, experiments, data compilation, analysis and wrote this manuscript.
Siraj-ud-Din: Guided whole research study. Helped in data compilation and analysis.
Author’s Contribution
This paper is a part of the major author’s contribution to the requirement of a Ph.D. Syeda Farzana conducted the experimental work, and Prof. Dr. Siraj-ud-Din guided her as a research supervisor during the complete process of research.
Conflict of interest
The authors have declared no conflict of interest.
References
AOAC. 2000. Official methods of analysis of AOAC. International 17th edition; Gaithersburg, MD, USA Association of Analytical Communities.
Agte, V., K. Tarwadi, S. Mengale and S. Chiplonkar. 2000. Potential of traditionally cooked green leafy vegetables as natural sources for supplementation of eight micronutrients in vegetarian diets. J. Food Comp. Anal., 13(6): 885-891. https://doi.org/10.1006/jfca.2000.0942
Allemann, J., S. Venter and E. Van den Heever. 1995. Indigenous vegetable research. Roodeplaat Bull., 45: 14-15.
Aruoma, O.I., 2003. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Fundam. Mol. Mechanis. Mutagen., 523: 9-20. https://doi.org/10.1016/S0027-5107(02)00317-2
Atta-ur-Rhman Choudhary, M. and W. Thomsen. 2001. Bioassay technique for drug development harwood academic publishers. https://doi.org/10.3109/9780203304532
Bamiro, F., K. Esuoso and O. Tairu. 1995. Comparative elemental contents (Cu, Ca, Zn, K, Mg, Ni, Fe and Cd) of seven various edible tubers in Nigeria. Pak. J. Sci. Ind. Res., 38: 316-318.
Batish, D.R., M. Kaur, H.P. Singh and R.K. Kohli. 2007. Phytotoxicity of a medicinal plant, Anisomeles indica, against Phalaris minor and its potential use as natural herbicide in wheat fields. Crop Prot., 26(7): 948-952. https://doi.org/10.1016/j.cropro.2006.08.015
Broyer, T., C. Johnson and R. Paul. 1972. Some aspects of lead in plant nutrition. Plant Soil, 36(1): 301-313. https://doi.org/10.1007/BF01373485
Burkill, H.M., 1985. The useful plants of West Tropical Africa. Vol. 1. Families AD: Royal Botanic Gardens.
Cheng, F. and Z. Cheng. 2015. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci., 6: 1020. https://doi.org/10.3389/fpls.2015.01020
Cordeau, S., M. Triolet, S. Wayman, C. Steinberg and J.P. Guillemin. 2016. Bioherbicides: dead in the water? A review of the existing products for integrated weed management. Crop Prot. 87: 44-49. https://doi.org/10.1016/j.cropro.2016.04.016
Dudai, N., A. Poljakoff-Mayber, A. Mayer, E. Putievsky and H. Lerner. 1999. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol., 25(5): 1079-1089. https://doi.org/10.1023/A:1020881825669
Duke, S.O., 2018. Glyphosate: The world’s most successful herbicide under intense scientific scrutiny. Pest Manage. Sci., 74: 1025-1026. https://doi.org/10.1002/ps.4902
Duke, S., F. Dayan, J. Romagni and A. Rimando. 2000. Natural products as sources of herbicides: Current status and future trends. Weed Res., 40(1): 99-111. https://doi.org/10.1046/j.1365-3180.2000.00161.x
Eslami, A., G.J. Khaniki, M. Nurani, M. Mehrasbi, M. Peyda and R. Azimi. 2007. Heavy metals in edible green vegetables grown along the sites of the Zanjanrood river in Zanjan, Iran. J. Biol. Sci., 7(6): 943-948. https://doi.org/10.3923/jbs.2007.943.948
Foy, C., R.T. Chaney and M. White. 1978. The physiology of metal toxicity in plants. Ann. Rev. Plant Physiol., 29(1): 511-566. https://doi.org/10.1146/annurev.pp.29.060178.002455
Gitte, T., M. Kare and A. Deshmukh. 2012. Ethno-medicinal studies on barks of some medicinal plants in Marathwada (MS), India. Recent Res. Sci. Technol., 4(10): 08-10.
Heap, I., 2018. The international survey of herbicide resistant weeds. http://www.weedscience.org.
Hseu, Z.Y., 2004. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol., 95(1): 53-59. https://doi.org/10.1016/j.biortech.2004.02.008
Hussain, F., I. Hameed, G. Dastagir, I. Khan and B. Ahmad. 2010. Cytotoxicity and phytotoxicity of some selected medicinal plants of the family Polygonaceae. Afr. J. Biotechnol., 9(5).
Hussain, J., F.U. Khan, R. Ullah, Z. Muhammad, N. Rehman, Z.K. Shinwari and S.M. Hussain. 2011. Nutrient evaluation and elemental analysis of four selected medicinal plants of Khyber Pakhtoonkhwa, Pakistan. Pak. J. Bot., 43(1): 427-434.
Ibrar, M. and N. Muhammad. 2011. Evaluation of Zanthoxylum armatum DC. for in-vitro and in-vivo pharmacological screening. Afr. J. Pharm. Pharmacol., 5(14): 1718-1723. https://doi.org/10.5897/AJPP11.405
Jarald, E.E. and S.E. Jarald. 2007. Textbook of pharmacognosy and phytochemistry. CBS Publisher and distributers, New Delhi, India. pp. 6.
Kaneez, F., M. Qadiruddin, M. Kalhoro, S. Khaula and Y. Badar. 2001. Determination of major and trace elements in Artemisia elegantissima and Rhazya stricta and their relative medicinal uses. Pak. J. Sci. Ind. Res., 44(5): 291-293.
Khan, H., M. Saeed, M.A. Khan, A. Dar and I. Khan. 2010. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J. Ethnopharmacol., 127(2): 521-527. https://doi.org/10.1016/j.jep.2009.10.003
Ladhari, A., F. Omezzine, M. Dellagreca, A. Zarrelli and R. Haouala. 2013. Phytotoxic activity of Capparis spinosa L. and its discovered active compounds. Allelopathy J., 32(2): 175-190
Lobo, V., A. Patil, A. Phatak and N. Chandra. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev., 4(8): 118. https://doi.org/10.4103/0973-7847.70902
Loutit, M., P. Bremer and J. Aislabie. 1988. The significance of the interactions of chromium and bacteria in aquatic habitats. Chromium Natl. Hum. Environ., 20: 317-334.
Merrill, A. and B. Watt. 1973. Energy Value of Foods: Basis and derivation (Agriculture Handbook No. 74). Washington: US government printing office.
Miliauskas, G., P.R. Venskutonis and T.A. VanBeek. 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem., 85: 231-237. https://doi.org/10.1016/j.foodchem.2003.05.007
Mncube, T.L., H.R. Mloza Banda. 2018. Evaluation of chemical and non-chemical weed control practices on weed communities and maize yield in two agroecological zones of Swaziland. Afr. J. Agric. Res., 13: 1708-1718. https://doi.org/10.5897/AJAR2018.13311
Morimoto, M., C.L. Cantrell, L. Libous-Bailey and S.O. Duke. 2009. Phytotoxicity of constituents of glandular trichomes and the leaf surface of camphorweed, Heterotheca subaxillaris. Phytochemistry, 70(1): 69-74. https://doi.org/10.1016/j.phytochem.2008.09.026
Muller, H.G. and G. Tobin., 1980. Nutrition and food processing. Dept. of Food Sci., Univ. of Leeds, Leeds, UK. Publisher Croom Helm Ltd, London, UK. pp. 302
Naresh, S. 2009. Crataeva adansonii (Sacred barna). [Photograph]. Retrieved from https://flickriver.com/photos/swami_naresh/4573746429/
Nesamvuni, C., N. Steyn and M. Potgieter. 2001. Nutritional value of wild, leafy plants consumed by the Vhavenda. South Afr. J. Sci., 97(1): 51-54.
Patil, S.G., A.S. Wagh, R.C. Pawara and S.M. Ambore. 2013. Standard tools for evaluation of herbal drugs: an overview. Pharma Innov., 2(9): 60-65.
Ryan, K.J. and C.G. Ray. 2004. Medical microbiology. McGraw Hill, 4: 370.
Saeed, M., N. Muhammad, H. Khan and S.A. Khan. 2010. Analysis of toxic heavy metals in branded Pakistani herbal products. J. Chem. Soc. Pak. 32(4): 471. https://doi.org/10.4314/tjpr.v10i4.16
Schmidt, M., 2008. Crataeva adansonii DC. [Photograph]. Retrieved from http://www.africanplants.senckenberg.de/root/index.php?page_id=78andid=427#image=4545
Sucman, E., M. Mahrova, J. Pac, M. Vavrova and P. Kettisch. 2008. Microwave assisted digestion method for the determination of cadmium, copper, lead, and zinc in biological materials. Electroanalysis: Int. J. Devoted Fundam. Pract. Aspects of Electroanalysis. 20(4): 386-389. https://doi.org/10.1002/elan.200604049
Teixeira, M.G., E.S. Alvarenga, D.T. Lopes and D.F. Oliveira. 2019. Herbicidal activity of isobenzofuranones and in silico identification of their enzyme target. Pest Manage. Sci., 75(12): 3331-3339. https://doi.org/10.1002/ps.5456
Tuzen, M., 2003. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem., 80(1): 119-123. https://doi.org/10.1016/S0308-8146(02)00264-9
Underwood, E., 1977. Copper. In: Trace elements in human and animal nutrition 4th edition, Academic Press. New York. 57-100. https://doi.org/10.1016/B978-0-12-709065-8.50007-9
Valke, D., 2011. Crataeva adansonii. [Photograph]. Retrieved from https://www.flickr.com/photos/91314344@N00/5656764673
Wallis, T., 1985. Textbook of pharmacognosy. 1957. J. and A. Churchill, London.
Westwood, J.H., R. Charudattan, S.O. Duke, S.A. Fennimore, P. Marrone and D.C. Slaughter. 2018. Weed Management in 2050: Perspectives on the future of weed science. Weed Sci. 66: 275-285. https://doi.org/10.1017/wsc.2017.78
Yang, Y., F. Zhang, H. Li and R. Jiang. 2009. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Mgt., 90(2): 1117-1122. https://doi.org/10.1016/j.jenvman.2008.05.004
Zafar, M., M.A. Khan, M. Ahmad, G. Jan, S. Sultana, K. Ullah and A. Nazir. 2010. Elemental analysis of some medicinal plants used in traditional medicine by atomic absorption spectrophotometer (AAS). J. Med. Plants Res., 4(19): 1987-1990. https://doi.org/10.5897/JMPR10.081
Zarnowski, R. and Y. Suzuki. 2004. Expedient Soxhlet extraction of resorcinolic lipids from wheat grains. J. Food Compos. Anal., 17(5): 649-663. https://doi.org/10.1016/j.jfca.2003.09.007
Zhang, Y., X. Yang, Y. Zhu, L. Li, Y. Zhang, J. Li and S. Qiang. 2019. Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol. Contr., 139: 104093. https://doi.org/10.1016/j.biocontrol.2019.104093
To share on other social networks, click on any share button. What are these?








