Toxicity of Imidacloprid Administered Alone and in Combination with Heavy Metal Lead on Silkworm, Bombyx mori (Lepidoptera: Bombycidae)
Toxicity of Imidacloprid Administered Alone and in Combination with Heavy Metal Lead on Silkworm, Bombyx mori (Lepidoptera: Bombycidae)
Sehrish Akram1, Abida Butt1* and Shakil Ahmad Khan2
1Department of Zoology, University of the Punjab, Lahore, Pakistan.
2Sericulture Research Lab, 108 Ravi Road, Lahore, Pakistan
ABSTRACT
In this study, we evaluated the single and joint toxicities of insecticide imidacloprid (commercial formulation and technical grade) and heavy metal lead (Pb) against 2nd, 3rd, 4th and 5th instars of silkworm. For treatment, leaf-dipping method was used and toxicity calculated after 48h exposure to the treatment. Both chemicals showed high toxicity against all instars of silkworm. However, results indicated that imidacloprid was more toxic for silkworm larvae compared to lead. The acute toxicity of imidacloprid formulation (IMF), imidacloprid technical grade (IMT) and Pb showed positive correlation with the concentration and negative with the age of larvae. From second to fifth instars, LC50 values of IMF ranges from 0.72 to 2.96, IMT from 0.86 to 2.75 and Pb from 1.93 to 6.88 mg/L. The toxicity of binary mixture of IMF + Pb showed synergism against all instars of silkworm. While, binary mixture of IMT + Pb displayed synergism, additive, and antagonism and antagonism activity in silkworm larvae in silkworm larvae. The results of our study provide useful data for the assessment of ecological risk of the tested compounds (single or mixture) against different silkworm instars.
Article Information
Received 23 June 2021
Revised 28 September 2021
Accepted 19 October 2021
Available online 11 February 2022
(early access)
Published 24 October 2022
Authors’ Contribution
AB conceived the idea and designed the experiment. SAK provided experimental basis for research. SA and SAK performed experiments. SA and AB analysed the data and wrote the article. AB revised the manuscript.
Key words
Insecticides, Heavy metal, Combined toxicity, Silkworm larvae, Instars
DOI: https://dx.doi.org/10.17582/journal.pjz/20210623070602
* Corresponding author: abidajawed.zool@pu.edu.pk
0030-9923/2023/0001-165 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Silkworm Bombyx mori L. (Lepidoptera: Bombycidae) is an economically important insect that was domesticated for more than 5000 years ago for silk production. It utilizes less valuable resources (mulberry leaves) in most effective manner to produce high quality silk. It’s silk and other products are used in textile, medical, cosmetic and food industries (Lee et al., 2011; Longvah et al., 2011; Zhang et al., 2011; Dutta et al., 2012; Gupta et al., 2015). It is a source of income generation in many areas of the world and play crucial role to increase the employment of the people.
Although silkworm is domesticated and reared in isolated insectaries but still exposed to different xenobiotics through food contamination. The major food of B. mori is mulberry (Morus alba) leaves, that is attacked by many insect pests like mites, grasshoppers, mealy bug, beetles, cutworms etc. (Singh and Saratchandara, 2002).To control these pests, different types of insecticides are sprayed on mulberry plants. Some other pesticides used in surrounding area also reach to mulberry plants through spray drift, runoff and leaching. Imidacloprid is widely used insecticide throughout the world to control chewing and sucking insect pests. It is a broad spectrum neonicotinoid insecticide with contact and systemic activity. In crop fields, it is used as foliar spray or seed treatment. Plants absorb it from the soil or leaves and translocate it to their different parts. In insects, imidacloprid bind with nicotinic acetylcholine receptors of central nervous system that cause paralysis and ultimately its death (Matsuda et al., 2001, 2005). Liu et al. (2018) reported low toxicity of imidacloprid against silkworm larvae as compared to pyrethroids. Yu et al. (2016) studied acute toxicity of different insecticides against silkworm larvae and reported high toxicity of imidacloprid based on LC50 values after 96 h exposure.
In few decades, heavy metals pollution in the environment has increased many folds due to industrialization and agriculture practices. Lead (Pb) is non-essential heavy metal. Its concentration in the air and soil is increasing due to deposition of pollutants from automobile, smelters, mining and paint industry (Zia et al., 2011). Long term persistence and accumulation of Pb in environment produce high risk for organisms that exposed to Pb directly or indirectly (Peterson et al., 2017). Mulberry plants absorb different heavy metals including Pb from the soil and accumulate them in their different parts (Zhou et al., 2015; Jiang et al., 2020). Silk worm larvae exposed to Pb through the consumption of contaminated mulberry leaves. The large quantity of consumed Pb passed through the gut of silkworm larvae and excreted in feces. While, some quantity is absorbed by the gut and later excreted from the body through spinning or pupal case (Jiang et al., 2012). Different detoxification processes also help to reduce the effects of Pb accumulation in silkworm larvae (Zhou et al., 2015).
Many studies have reported toxicity of many pesticides administered alone and in combination with heavy metals against silkworm larvae (Zhang et al., 2008; Zhou et al., 2015; Yu et al., 2016; Meng et al., 2017; Liu et al., 2018). However, effect of insecticide and heavy metal mixture on the survival, biology and physiology on silkworm larvae is not reported according to our knowledge. The objective of this study was to assess single and joint toxicity of imidacloprid and Pb against different instars of silkworm. The results of this study will provide useful guidelines to assess the harmful effects of xenobiotics and their mixtures against silkworms and other nontarget organisms.
MATERIALS AND METHODS
Insects
The eggs of silkworm (Bulgarian hybrid H1*KK*G2*V2) were obtained from Sericulture Department, 108 Ravi Road, Lahore, Pakistan. These eggs were reared in the laboratory at 25±2 °C, 70±5% relative humidity and photoperiod of 12:12 h (L: D). Fresh mulberry leaves were harvested from untreated mulberry plants present in the University of the Punjab, Lahore, Pakistan and fed to the silkworm larvae ad libitum.
Chemicals
In present study, both technical grade and commercial formulations of the imidacloprid were used. Commercial formulation of imidacloprid (Confidor 200 SC) was purchased from local office of Bayer. Technical grade imidacloprid (CAS No. 138261-41-3) and lead chloride (PbCl2, CAS No. 7758-95-4) were purchased from Sigma Aldrich, USA. Stock solution of Imidacloprid formulation (IMF) and imidacloprid technical grade (IMT) was prepared at field application rate in acetone and lead chloride at rate of 20 mg/L in distilled water. All stock solutions were stored at 4 °C till further used. Each stock solution was diluted with distilled water to the required concentrations of the bioassays.
The binary mixtures of imidacloprid commercial formulation + lead chloride (IMF+PbCl2), and imidacloprid technical grade + lead chloride (IMT+PbCl2) were prepared by mixing the two solutions at their LC50: LC50 ratio.
Bioassay method
To assess the toxicity of above mentioned chemicals, 2nd, 3rd, 4th, and 5th instars of silkworm were exposed to a series of IMF, IMT and Pb concentrations and to their binary combinations at LC50: LC50 ratio. Before experiment, preliminary bioassays were performed to determine the concentrations of each chemical that cause 0-100% mortality in silkworm larvae after 48h of exposure. On the basis of this study, six concentrations were designed for bioassays to calculate LC50 values for each chemical.
For assays, leaf dip method described by Yu et al. (2011) was used. Fresh and healthy mulberry leaves were plucked from mulberry trees present in the vicinity of University of the Punjab, Lahore, Pakistan and washed with water and air dried. These clean mulberry leaves were immersed in required concentration of each chemical for 30s, drained and spread on filter paper to dry under fan. The treated mulberry leaves were placed in wooden rearing trays (dimension 25cm x 15cm). Thirty same size and same age silkworm larvae were placed in each tray and fed with treated mulberry leaves for 48h. Assay for each concentration of a chemical was replicated four times. Mortality of the silkworm larvae was recorded after 24 and 48h of exposure. Control group silkworms were fed with water treated mulberry leaves that contain acetone at a concentration present in the highest dose of insecticide used in bioassay. The bioassay conditions were same as described above for the rearing of silkworm.
Data analysis
The silkworm larvae that did not show any movement on touching with camel hair brush were considered dead. The mortality of silkworm larvae after 48 h of exposure to treatments was used to calculate LC50 and median effect using Compusyn software (Chou and Martin, 2005). The following formula was used:
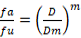
Where fa is the fraction of the total organism affected by the dose D; fu is the fraction of the total organism remains unaffected by the dose D; Dm is concentration of chemicals at which 50% mortality occurs (LC50); m is coefficient that define the shape of dose-mortality curve. The value of m may be < 1, = 1, > 1 that represent flat sigmoidal, hyperbolic, and sigmoidal dose-mortality curves, respectively.
To identify and quantify the interaction between insecticide and heavy metal, data was analyzed using formula of combination index (Chou, 2006). The combination index (CI) values for binary mixtures were also calculated using compusyn software. The CI value may be >1, <1 or =1 that indicate antagonism, synergism and additive effect of binary mixture, respectively. The formula used to calculate n-chemicals combinations for x% mortality is given below:
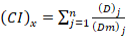
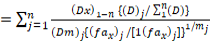
Where (Dx)1-n is the sum of the concentrations of n chemicals that cause x% mortality due to mixture, {(D)j
/ Σn1 (D)} is the fraction of individual concentration of n chemicals that cause x% mortality and {(Dm)j {(fax)j / [1(fax)j ]}1/mj is the concentration of single chemical cause x% mortality.
General linear model was used to compare the acute toxicity of IMF, IMT and Pb individually and their mixtures. ANOVA followed by Tukey’s test was used to compare the LC50 values of a chemical in different instars. Pearson correlation was used to find relationship between mortality and concentration of chemical, and instar of silkworm.
On the basis of LC50 values, four toxicity classes of chemicals were devised following Yu et al. (2016). These were (i) extreme (LC50< 0.5 mg/L), (ii) high (LC50 > 0.5- 20 mg/L), (iii) moderate (LC50 > 20-200 mg/L), and low (LC50> 200 mg/L) toxicity producing chemicals.
RESULTS
In the study, mortality rate of silkworm larvae in control groups ranged from 0–4% which depicted the credibility of the experiments. The concentration-mortality curve parameters (Dm, m and r) of the single and binary mixture of Pb, IMF, and IMT against 2nd, 3rd, 4th, and 5th in stars of silkworm are shown in Tables I and II. For all single and combination assays, the values of m were >1 which represented that the sigmoidal curves of median-effect equation fit best on our data (Fig. 1). For all assays, r values > 0.90 also confirmed that sigmoid curve fit best on our data. According to LC50 values (Dm), the toxicity of all chemicals showed positive correlation with the concentration (Table 1) and negative with the age of silkworm larvae (IMF r2 = 96.6%; IMT r2 = 90.7%; Pb r2 = 98.4%). In single chemicals decreasing order of toxicity was IMF = IMT > Pb in all instars after 48 h of exposure (2nd instar, F2,9 =214.65; 3rd instar, F2,9 = 196.40; 4th instar, F2,9 = 78.72 and 5th instar, F2,9 = 137.61, P<0.001 for all). The LC50 values of IMF and IMT did not differ significantly in all instars.
The susceptibility of binary mixtures also showed negative correlation with the age of silkworm larvae (r2 = 92.3% for IMF+Pb and r2 = 95.7% for IMT+Pb; P< 0.001; Table II). For all instars, binary mixture of IMF+Pb was more toxic than IMT+Pb (F1,24 =72.80, P<0.001). CI values were calculated at LC10, LC50 and LC90 to assess the behavior of each mixture (Table II). The binary mixture of IMF+Pb showed strong synergistic effect in 2nd and 3rd instars larvae of silkworm at all affective levels (fa). However, 4th and 5th instars showed moderate to low level synergism with the increase of fa (Fig. 2). The binary mixture of IMT +Pb showed strong synergistic effect at all fa values in 2nd instar of silkworm. In 3rd, 4th and 5th instars, binary mixture of IMT +Pb showed strong to moderate synergism at low fa values and additive to low antagonism at high fa values (Fig. 2).
Table I. LC50 values (Dm) with dose-mortality curve coefficient (m) against different instars of silkworm B. mori after 48h of exposure.
|
Chemicals |
Instar |
Dm (mg/L) |
m |
r |
Toxicity grade |
|
Pb |
2nd |
1.93d ± 0.121 |
2.03± 0.15 |
0.97 |
Highly toxic |
|
3rd |
2.28c ± 0.163 |
2.72± 0.14 |
0.94 |
Highly toxic |
|
|
4th |
4.45b ± 0.392 |
3.30 ± 0.25 |
0.97 |
Highly toxic |
|
|
5th |
6.88a ± 0.576 |
3.85± 0.49 |
0.98 |
Highly toxic |
|
|
IMT |
2nd |
0.86c ± 0.082 |
2.06± 0.11 |
0.93 |
Highly toxic |
|
3rd |
1.09c ± 0.071 |
1.70± 0.14 |
0.98 |
Highly toxic |
|
|
4th |
2.39b ± 0.186 |
2.89± 0.21 |
0.99 |
Highly toxic |
|
|
5th |
2.75a ± 0.162 |
2.57± 0.38 |
0.98 |
Highly toxic |
|
|
IMF |
2nd |
0.72d ± 0.013 |
2.31± 0.14 |
0.99 |
Highly toxic |
|
3rd |
1.24c ± 0.029 |
2.26± 0.28 |
0.96 |
Highly toxic |
|
|
4th |
2.20b ± 0.196 |
2.21± 0.38 |
0.99 |
Highly toxic |
|
|
5th |
2.96a ± 0.216 |
3.64± 0.52 |
0.99 |
Highly toxic |
IMT, imidacloprid technical grade; IMF, imidacloprid formulation; Pb, lead.
Table II. Values of LC50 (Dm), dose-mortality curve coefficient (m) and combination index of Lead and imidacloprid (Technical and formulation) mixture against different instars of silkworm B. mori after 48h of exposure.
|
Chemicals |
Instar |
Dm (mg/L) |
m |
r |
Combination Index values (CI) |
||
|
LC10 LC50 LC90 |
|||||||
|
Pb + IMT |
2nd |
0.29d ±0.13 |
1.48±0.43 |
0.99 |
0.11 |
0.17 |
0.26 |
|
3rd |
0.74c ±0.24 |
1.25±0.27 |
0.91 |
0.25 |
0.50 |
1.00 |
|
|
4th |
2.19b ±0.47 |
2.05±0.65 |
0.97 |
0.44 |
0.70 |
1.11 |
|
|
5th |
3.55a ±0.42 |
2.35±0.71 |
0.98 |
0.72 |
0.90 |
1.22 |
|
|
Pb + IMF |
2nd |
0.12c±0.06 |
1.86±0.34 |
0.95 |
0.09 |
0.12 |
0.20 |
|
3rd |
0.37c±0.19 |
1.71±0.23 |
0.94 |
0.15 |
0.23 |
0.35 |
|
|
4th |
1.77b±0.26 |
2.30±0.59 |
0.99 |
0.53 |
0.55 |
0.61 |
|
|
5th |
2.83a±0.74 |
2.79±0.64 |
0.96 |
0.56 |
0.68 |
0.83 |
|
For abbreviations see Table I.
According to the toxicity grades, all chemicals were highly toxic against all instar of silkworm on the basis of 48 h of exposure. The binary mixture of IMF+Pb was extremely toxic for 2nd and 3rd instars and highly toxic for 4th and 5th instars of silkworm. While, the binary mixture of IMT+Pb was extremely toxic for 2nd in star and highly toxic for 3rd, 4th and 5th instars of silkworm.
DISCUSSION
In recent years, mulberry leaves have been contaminated with different types of xenobiotics including different insecticides and heavy metals. Insecticide Imidacloprid is frequently used in mulberry orchards to control infestation of different insect pests and reach to silk worm through contaminated mulberry leaves (Sun et al., 2012; Yu et al., 2015). During the study, both commercial formulations and technical grade imidacloprid was used to assess the toxicity against B. mori. The formulation of an insecticide contains both active ingredients and inert ingredients like surfactants, stabilizers, dyes, etc. These inert ingredients make formulated insecticides safer, more effective and easier to handle. Sometime, these inert ingredients cause increase in the acute toxicity of insecticides (Puglis and Boone, 2011). So, to fully understand the toxicity of insecticides against nontarget organisms, it is imperative to study their formulations also. In our study, both imidacloprid technical grade and formulation showed high toxicity against all instars of B. mori. Luo et al. (2011) reported 0.256 mg/liter LC50 value of imidacloprid against silkworm after 96-h exposure. Yu et al. (2016) also reported imidacloprid as highly toxic insecticide against B. mori. In their experiment LC50 value of imidacloprid against 2nd instar larvae of B. mori after 48h exposure was 2.66 (2.50–2.84) mg/liter. However, in our study, LC50 value of same instar was more than reported values of Luo et al. (2011) and less than Yu et al. (2016). In present study, we also recorded high toxicity of Pb against all instars of B. mori. Zhou et al. (2015) reported no mortality in B. mori larvae that consumed
high Pb (60 mg/kg) contaminated mulberry leaves. Most of the Pb consumed by silkworm larvae removed through feces and a small quantity absorbed through gut epithelium (Jiang et al., 2012). Pb assimilated through gut stored in different organs and removed from the body through molting and spinning (Zhou et al., 2015). Theses variations in susceptibility of imidacloprid and Pb could be explained due to difference of silkworm species, physiological conditions of studied organisms and environmental factors (Zhang et al., 2008).
Present study showed dose dependent increase in the mortality of B. mori larvae when exposed to different concentrations of imidacloprid and Pb. High concentrations of insecticides or any other xenobiotic is more toxic to organisms because their accumulations in body inhibit activities of different detoxification enzymes (Zhu et al., 2014). The age dependent increase in LC50 values indicated that toxicity of imidacloprid and Pb was inversely proportional to the age of larvae. Many studies have reported higher susceptibility of early instar larvae compared with older instars against different xenobiotics (Hornby and Gardner, 1987; Prabhaker et al., 1989; Prabhaker and Toscano, 2007). Less susceptibility of neonicotinoid thiamethoxam in fifth instar larvae of the Indian meal moth, Plodia interpunctella, than second instar larvae were recorded by Yue et al. (2003). In many studies, body size was positively related with the capacity of organisms to withstand the stress caused by the xenobiotics. Smaller organisms have higher feeding and metabolic rate and accumulate more xenobiotics than larger ones (Vesela andVijverberg, 2007).
In nature organism is mostly exposed to a mixture of xenobiotics such as pesticides, heavy metals, and other chemicals. The combinations of different compounds modify each other’s effects that interfere erroneously and undesirably with the activities of biological system. The modulated behavior of combination compounds can be additive, synergistic and antagonistic. Some studies have reported joint toxicity of different insecticides (Zhang et al., 2008, 2010; Tatum et al., 2011; Yu et al., 2016; Yang et al., 2017) and heavy metals (Wu et al., 2016) to non- target organisms. However, no study was performed to assess the combination effect of insecticides and heavy metals against silkworm larvae. Our results showed that binary combination of imidacloprid and Pb exerted synergistic effect on all instars of silkworm. Synergistic effect in this combination of compound may be due to their mode of action on different biological systems, as both chemicals differ in their structures (Thany et al., 2006). The related variables may include synthesis of toxic metabolites, inhibition of detoxifying mechanism, changes in the distribution, enhanced uptake via biological membranes, and decrease in excretion (Wu et al., 2016). Another possible explanation would be reduced protein formation in the treated silkworms that consequently decreased the production of particular detoxifying enzymes like GST and Cytochrome P450 (Wang et al., 2013) ultimately making the silkworms more vulnerable to the xenobiotics. However, the synergistic effect might be explained by the fact that toxicants may somehow combine to increase toxicity by an unknown way of action (Rodea-Palomares et al., 2010). The combination of IMT+Pb showed moderate synergistic effect at low toxicity values and moderate antagonistic at higher toxicities values in 3rd, 4th and 5th instar larvae. It is possible that these chemicals competed for uptake through same binding sites and suppressed the toxic effect of each other (González-Pleiter et al., 2013).
CONCLUSIONS
Our results indicate that combination of different compounds present in nature can increase the risk to silkworm industry. Mulberry plants can uptake many heavy metals and chemicals from the soil or air that ultimately accumulate to mulberry leaves. These contaminated leaves effect the population of silkworm and reduce their production of silk. For risk assessment, it is necessary to estimate acute and residual toxicity of different combinations of compounds present in the environment to revise the existing standards of pollution management.
ACKNOWLEDGEMENTS
We thank the Head, Sericulture Research Lab, Ravi Road, Lahore for providing necessary facilities for this research.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Chou, T.C. and Martin, N., 2005. CompuSyn for drug combinations PC software and user’s guide a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. ComboSyn, Paramus, NJ.
Chou, T.C., 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev., 58: 621–681. https://doi.org/10.1124/pr.58.3.10
Dutta, A., Dutta, S. and Kumari, S., 2012. Growth of poultry chicks fed on formulated feed containing silkworm pupae meal as protein supplement and commercial diet. J. Anim. Feed Res., 2: 303–307.
González-Pleiter, M., Gonzalo, S., Rodea-Palomares, I., Leganés, F., RosaL, R., Boltes, K., Marco, E. and Fernández-Piñas, F., 2013. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: implications for environmental risk assessment. Water Res., 47: 2050–2064. https://doi.org/10.1016/j.watres.2013.01.020
Gupta, D., Chaudhary, H. and Gupta, C., 2015. Sericin-based polyester textile for medical applications. J. Text. Inst., 106: 366–376. https://doi.org/10.1080/00405000.2014.922244
Hornby, J.A. and Gardner, W.A., 1987. Dosage mortality response of Spodoptera frugiperda (Lepidoptera: Noctuidae) and other noctuid larvae to beta-exotoxin of Bacillus thuringiensis. J. econ. Ent., 80: 925–929. https://doi.org/10.1093/jee/80.4.925
Jiang, L., Wang, G., Cheng, T., Yang, Q., Jin, S., Lu, G., Wu, F., Xiao, Y., Xu, H., and Xia, Q., 2012. Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Arch. Virol., 157: 1323–1328. https://doi.org/10.1007/s00705-012-1309-8
Jiang, Y., Jiang, S., Yan, X., Qin, Z., Jia, C., Li, Z.J., and Huang, R., 2020. The mobility of cadmium and lead in the soil-mulberry-silkworm system. Chemosphere, 242: 125–179. https://doi.org/10.1016/j.chemosphere.2019.125179
Lee, K., Kweon, H., Yeo, J., Woo, S., Han, S. and Kim, J., 2011. Characterization of tyrosine-rich Antheraea pernyi silk fibroin hydrolysate. Int. J. Biol. Macromol., 48: 223–226. https://doi.org/10.1016/j.ijbiomac.2010.09.020
Liu, Y., Zhang, H., He, F., Li, X., Tan, H., and Zeng, D., 2018. Combined toxicity of chlorantraniliprole, lambda-cyhalothrin, and imidacloprid to the silkworm Bombyx mori (Lepidoptera: Bombycidae). Environ. Sci. Pollut. Res., 25: 22598–22605. https://doi.org/10.1007/s11356-018-2374-7
Liu, Y.M., Liu, S.H., Zhang, H., Gu, Y.P., Li, X.S., He, M.Y., and Tan, H.H., 2017. Application of the combination index (CI)-isobologram equation to research the toxicological interactions of clothianidin, thiamethoxam, and dinotefuran in honeybee, Apis mellifera. Chemosphere, 184: 806–811. https://doi.org/10.1016/j.chemosphere.2017.06.045
Longvah, T., Mangthya, K. and Ramulu, P., 2011. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Fd. Chem., 128: 400–403. https://doi.org/10.1016/j.foodchem.2011.03.041
Luo, Y.J., Xie, D.Y., Chai. J.P., Liu, Y.G., Huang. P. and Ding, W., 2011. Toxicity and effect of sublethal dosage of several common pesticides to growth and development of the silkworm, Bombyx mori. Sci. Sericult., 37: 142–146.
Matsuda, K., Buckingham, S.D., Kleier, D., Rauh, J.J., Grauso, M. and Sattelle, D.B., 2001. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci., 22: 573–580. https://doi.org/10.1016/S0165-6147(00)01820-4
Matsuda, K., Shimomura, M., Ihara, M., Akamatsu, M. and Sattelle, D.B., 2005. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci. Biotechnol. Biochem., 69: 1442–1452. https://doi.org/10.1271/bbb.69.1442
Meng, X., Zhu, F. and Chen, K., 2017. Silkworm: A promising model organism in life science. J. Insect Sci., 17: 1–6.
Peterson, E.K., Wilson, D.T., Possidente, B., McDaniel, P., Morley, E.J., Possidente, D., Hollocher, K.T., Ruden, D.M. and Hirsch, H.V., 2017. Accumulation, elimination, sequestration, and genetic variation of lead (Pb2+) loads within and between generations of Drosophila melanogaster. Chemosphere, 181: 368–375. https://doi.org/10.1016/j.chemosphere.2017.04.091
Prabhaker, N. and Toscano, N.C., 2007. Toxicity of the insect growth regulators, buprofezin and pyriproxyfen, to the glassy winged sharpshooter, Homalodisca coagulata say (Homoptera: Cicadellidae). Crop Prot., 26: 495–502. https://doi.org/10.1016/j.cropro.2006.04.019
Prabhaker, N., Toscano, N.C. and Coudriet, D.L., 1989. Susceptibility of the immature and adult stages of the sweetpotato whitefly (Homoptera: Aleyrodidae) to selected insecticides. J. Econ. Entomol., 82: 953–958. https://doi.org/10.1093/jee/82.4.953
Puglis, H.J. and Boone, M.D., 2011. Effects of technical-grade active ingredient vs. Commercial formulation of seven pesticides in the presence or absence of radiation on survival of green frog tadpoles. Arch. environ. Contam. Toxicol., 60: 145–155. https://doi.org/10.1007/s00244-010-9528-z
Rodea-Palomares, I., Petre, A.L., Boltes, K., Leganes, F., Perdigon-Melon, J.A., Rosal, R. and Fernandez-Pinas, F., 2010. Application of the combination index (CI)-isobologram equation to study the toxicological interactions of lipid regulators in two aquatic bioluminescent organisms. Water Res., 44: 427–438. https://doi.org/10.1016/j.watres.2009.07.026
Singh, R.N. and Saratchandra, B., 2002. An integrated approach in the pest management in sericulture. Int. J. Ind. Ent., 5: 141–151.
Sun, X., Van-Der-Valk, H., Jiang, H., Wang, X., Yuan, S., Zhang, Y., Roessink, I. and Gao, X., 2012. Development of a standard acute dietary toxicity test for the silkworm (Bombyx mori L.). Crop Prot., 42: 260–267. https://doi.org/10.1016/j.cropro.2012.07.021
Tatum, V.L., Borton, D.L., Streblow, W.R., Louch, J. and Shepard, J.P., 2011. Acute toxicity of commonly used forestry herbicide mixtures to Ceriodaphnia dubia and Pimephales promelas. Environ. Toxicol., 27: 671–684. https://doi.org/10.1002/tox.20686
Thany, S.H., Lenaers, G., Raymond-Delpech, V., Sattelle, D.B. and Lapied, B., 2006. Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci., 28: 14–22. https://doi.org/10.1016/j.tips.2006.11.006
Vesela, S. and Vijverberg, J., 2007. Effect of body size on toxicity of zinc in neonates of four differently sized Daphnia species. Aquat. Ecol., 41: 67–73. https://doi.org/10.1007/s10452-006-9050-6
Wang, Y.H., Gu, Z.Y., Wang, J.M., Sun, S.S., Wang, B.B., Jin, Y.Q., Shen, W.D. and Li, B., 2013. Changes in the activity and the expression of detoxification enzymes in silkworms (Bombyx mori) after phoxim feeding. Pestic. Biochem. Physiol., 105: 13–17. https://doi.org/10.1016/j.pestbp.2012.11.001
Wu, X., Cobbina, S.J., Mao, G., Xu, H., Zhang, Z. and Yang, L., 2016. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res., 23: 8244–8259. https://doi.org/10.1007/s11356-016-6333-x
Yang, G.L., Chen, C., Wang, Y.H., Peng, Q., Zhao, H.Y., Guo, D.M., Wang, Q. and Qian, Y.Z., 2017. Mixture toxicity of four commonly used pesticides at different effect levels to the epigeic earthworm Eisenia fetida. Ecotox. Environ. Saf., 142: 29–39. https://doi.org/10.1016/j.ecoenv.2017.03.037
Yu, R.X., Wang, Y.H., Hu, X.Q., Wu, S.G., Cai, L.M. and Zhao, X.P., 2015. Individual and joint acute toxicities of selected insecticides against Bombyx mori (Lepidoptera: Bombycidae). J. econ. Ent., 109: 327–333. https://doi.org/10.1093/jee/tov316
Yu, R.X., Wang, Y.H., Hu, X.Q., Wu, S.G., Cai, L.M., and Zhao, X.P., 2016. Individual and joint acute toxicities of selected insecticides against Bombyx mori (Lepidoptera: Bombycidae). J. econ. Ent., 109: 327–333. https://doi.org/10.1093/jee/tov316
Yu, R.X., Wang, Y.H., Wu, S.G., Wu, C.X., Chen, L.P., Cang, T. and Zhao, X.P., 2011. Acute toxicity and risk assessment of 21 fungicides to the larvae of Bombyx mori. Asian J. Ecotoxicol., 6: 643–648.
Yue, B., Wilde, G.E. and Arthur, F., 2003. Evaluation of thiamethoxam and imidacloprid as seed treatments to control European corn borer and Indian meal moth (Lepidoptera: Pyralidae) Larvae. J. econ. Ent., 96: 503–509. https://doi.org/10.1093/jee/96.2.503
Zhang, Z., Wang, D., Chi, Z., Liu, X. and Hong, X., 2008. Acute toxicity of organophosphorus and pyrethroid insecticides to Bombyx mori. J. econ. Ent., 101: 360–364. https://doi.org/10.1093/jee/101.2.360
Zhang, Z.T., Zhang, H.W., Fang, X.D., Wang, L.M., Li, X.X., Li, Y.F., Sun, X.W., Carver, J., Simpkins, D., Shen, J. and Weisberg, M., 2011. Cosmetic outcome and surgical site infection rates of antibacterial absorbable (Polyglactin 910) suture compared to Chinese silk suture in breast cancer surgery: a randomized pilot research. Chin. Med. J., 124: 719–724.
Zhang, Z.Y., Yu, X.Y., Wang, D.L. and Yan, H.J., 2010. Acute toxicity to zebrafish of two organophosphates and four pyrethroids and their binary mixtures. Pest Manage. Sci., 66: 84–89. https://doi.org/10.1002/ps.1834
Zhou, L., Zhao, Y., Wang, S., Han, S. and Liu, J., 2015. Lead in the soil mulberry (Morus alba L.) silkworm (Bombyx mori) food chain, translocation and detoxification. Chemosphere, 128: 171–177. https://doi.org/10.1016/j.chemosphere.2015.01.031
Zhu, W.Y., Schmehl, D.R., Mullin, CA. and Frazier, J.L., 2014. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS One, 9: 77547. https://doi.org/10.1371/journal.pone.0077547
Zia, M.H., Codling, E.E., Scheckel, KG. and Chaney, R.L., 2011. In vitro and in vivo approaches for the measurement of oral bioavailability of lead (Pb) in contaminated soils: A review. Environ. Pollut., 159: 2320–2327. https://doi.org/10.1016/j.envpol.2011.04.043
To share on other social networks, click on any share button. What are these?










