The Effect of Genotype on Performance and Egg Quality Traits of the Naked-Neck, Normal-Feathered, and Exotic Tetra H Chickens
Research Article
The Effect of Genotype on Performance and Egg Quality Traits of the Naked-Neck, Normal-Feathered, and Exotic Tetra H Chickens
Abiyu Tadele 1,2*, Gebreyohannes Berhane2, Wondmeneh Esatu3
1Bonga University, College of Agriculture and Natural resources, P.O. Box 334, Bonga, Ethiopia; 2Addis Ababa University, College of Veterinary Medicine and Agriculture, P.O. Box 34, Bishoftu, Ethiopia; 3International Livestock Research Institute (ILRI), P. O. Box: 5689, Addis Ababa, Ethiopia.
Abstract | Studies on the performance and egg quality traits of chicken are essential for consumers and chicken husbandry, helping them to anticipate yield, market, and exploitation of the genotypes in future breeding plans. The main ecotypes in Southwest Ethiopia are the indigenous naked-neck and normal-feathered chicken genotypes, and the exotic Tetra H, a dual-purpose genotype that was imported from Hungary. Data were collected from 168 hens of the three genotypes kept under intensive management for 20 weeks. The Tetra H genotype had significantly greater (p < 0.05) body weight, feed consumption, hen-housed egg production, egg mass, and a lower (better) feed conversion ratio than the other genotypes. Similarly, the naked-neck chicken genotype outperformed the normal-feathered chicken in terms of age at first egg, body weight, egg number per bird, hen-housed egg output, and egg mass. Furthermore, Tetra H had a 29.8% higher body weight at the first egg and a 20.72% higher body weight at the first egg than the normal-feathered and naked-neck genotypes, respectively. For the assessment of egg quality traits, 240 fresh eggs from the three genotypes were sampled. The findings revealed that, except for yolk breadth and color, the Tetra H genotype had considerably better (p < 0.05) egg quality characteristics than the other genotypes. Internal and external egg quality characteristics were found to have positive and significant associations among the genetic group of chickens, demonstrating that choosing birds with bigger eggs increases the proportion of edible eggs. As a result, our findings will help to develop more effective breeding programs for these three chicken genotypes by providing valuable insights into the genetic basis of chicken performance and egg quality.
Keywords | Age at first egg; Body weight, Correlation coefficients; Egg mass, Egg quality traits
Received | April 04, 2023; Accepted | April 25, 2023; Published | June 15, 2023
*Correspondence | Abiyu Tadele, Bonga University, College of Agriculture and Natural resources, P.O. Box 334, Bonga, Ethiopia; Email: abiyu.tadele@yahoo.com
Citation | Tadele A, Berhane G, Esatu W (2023). The effect of genotype on performance and egg quality traits of the naked-neck, normal-feathered, and exotic tetra h chickens. J. Anim. Health Prod. 11(2): 222-233.
DOI | http://dx.doi.org/10.17582/journal.jahp/2023/11.2.222.233
ISSN | 2308-2801

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The socioeconomic well-being of people in developing countries depends on the production of livestock, specifically chickens (Melesse, 2014; Melesse et al., 2021). Due to their short generation interval and adaptability in a wide range of agro-ecological settings, chickens are a widely distributed avian species worldwide (Markos et al., 2014). Domestic chickens are the most commonly kept animal species in the world because they provide low-income rural households with high-quality protein and income (Herrero et al., 2013). This is because chickens have useful characteristics such as disease resilience, adaptation to harsh conditions, and the ability to use low-quality feed (Assefa, 2019).
The entire poultry population in Ethiopia is believed to be 57 million, with 78.85% being indigenous chickens raised primarily by smallholder rural farmers in a scavenging management system. The rest 12.02% and 9.11% of chickens are hybrid and exotic (CSA, 2021). Indigenous chickens, which are mostly kept by smallholder rural communities, are chosen for their adaptive characteristics either naturally or by farmers who keep them and are frequently poor in egg production, late maturation, and extended broodiness (Negassa et al., 2014). A previous study showed that indigenous naked-neck chicken locally known as “Angete Melata” (Melesse et al., 2010) has a good heat dissipation mechanism and is well adapted to the harsh environment and poor nutrition, and is highly resistant to disease, and superior to indigenous normal-feathered and exotic egg-type or exotic naked-neck counterparts in terms of growth rate, egg production, egg, and meat yield traits (Islam & Nishibori, 2009). The Tetra H chicken genotype is dual-purpose rural hybrid chicken that originated in Hungary and is popular in Europe and neighboring countries. It is characterized by calm characteristics, straw and wheat-colored feathers with black tail feathers. Its heavy weight with good conformations makes the chicken ready for slaughter at 10-12 weeks of age with a body weight over 1.6 kg, while females start egg-laying at 21-22 weeks of age (Guide, 2013; Gerzilov et al., 2018). As a result of these, the Tetra H chicken genotype was introduced in Ethiopia to improve the performance of the indigenous chicken genotype through crossbreeding (Tadele et al., 2023).
Numerous studies have shown that crossbreeding improves the productive and reproductive performances of chicken genotypes (Padhi, 2016). Because of the ongoing population growth and desire for high-quality protein in developing nations, the chicken industry has received a lot of attention and is anticipated to satisfy the community’s requirements in a relatively short period (Geleta et al., 2013; Melesse, 2014). Many variables affect egg production, including poultry strain, nutrition, mortality, culling, health and management practices, age at the first lay, peak lay, and lay persistency (Farooq et al., 2002; Amin & Nawawi, 2013). Egg quality traits, on the other hand, refer to various features of an egg that determine its suitability for human consumption (Sreenivas et al., 2013; Kumar et al., 2022). In developed nations, eggs are sold based on several parameters which determine the price of eggs (Berkhoff et al., 2020). The egg quality parameters are of great importance for egg industries which determine the grading, price, hatchability, chick weight, and consumer preferences (Farooq et al., 2002; Sreenivas et al., 2013; Kumar et al., 2022), and due to these, the poultry breeding industries are more focused on egg quality traits. Due to these, studies carried out by several scholars in Ethiopia and other parts of the world gave poultry breeders and researchers enormous opportunities.
As a result, (Guide, 2013; Padhi, 2016) evaluated the egg quality traits of indigenous chickens and observed the potential of these genotypes for future improvements. Similarly, (Fereja et al., 2016) and (Assefa et al., 2019) examined local and exotic chicken egg quality traits and discovered the presence of genetic variations among the chicken genotypes. Thus, evaluating egg quality is crucial for maintaining good egg production (Nolte et al., 2021). The reproductive, productive, and egg-quality traits of the indigenous normal-feathered chicken populations under farmers’ management conditions have been well documented in Southwest Ethiopia (Geleta et al., 2013; Sreenivas et al., 2013; Kumar et al., 2022). However, the effect of genotype on performance and egg quality traits in these genotypes remains poorly understood under intensive system. In this study, we investigated the effect of genotype on performance and egg quality traits in Naked-Neck, Normal-Feathered, and Exotic Tetra H chickens genotypes in Kaffa Zone, Southwest Ethiopia.
Materials and Methods
Ethical Statement
All trial procedures were approved by the College of Agriculture and Veterinary Medicine, Addis Ababa University Animal Research Review committee with certificate Ref.No:VM/ERC/02/01/14/2022.
Description of the study area
This research was carried out in Kaffa Zone, Southwest Ethiopia which is located between 6o24’ to 8o13’ North latitude and 35o30’ to 36o46’ East longitude. Kaffa Zone is divided into twelve districts and four administrative towns and situated within an altitude ranging from 500-3000 (meters above sea level) m.a.s.l. The average annual temperature ranges from 10.1 oC to 27.5 oC. The annual rainfall varies from 1001-2200 mm (Tadele et al., 2018; 2023).
Sampling Methods and data collection
The data were collected from the three chicken genotypes, where the same batch of 99 pullets and 13 cocks of average weight were added to the existing experiment (Tadele et al., 2023) at the 19th week of age. Then, 196 chickens were randomly assigned to one of three treatment groups: normal-feathered (n = 56; 8 males and 48 females), naked-neck (n = 56; 8 males and 48 females), and Tetra H (n = 84; 12 males and 72 females). In a completely randomized design (CRD), each treatment group was replicated four times with 12 hens and 2 cocks per replicate in the naked-neck and normal-feathered chicken genotypes and 18 hens and 3 cocks in the Tetra H chicken in a 1:6 ratios. The birds were given layer mash comprising 17.3% crude protein and 2,856 kcal/kg metabolizable energy (Tadele et al., 2023), managed in a deep-liter system under the same management conditions, and vaccinated following the National veterinary Institute (NVI) recommendations (Gide et al., 2020).
The study consisted of body weight and age at first egg, egg production, and egg quality assessments of the normal-feathered, naked-neck, and Tetra H chicken genotypes. Data on egg production were collected throughout the laying period and were calculated as a percent of hen-housed egg production (HHEP). Weighing egg samples from each genotypic group yielded the mean egg weight. The eggs were weighed beginning at 24 weeks of age and every week thereafter. The feed conversion ratio was calculated as the amount of feed consumed (g) to produce a dozen eggs and the percent of hen-housed egg production (HHEP) was calculated following (M. A. Islam et al., 2022)
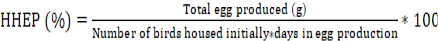
Following FAO (2012) standards, 240 fresh eggs were collected from the three chicken genotypes (80 eggs from each genotype; 20 eggs per replicate) for the evaluation of external and internal egg quality characteristics. The eggs were then individually weighed with a sensitive balance (Fig. 2a). The width and length of the egg were then measured with a digital caliper (Fig. 2b). After measuring the external egg quality, each egg was cautiously opened on a flat dish to determine the most important egg quality characteristics. The inner shell membrane was removed from three typical regions (top, middle, and bottom) and the shell thickness (in mm) was measured using a digital caliper (Fig.2d). A sensitive balance was used to measure albumen/or yolk and shell weight in gram (Fig. 2a & 3a). Albumen and yolk height (mm) were determined using a tripod micrometer and yolk width in mm was measured using a digital caliper (Fig. 3c) and yolk color fan with 1-15 calibrated color fans were used to determine yolk color (Fig. 3b).
The egg shape index (%) was computed by dividing egg width by egg length x 100. The egg shape index (SI) was determined based on the equation described by (Anderson et al., 2004). The egg shape index was also classified as sharp egg where (SI < 72), standard (SI = 72-76), and/or round egg (SI > 76), (Vekić et al., 2022) and was computed following the formula given by (Nolte et al., 2021). The shell ratio was calculated as shell weight divided by egg weight x 100, following the equation given by (Vekić et al., 2022). The Yolk index (%) was computed by dividing yolk height by yolk width x 100, according to the formula described by (Kul & Seker, 2004). Haugh (HU) unit was calculated by fitting the average albumen height and egg weight into the following equation; provided by (Anderson et al., 2004); where AH is the albumen height in mm and EW is the weight of the egg in g.
Statistical analysis
The general linear model procedure of Statistical Analysis Systems (SAS) Software was used to analyze the data (SAS, 2012). One-way ANOVA was used to examine variations in response variables among the three genotypes. When the F-test declared significant differences at (p < 0.05), Tukey HSD Test was used to distinguish differences between treatment means. The models are;
Model 1. 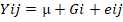 ; Where: Yij = represents the j observation in the ith genotype level; µ = overall mean; Gi = genotype effect; and eij = random error.
; Where: Yij = represents the j observation in the ith genotype level; µ = overall mean; Gi = genotype effect; and eij = random error.
Model 2. 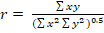
Where r= correlation coefficient, 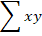 ,
, 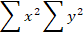 = sum of variables
= sum of variables
Table 1: Reproductive and Productive traits of the three chicken genotypes
|
Parameters |
Treatment groups |
|||
| Normal-feathered |
Naked-neck |
Tetra H |
p-value |
|
| Body weight (g) at 20 weeks |
991.7±24.6b |
1120±80.2b |
1412.7±66.8a |
0.007 |
| Age at first egg (days) |
161±2.48a |
152.3±3.04b |
150.2±2.25b |
0.006 |
| Body weight at first egg (g) |
1033.8±29.7b |
1168.2±36.2b |
1458.2±57.3a |
0.037 |
| Average body weight (g) at 40 weeks |
1285.2 ±19.8b |
1317± 17.1b |
1637.9±30.3a |
0.001 |
| Total eggs/hen/140 days |
51.0±1.158b |
54.8 ±1.277b |
95.4±1.247a |
0.001 |
| Daily feed intake (g) |
117.6±0.097a |
116.3±0.150b |
117.9±0.123a |
0.001 |
| Feed conversion ratio |
3.41±0.108a |
3.45±0.117a |
1.68±0.052b |
0.001 |
| HHEP% |
36.4±0.827b |
39.1±0.912b |
68.2±0.891a |
0.001 |
| EM (g/hen/day) |
16.1±0.389b |
17.6±0.431b |
37.7±0.553a |
0.001 |
a,b Row means with different superscript letters are significantly different (p < 0.05)
SE = Standard error; HHEP%, Percent of hen-housed egg production; EM, egg mass
Table 2: The mean SE external egg parameters of eggs collected from chicken genotypes.
|
External egg quality traits |
Treatment Groups |
|||
| Normal-feathered |
Naked-neck |
Tetra H |
p-value |
|
| Egg weight (g) |
43.7±0.336b |
44.3±0.344b |
56.8±0.211a |
0.001 |
| Egg length (mm) |
52.3±0.273b |
52.6±0.216b |
56.2±0.353a |
0.001 |
| Egg width (mm) |
38.8±0.137b |
39.0±0.152b |
43.1±0.228a |
0.001 |
| Egg shape index (%) |
74.4±0.477b |
74.2±0.369b |
76.7±0.329a |
0.001 |
| Shell weight (g) |
5.33±0.049b |
5.52±0.053b |
6.02±0.084a |
0.001 |
| Shell thickness (mm) | 0.423±0.006 | 0.431±0.006 | 0.424±0.007 | 0.651 |
| Shell ratio % |
12.2±0.0907a |
12.4± 0.079a |
10.6±0.152b |
0.001 |
a,b Row means with different superscript letters are significantly different (p < 0.05)
SE = Standard error
Table 3: Internal traits of eggs collected from three chicken genotypes (Mean SE)
|
Internal Egg traits |
Treatment Groups |
|||
| Normal-feathered |
Naked-neck |
Tetra H |
P-value |
|
| Albumen weight (g) |
22.9±0.254b |
23.3±0.251b |
33.9±0.104a |
0.001 |
| Yolk weight (g) |
15.4±0.084b |
15.5±0.077b |
16.8±0.208a |
0.001 |
| Albumen height (mm) |
5.42±0.084b |
5.27±0.096b |
8.55±0.165a |
0.001 |
| Yolk height (mm) |
15.0±0.128b |
15.1±0.089b |
16.1±0.075a |
0.001 |
| Yolk width (mm) | 38.7 ± 0.113 | 38.7±0.126 | 38.4 ± 0.144 | 0.070 |
| Yolk index (%) |
38.8 ± 0.314b |
38.1± 0.252b |
41.9 ± 0.232a |
0.001 |
| Haugh unit (%) |
78.6±0.609b |
77.2±0.645b |
92.7±0.963a |
0.001 |
| Yolk color (1-15) | 10.1 ±0.050 | 10.2±0.097 | 10.3±0.039 |
0.131 |
a,b Means across a raw with different superscript letters are significantly different (p < 0.05)
SE = Standard error
Table 4: Phenotypic correlation of external and internal egg quality traits of the three chicken genotypes
| Genotypes |
Traits |
EW |
EL |
EWD |
SW |
ST |
AW |
YW |
AH |
YH |
| Normal-Feathered |
EW |
1.00 | 0.511** | 0.827** | 0.615** | 0.242 | 0.966** | 0.715** | 0.109 | 0.140 |
| Naked-neck | 1.00 | 0.564** | 0.853** | 0.762** | 0.136 | 0.971** | 0.778** | 0.645** | 0.563** | |
| Tetra H | 1.00 | 0.114 | 0.119 | 0.079 | 0.119 | 0.414** | 0.779** | -0.063 | 0.061 | |
| Normal-Feathered |
EL |
1.00 | 0.227 | 0.167 | 0.005 | 0.762** | 0.644** | 0.077 | 0.218 | |
| Naked-neck | 1.00 | 0.23964 | 0.464** | 0.059 | 0.535** | 0.455** | 0.577** | 0.300* | ||
| Tetra H | 1.00 | 0.726** | 0.358** | -0.221 | 0.247 | -0.152 | 0.383** | 0.509** | ||
|
Normal-Feathered |
EWD |
1.00 | 0.607** | 0.297* | 0.762** | 0.644** | 0.077 | 0.218 | ||
| Naked-neck | 1.00 | 0.700** | 0.187 | 0.813** | 0.678** | 0.508** | 0.536** | |||
| Tetra H | 1.00 | 0.288* | 0.080 | 0.157 | -0.074 | 0.374** | 0.482** | |||
| Normal-Feathered |
SW |
1.00 | 0.446 | 0.495** | 0.369* | -0.147 | 0.146 | |||
| Naked-neck | 1.00 | 0.295* | 0.651** | 0.596** | 0.571** | 0.278* | ||||
| Tetra H | 1.00 | -0.007 | 0.088 | -0.368* | 0.189 | 0.382** | ||||
| Normal-Feathered |
ST |
1.00 | 0.193 | 0.119 | -0.148 | 0.235 | ||||
| Naked-neck | 1.00 | 0.105 | 0.06387 | 0.17758 | -0.0147 | |||||
| Tetra H | 1.00 | -0.191 | 0.219 | -0.067 | 0.056 | |||||
| Normal-Feathered |
AW |
1.00 | 0.544** | 0.175 | 0.017 | |||||
| Naked-neck | 1.00 | 0.634** | 0.597** | 0.601** | ||||||
| Tetra H | 1.00 | -0.114 | -0.057 | -0.004 | ||||||
| Normal-Feathered |
YW |
1.00 | -0.007 | 0.424** | ||||||
| Naked-neck | 1.00 | 0.546** | 0.361** | |||||||
| Tetra H | 1.00 | -0.112 | -0.090 | |||||||
| Normal-Feathered |
AH |
1.00 | -0.115 | |||||||
| Naked-neck | 1.00 | 0.621** | ||||||||
| Tetra H | 1.00 | 0.547** | ||||||||
|
Normal-Feathered |
YH |
1.00 | ||||||||
| Naked-neck | 1.00 | |||||||||
| Tetra H | 1.00 |
EW, Egg weight; EL, Egg length; EWD, Egg width; SW, Shell weight; ST, Shell thickness; AW, Albumen weight; YW, Yolk weight; AH, Albumen height; YH, Yolk height; sig. **p < 0.01; *p < 0.05.
Results
Reproductive and productive traits
The Tetra H and naked-neck pullets started laying eggs earlier than the normal-feathered pullets, and there were significant differences in age at first egg (p < 0.05) among the chicken genotypes (Table 1). During the experiment, the Tetra H chicken showed that its live body weight increased with age (Fig. 1). In comparison to Tetra H and normal-feathered chicken genotypes, the naked-neck chickens had reduced average daily feed intake values by 1.37%. Tetra H hens significantly outperformed the normal-feathered and naked-neck chicken genotypes in terms of hen-housed egg production and feed consumption (p < 0.05) (Table 1). In terms of egg mass, Tetra H chickens did noticeably better (p < 0.05) than the other genotypes.
External and internal egg quality traits
External egg quality traits: The characteristics of external egg quality are shown in Table 2 and Fig. 2. In this findings, the Tetra H chicken had higher values for the majority of external egg quality traits, except for shell thickness, which did not differ significantly (p > 0.05) among the three chicken genotypes. The average egg weight, length, width, shell weight, and shape index all differed significantly (p < 0.01) among the genetic group of chickens. The average egg weight in the current study was 43.7 g, 44.3 g, and 56.8 g for the normal-feathered, naked-neck, and Tetra H chicken, respectively. The Tetra H chicken genotype recorded better than the other chicken genotypes in terms of egg length, which differed among chicken genetic groups.
Internal egg quality traits: The average albumen and yolk weight, albumen and yolk height, and Haugh unit varied considerably (p < 0.01) among the chickens in the genetic group, as shown in Table 3. The Tetra H chicken genotype had a substantially higher (p < 0.01) value for every trait evaluated aside from yolk width and color. The typical albumen weight in the current study was 22.9 g, 23.3 g, and 33.9 g for the normal-feathered, naked-neck, and Tetra H chicken respectively. Yolk weight for the Tetra H chicken genotype was considerably heavier (p < 0.01) than the chicken genotypes in the naked neck and normal feathers. However, there were no noticeable differences in the egg yolk width values among the genetic group of chickens (p > 0.05). The Haugh unit values in the current study showed significant differences (p < 0.01) among chicken genetic groups, with the Tetra H chicken genotype having a higher value.
Phenotypic Correlation of External and internal egg traits: Except for shell thickness, the present study found that among chicken genotypes, egg weight, and external egg characteristics had a strong and significant (p < 0.01) positive association (Table 4). The association between egg weight and egg length was significant (p < 0.01) for the naked-neck (r = 0.853) and normal-feathered (r = 0.827) chicken genotypes but non-significant (r = 0.119) for the Tetra H chicken. The internal egg quality characteristics of albumen and yolk weight were considerably (p < 0.01) associated with egg weight among the genetic group of chickens. In the Tetra H genotype, egg length was significantly (p < 0.01) correlated with egg width, shell weight, albumen, and yolk height, and albumen and yolk weight in the naked-neck and normal-feathered chicken genotypes.
There were positive and significant (p < 0.01) associations between egg width and eggshell weight within the genetic group of chickens. However, there were no significant (p > 0.05) associations between egg width, albumen, or yolk height in the normal-feathered chicken genotype. Albumen weight and yolk weight were considerably linked for the three chicken genotypes (p < 0.01). In the normal-feathered and naked-necks, albumen weight was found to be significantly associated with yolk weight, but not in the Tetra H genotype. Yolk weight and yolk height were significantly (p < 0.01) associated with the naked-neck and normal-feathered genotypes but not in the Tetra H genotype. In the naked-neck and Tetra H genotypes, albumen height was found to be significantly associated with yolk height, but not in the normal-feathered genotype.
Discussion
Reproductive and productive performance
The age of the first egg is an important economic trait in poultry production as it determines the productivity of the flock (Mohanta et al., 2018). In the present study, significant differences were found in the genetic group of chickens for age at sexual maturity, and the Tetra H (150 days) and naked-neck (153 days) laid eggs earlier than the normal-feathered chicken (161 days). The Tetra H chicken began laying eggs at 21-22 weeks, as recommended by the producer Babolna TETRA (Guide, 2013). However, the findings reported by (Gerzilov et al., 2018) were 117 and 134 days age at first egg in the first and second experiments in the Tetra H chicken genotype, respectively, which were even earlier than the producer Babolna TETRA (Guide, 2013) guidelines. The age of the first egg in the naked-neck and normal-feathered chicken genotypes reported by (Adomako et al., 2010), was comparable with the naked-neck, however earlier than the reported values from the normal-feathered than the current study.
The Tetra H genotype’s body weight (1412.7 g) at 20 weeks was 29.8% higher than the normal-feathered and 20.72% higher than the naked-neck, but 18.1% lower than the manufacturer’s Babolna TETRA (1725 g) at the same age (Guide, 2013). Similarly, Almasi et al. (2012) reported a higher live body weight (2215 g) of Tetra H females at 12 weeks of age and which was even higher than the manufacturer value by 22.12% (Guide, 2013). The observed variations in live weight may be due to environmental, nutritional, and other management factors; however, in the current study, the observed variations are due to genetic differences and agreed with the reports of (Tadele et al., 2023). On the other hand, body weight is associated with egg production performance and age at sexual maturity, and thus, the Tetra H genotype laid earlier than the other chicken genotype due to their higher body weight (Gerzilov et al., 2018), and the naked-neck was also relatively higher in body weight and laid earlier than their normal-feathered counterparts. As shown in Table 1, genotype had a significant influence on average hen-housed egg production during the laying experimental period, with higher values recorded in the Tetra H chicken. These results are in agreement with the observations of (Islam & Nishibori, 2009; Yonas et al., 2019) who reported the genotype affects hen-housed egg production. When compared to the other genotypes, the naked-neck chicken had the lowest feed consumption. These findings are consistent with earlier research, which found that the naked-neck genotype consumes less feed than other chicken genotypes (Tadele et al., 2023).
External egg quality traits
The result of egg weight recorded from the Tetra H chicken genotype (56.8 g) in the present study was significantly heavier (p < 0.05) than the two chicken genotypes. These results are in agreement with the findings of (Fereja et al., 2016) and (Yonas et al., 2019) who reported heavier egg weight values from exotic chickens than the indigenous chicken genotype. Consistent with the present study, the result observed in Nigeria, the egg weight of exotic chicken had significantly higher (59.6 g) for the Dominant blue chicken than the indigenous normal-feathered (52.33 g) and Naked neck genotypes (52.70 g) (Issn, 2013) and the results were higher than the observed egg weight values from the current indigenous normal-feathered (43.7 g) and naked neck (44.3 g) chicken genotypes. The egg weight values reported by (Markos et al., 2017a) in the highlands of the Tigray region (43.7 g) and (Edmew et al., 2018) in Bench Maji Zone, Southwest Ethiopia (43.9 g) were consistent with the result observed in the naked-neck and normal-feathered chicken genotypes of the present study.
However, various scholars in Ethiopia and other parts of the world also reported lower egg weights of indigenous chickens than in the present study. Accordingly, (Melesse et al., 2013) found lower egg weights of indigenous chickens in different agroecology (39.4 g, 40.2 g, and 39.3 g, from the lowland, midland, and highland) of Ethiopia. Assefa et al. (2019) also reported 41.1 g and 39.5 g from low and midland agroecology. Accordingly (Hussain et al., 2013) in Pakistan reported 41.05 g of egg weight of indigenous chicken, and (Liswaniso et al., 2020) reported 41.7 g and 40.59 g for Assel and Kadaknath chicken genotypes. The higher egg weight value recorded from the Tetra H chicken genotype in the current study might be associated with the heavier body size of the Tetra H chicken genotype. According to (Alex, 2001), report egg weight of a chicken was proportional to body weight, indicating the heavier body weight of a chicken would likely produce big-sized eggs as they can consume more feed. This was also a similar scenario to the report of (Issn, 2013) who reported that egg weight was higher for heavy chickens than for light chicken breeds. In agreement with this, (Liswaniso et al., 2020) reported a positive relationship between chicken body weight and egg weight. As a result, the lower egg weight exhibited by the indigenous normal-feathered and naked-neck chicken genotypes in the current research was associated with the genotypes’ lower body weight (Tadele et al., 2023), and thus, the selection of chickens with heavier body weight would produce superior egg weight (Tongsiri et al., 2019).
The Tetra H chicken’s average egg length (56.2 mm) was substantially greater (p < 0.05) than the other two chicken genotypes. The differences in egg length found may be related to genetic composition (Issn, 2013). The current study’s findings agreed with those of (Yonas et al., 2019) from Hawassa and Yirgalem Town, who reported egg length values of 55.7 mm and 55.59 mm from Sasso and Bovan chicken, respectively. Consistent with this, (Assefa et al., 2019) reported average egg length values of 54.8 mm, 55 mm, and 53.2 mm from Bovan, Sasso, and Koekoek chickens, respectively. In line with this, the result reported from Nigeria was consistent with the current study, which reported the exotic Dominant blue had a 58.8 mm higher egg length value than the indigenous normal-feathered (55.0 mm) and naked-neck (55.8 mm), chicken genotypes (Issn, 2013). In the current study, egg length observed from indigenous normal-feathered (52.3 mm) and naked-neck (52.6) mm chickens was comparable with the observations of (Kumar et al., 2022), for Assel (51.6 mm) and Kadaknath (51.3 mm) native chickens. However, (Yonas et al., 2019) reported comparatively higher egg length (54.6 mm) and (Hussain et al., 2013) reported 55.5 mm of egg length from native chicken.
The egg width observed in the present study showed a significant difference (p < 0.05) among the genotypes, with a higher value (43.1 mm) recorded from the Tetra H chicken. Consistent with the current study, previous comparative studies from exotic chicken genotypes showed higher egg width values than the indigenous chicken genotype. Based on this (Kebede et al., 2019) reported 42 mm from Exotic chicken in Metekel Zone and (Assefa et al., 2019) also reported the egg width value from exotic ranged from 39-41.2 mm in the low and mid-agro-ecologies of the Sidama Zone. Similarly, the results reported in Nigerian Dominant blue chicken exhibited higher (44.3 mm) than the indigenous normal-feathered (42.1 mm) and naked-neck (42.7 mm), chicken genotypes, respectively (Issn, 2013). However, the egg width reported by (Kumar et al., 2022) from Aseel (38.5 mm) and Kadaknath (37.9 mm) native chicken, and (Yonas et al., 2019) observed egg width values of 40.6 mm were in agreement with the values recorded from indigenous chicken genotypes.
The egg shape index in the current study varied significantly among the genetic group of chickens with higher values recorded from the Tetra H chicken genotype. The Tetra H chicken had a significantly higher egg shape index of 76.7 % and indigenous normal-feathered (74.4 %) and naked-neck (74.2 %). The result of the current study was consistent with the values reported by various scholars in Ethiopia and other parts of the world (Nkukwana, 2018; Vekić et al., 2022). Similarly, the shape index reported from Aseel (74.75 %) and Kadaknath (74.02%) chicken breeds were consistent with the result recorded from indigenous normal-feathered and naked-neck chickens in the current study. However, a relatively higher shape index value than the indigenous chicken in the current study was reported by (Haunshi et al., 2011) and (Valavan et al., 2016), which were 76.39 % and 77.23 %, respectively. Similarly (Ahmad et al., 2013) also found 77.25 % to 83.87 % of the shape index from indigenous chicken. Since the egg shape index was a good indicator of the similarity of the egg size and the higher shape index indicates the uniformity of the eggs (Agarwal et al., 2021). Thus, the shape index observed from the indigenous naked-neck and normal-feathered chicken genotypes falls within the standard egg size (72-76 %) and the Tetra H falls with a round egg size > 76 % (Duman et al., 2016).
The average eggshell thickness values obtained from the current study (0.43 mm) were in agreement with the findings of (Abdurehman & Urge, 2016) who reported 0.40 mm for Nigerian Dominant Black female chickens, and (Rajaravindra et al., 2015) for homozygous and heterozygous naked-neck chicken genotypes. Consistently, the eggshell thickness reported in Nigerian indigenous normal-feathered and exotic dominant blue chicken reported by (Issn, 2013), 0.42 mm agrees with the present study. However, lower eggshell thickness values were reported in various studies (Abdurehman & Urge, 2016) observed a 0.29 mm in Eastern Hararghe Ethiopia, (Yonas et al., 2019) in Yirgalem and Hawassa Ethiopia reported in the range of 0.19-0.32 mm, and (Van et al., 2020) from Vietnam reported 0.22-0.23 mm for indigenous chicken and (Liswaniso et al., 2020) from Zambia reported 0.34 mm and (Issn, 2013) and (Issn, 2013) observed 0.36 mm in Nigerian indigenous normal-feathered chicken. On the other hand, higher eggshell thickness values of 0.59 mm were reported (Abdurehman & Urge, 2016) for the Fulani chicken Ecotype and Dominant black chicken (0.50 mm) in Nigeria (Issn, 2013). According to (Liswaniso et al., 2020) the eggshell thickness of 0.33 mm was supposed to be strong enough to resist minimal handling and thus, the average eggshell thickness recorded in the present study (0.43 mm) was able to withstand handling during storage and transportation.
Internal egg quality traits
The egg yolk weight result of the present study differed considerably between genetic groups of chicken, with the highest weight observed from exotic Tetra H chicken. This was consistent with the findings of (Issn, 2013), who found that exotic Dominant blue had substantially higher egg yolk weight than indigenous normal-feathered and naked-neck genotypes in Nigeria. The egg yolk weight of the indigenous normal-feathered and naked-neck chicken genotype found in this research was similar to several studies. Accordingly, (Edmew et al., 2018) reported 15.1 g, and (Abdurehman & Urge, 2016) observed, 15.05 g was consistent with the results observed from indigenous normal-feathered (15.3 g) and naked-neck (15.6 g) chicken genotypes. Similarly, (Markos et al., 2017) reported 16.5 g egg yolk weight in highland agro-ecologies of the Tigray region, Ethiopia, and (Van et al., 2020) in Vietnam indigenous Ho chicken reported 16.76 g and these were in agreement with the current result observed from Tetra H chicken genotype and (Liswaniso et al., 2020) in Zambia also reported 16.92 g from indigenous chicken. However, higher egg yolk weight was reported in Nigerian indigenous normal-feathered (17.55 g), naked-neck (18.2 g), Dominant black (18.1 g), and Dominant blue (19.5 g) chicken genotypes (Issn, 2013) and 17.44 g from indigenous Dong Tao chicken in Vietnam (Van et al., 2020) than the current study. A lower egg yolk weight 13.75 g was reported from indigenous chicken in Pakistan (Hussain et al., 2013).
The yolk height values observed from indigenous normal-feathered (15 mm) and naked-neck chickens (15.1 mm) were consistent with the values reported from Aseel native chicken (15.3 mm) in India (Kumar et al., 2022). A higher egg yolk height of 18.22 mm was reported in India (Yonas et al., 2019) than in the current study. However, lower yolk height values were reported in various studies (Issn, 2013) and from Kadaknath native chicken in India (Kumar et al., 2022). In the present study albumen height observed from indigenous normal-feathered, naked neck and Tetra H was found to be 5.42 mm, 5.27 mm, and 8.55 mm, respectively. The albumen height values reported by (Yonas et al., 2019) from Yirgalem and Hawassa towns in Ethiopian local and exotic chicken genotypes were consistent with the present study. However, the albumen height reported by (Hussain et al., 2013) was 5.2 mm, and Kadaknath native chickens 5.52 mm (Kumar et al., 2022) agreed with the reported values from indigenous normal-feathered and naked-neck chickens. The albumen weight in the present study was varied significantly among the genetic group of chicken and higher values recorded from exotic Tetra H chicken. The results reported from Nigeria indicated the albumen weight recorded from the Dominant black (33.8 g) was higher than the reported values of the naked-neck (30.05 g) chicken genotype (Issn, 2013). However, the results of albumen weight observed from indigenous normal-feathered and naked-neck chickens in the present study were in agreement with various studies reported from indigenous chickens (Issn, 2013; Vekić et al., 2022).
The Haugh unit (HU) value was mainly influenced by albumen height and egg weight (Assefa et al., 2019) and in the present study, a higher value was reported from exotic Tetra H (92.7). This result was in agreement with the reports of various studies (Islam & Dutta, 2010; Tadele et al., 2018), which reported the exotic chicken genotype reported to have higher HU values than the local chicken genotypes. The HU value reported by (Rath et al., 2015a) in Indian White leghorn chicken (92.0) was consistent with the value reported in Tetra H chicken. On the other hand, the HU values reported from the East Shewa Zone of Ethiopia from exotic chicken maintained in various management conditions showed significant differences with higher HU observed from intensively managed chicken (Woldegiorgiss et al., 2015). Similarly, higher HU value was reported from intensively managed chicken breeds in Tanzania (Guni et al., 2021). However, the Haugh unit values observed in the normal-feathered (78.6) and naked-neck (77.2) chicken genotypes in the current study were higher than the values reported by various scholars (Almasi et al., 2012; Geleta et al., 2013; Issn, 2013; Padhi, 2016). In addition, lower Haugh unit values were also reported by (Yonas et al., 2019) who reported HU value of 74.9 from Hawassa town and (Hussain et al., 2013) from Indigenous chicken in Pakistan reported 71.4, and (Kumar et al., 2022) studied 63.97 from Indian Kadaknath native chicken.
Phenotypic correlation of external and internal egg traits
In the present study, there were strong, positive, and significant correlations between egg weight and albumen and yolk weight were observed among the genetic group of chickens. Strong correlation coefficients found between egg weight and, albumen and yolk weight in the three chicken genotypes were consistent with the findings of (Kgwatalala et al., 2016), who found strong, positive correlations between egg weight and albumen weight (r = 0.892 and r = 0.907) in the naked-neck and normal-feathered, respectively). Positive and significant (p < 0.01) correlations were also observed between egg weight and in most of the traits considered except shell thickness in the three chicken genotypes and non-significant correlations were recorded between egg weight and albumen and yolk height in the normal-feathered and Tetra H chicken genotypes. These results agree with the values reported by (Bobbo et al., 2013) for the naked-neck genotype except for shell thickness which reported significant values. A significant positive correlation (p < 0.01) was found between egg weight and albumen and yolk weight in the three chicken genotypes compares favorably with the result reported by (Assefa et al., 2019; Kebede et al., 2019).
The positive and significant correlations observed between egg width and sell weight among the genetic group of chickens were in the same scenario as the observations of (Vekić et al., 2022). However, non-significant correlations were recorded between egg width and yolk and albumen height for the normal-feathered chicken genotype and agree with (Bobbo et al., 2013). For the normal-feathered and naked-neck chicken genotypes, albumen weight was significantly (p < 0.01) correlated with yolk weight, which was consistent with the findings of (Rath et al., 2015; Tongsiri et al., 2019). Albumen and yolk weight were significantly (p < 0.01) correlated with albumen and yolk height in the naked-neck genotype, and albumen height was significantly (p < 0.01) correlated with yolk height in the naked-neck and Tetra H chicken genotypes and the results are in the same scenario with the observations of several scholars (Haunshi et al., 2011; Rath et al., 2015). Thus, selecting a chicken genotype for increased egg weight will eventually result in increased weight of the various egg components.
Conclusion
This study found that the Tetra H chicken genotype performs better than the other chicken genotypes in terms of age at first egg and body weight, egg production, and egg mass. Except for yolk width and color, the Tetra H genotype was found to have significantly higher internal and external egg quality traits than the other genotypes. Likewise, the naked-neck chicken genotype was better than the normal-feathered chicken when considering the age of the first egg, body weight, and egg production, although they were not statistically different. The positive and significant correlations discovered between internal and external egg quality traits confirm that selecting chickens with larger eggs increases the portions of the edible eggs of chicken. Thus, more research is needed to evaluate the crossbreeding performances of the Tetra H with the naked neck and normal-feathered chicken genotypes to fully commercialize the Tetra H chicken and improve the indigenous genotypes for future selection and improvements.
Acknowledgments
The authors would like to thank Bonga University for providing a study leave for the first author’s Ph.D. study. We would also like to thank those professionals and attendants who participated during the data collection period.
Conflict of interest
The authors declare that they have no conflict of interest.
novelty statement
Efforts to enhance food security in Ethiopia have primarily focused on distributing exotic chicken genotypes, neglecting the potential of indigenous chicken genotypes. Performance and lack of reliable information hinder breeding and improvement of these indigenous genotypes, as well as the commercialization of the Tetra H genotype. Therefore, this study provides valuable insights for researchers and poultry producers, providing them with reliable evidence regarding the performance of these chicken genotypes.
Author contribution
Abiyu Tadele: Conceptualization, methodology/study design, data collection, analysis, writing original draft, review and editing; Gebreyohannes Berhane: Conceptualization; supervision; editing and modifying the manuscript; Wondmeneh Esatu: Conceptualization; supervision; editing and modifying the manuscript.
References
Abdurehman A., Urge M. (2016). Evaluation of fertility, hatchability, and egg quality of rural chicken in Gorogutu District, eastern Hararghe, Ethiopia. Asian J. Poult. Sci., 10(2): 111–116. https://doi.org/10.3923/ajpsaj.2016.111.116
Agarwal S., Prasad S., Kumar R., Naskar S., Chandra S. (2021). Evaluation of egg quality traits of indigenous chicken of Chotanagpur plateau of Jharkhand under the intensive system. 10(8): 637–639.
Ahmad S., Hussain J., Akram M., Aslam F., Mahmud A., Mehmood S., Usman M., Mustafa G. (2013). Original article Comparative study on productive performance and hatching traits of three age groups of indigenous Mushki Aseel chickens. 2(May): 146–149.
Alex O. (2001). Factors affecting controlling egg size. Poultry Specialist, Department of Agriculture and Marketing. Nova Scotia, Webmaster.
Almasi A., Suto Z., Budai Z. (2012). Effect of Age , Sex and Strain on Growth , Body Composition and Carcass Characteristics of Dual Purpose Type Chicken. Wpc, 47–50.
Amin M. R., Nawawi S. A. (2013). Behaviour of laying curve in Babcock-380 brown commercial layers in Kelantan, Malaysia. IOSR J. Agricult. Vet. Sci., 5(2): 77–82.
Anderson K.E., Tharrington J.B., Curtis P.A., Jones F.T. (2004). Shell charateristics of eggs from historic strains of single comb White Leghorn chickens. In Int. J. Poult. Sci. 3(1): 17–19).
Assefa H. (2019). The role of poultry for poor livelihoods in Ethiopia. 4(3); 1–4.
Assefa S., Melesse A., Banerjee S. (2019). Egg Production and Egg Quality Traits of Local and Exotic Chicken Breeds Reared in Two Agroecologies under Traditional Management System. Res. J. Food Nutrit., 3(1): 11. https://www.sryahwapublications.com/research-journal-of-food-and-nutrition/pdf/v3-i1/3.pdf
Bobbo A.G., Baba S.S., Yahaya M.S. (2013). Egg quality characteristics of three phenotypes of local chickens in Adamawa State. IOSR J. Agricult. Vet. Sci., 4(2): 13–21.
CSA. (2021). Agency Agricultural Sample Survey 2020 / 21 [ 2013 E . C .] report on livestock and livestock characteristics. II(February).
Duman M., Sekeroglu A., Yildirim A., Eleroglu H., Camci O. (2016). Relation between egg shape index and egg quality characteristics. Euro. Poult. Sci., 80(1): 1–9.
Edmew W., Melesse A., Beyan M., Taye M. (2018). Assessing the performance, egg quality, and carcass characteristics of indigenous chickens reared under traditional management system. Int. J. Res. Stud. Agricult. Sci. (IJRSAS). 4(10): 27–35. https://doi.org/10.20431/2454-6224.0410004
Farooq M., Mian M.A., Durrani F.R., Syed M. (2002). Egg production performance of commercial laying hens in Chakwal district, Pakistan. Livest. Res. Rural Develop., 14(2): 31–39.
Fereja G.B., Negari B., Urge M., Ameha N. (2016). the Study of Egg Quality on Chicken Village in Chelliya District Western Shoa, Ethiopia. Int. J. Res. -GRANTHAALAYAH., 4(2): 46–51. https://doi.org/10.29121/granthaalayah.v4.i2.2016.2811
Gerzilov V., Boncheva V., Petrov P. (2018). Egg production from dual purpose hen genotypes reared in a free range system. Bulgarian J. Agricult. Sci. 24(1): 119–125. https://www.nvi.com.et/wp-content/uploads/2017/11/NVI-Product-catalog-4-1.pdf.
Gide A., Zhou Yang, Wang, Muna L. N., Verifikasi S., Kayu L., Berbasis N., Manajemen U., Milik (2020). National Veterinary institute product catalogue. https://www.scribd.com/document/465095053/NVI-Product-catalog-4-1.pp.20-32.
Guni F.S., Mbaga S.H., Katule A.M., Goromela E.H. (2021). Performance evaluation of Kuroiler and Sasso chicken breeds reared under farmer management conditions in highland and lowland areas of Mvomero district, Eastern Tanzania. Trop. Anim. Health Prod., 53(2): 53–59. https://doi.org/10.1007/s11250-021-02693-4
Haunshi S., Niranjan M., Shanmugam M., Padhi M.K., Reddy M.R., Sunitha R., Rajkumar U., Panda A.K. (2011). Characterization of two Indian native chicken breeds for production, egg and semen quality, and welfare traits. Poult. Sci., 90(2): 314–320. https://doi.org/10.3382/ps.2010-01013
Herrero M., Havlík P., Valin H., Notenbaert A., Rufino M.C., Thornton P.K., Blümmel M., Weiss F., Grace D., Obersteiner M. (2013). Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proceed. National Acad. Sci. United States America., 110(52): 20888–20893. https://doi.org/10.1073/pnas.1308149110
Institute S.A.S. (2012). SAS/OR 9.3 User’s Guide: Mathematical Programming Examples. SAS institute.
Islam M.A., Das N.C., Nishibori M. (2022). Effect of extruded eggshell, limestone and oyster shell on egg production performance of laying hens. J. Agricult. Crop Res., 10(3): 43–51.
Islam M.A., Nishibori M. (2009). Indigenous naked neck chicken: A valuable genetic resource for Bangladesh. World’s Poult. Sci. J., 65(1): 125–138. https://doi.org/10.1017/S0043933909000010
Issn H. (2013). Egg Quality Traits of Indigenous and Exotic Chickens As Influenced By Specific Genes. 3(1): 53–58.
Kebede T., Betseha S., Melesse A. (2019). Assessment of Morphological, Egg Quality and Carcass Characteristics of Local and Exotic Chickens Reared in Two Districts of Metekel Zone Ethiopia. Sumerianz J. Agricult. Vet., 2(12): 164–171.
Kgwatalala P., Molapisi M., Thutwa K., Sekgopi B., Selemoge T., Nsoso S. (2016). Egg quality characteristics and phenotypic correlations among egg quality traits in the naked neck , normal and d .Int. J. Environ. Agricult. Res., 2(8): 96–105.
Kul S., Seker I. (2004). Phenotypic correlations between some external and internal egg quality traits in the Japanese quail (Coturnix coturnix japonica). Int. J. Poult. Sci., 3(6): 400–405.
Kumar M., Dahiya S.P., Ratwan P., Sheoran N., Kumar S., Kumar N. (2022). Assessment of egg quality and biochemical parameters of Aseel and Kadaknath indigenous chicken breeds of India under backyard poultry farming. Poult. Sci., 101(2): 101589. https://doi.org/10.1016/j.psj.2021.101589
Markos S., Belay B., Astatkie T. (2017a). Evaluation of egg quality traits of three indigenous chicken ecotypes kept under farmers’ management conditions. Int. J. Poult. Sci., 16(5): 180–188. https://doi.org/10.3923/ijps.2017.180.188
Markos S., Belay B., Dessie T. (2014). Village chicken production constraints and opportunities in western zone of Tigray, northern Ethiopia. J. Biol., Agricult. Healthcare., 4(27): 232–245. http://www.iiste.org/Journals/index.php/JBAH/article/view/18654/18930
Melesse A., Maak S., von Lengerken G. (2010). Effect of long-term heat stress on egg quality traits of Ethiopian naked neck chickens and their F1 crosses with Lohmann White and New Hampshire chicken breeds. Livest. Res. Rural Develop. 22(4).
Melesse A., Tadele A., Assefa H., Taye K., Kebede T., Taye M., Betsha S. (2021). Assessing the Morphological Diversity of Ethiopian Indigenous Chickens Using Multivariate Discriminant Analysis of Morphometric Traits for Sustainable Utilization and Conservation. Poult. Sci. J., 9(1): 61–72. https://doi.org/10.22069/psj.2021.18469.1644
Melesse A., Worku Z., Teklegiorgis Y. (2013). Assessment of the prevailing handling and quality of eggs from scavenging indigenous chickens reared in different agro-ecological zones of Ethiopia. J. Environ. Occupat. Sci., 2(1): 1. https://doi.org/10.5455/jeos.20130104091334
Negassa D., Melesse A., Banerjee S. (2014). Phenotypic characterization of indigenous chicken populations in Southeastern Oromia Regional State of Ethiopia. Anim. Genet. Resources/Ressour. Génétiques Animales/Recursos Genéticos Anim., 55: 101–113. https://doi.org/10.1017/s2078633614000319
Nguyen Van D., Moula N., Moyse E., Do Duc L., Vu Dinh T., Farnir F., Van D.N., Moula N., Moyse E., Duc L. Do, Dinh T.V., Farnir F. (2020). Productive performance and egg and meat quality of two indigenous poultry breeds in Vietnam, Ho and Dong Tao, fed on commercial feed. Animals., 10(3): 408. https://doi.org/10.3390/ani10030408
Nolte T., Jansen S., Weigend S., Moerlein D., Halle I., Simianer H., Sharifi A.R. (2021). Genotypic and dietary effects on egg quality of local chicken breeds and their crosses fed with faba beans. Animals, 11(7): 1–17. https://doi.org/10.3390/ani11071947
Rajaravindra K.S., Rajkumar U., Rekha K., Niranjan M., Reddy B.L.N., Chatterjee R.N. (2015). Evaluation of egg quality traits in a synthetic coloured broiler female line. J. Appl. Anim. Res., 43(1): 10–14. https://doi.org/10.1080/09712119.2014.883319
Rath P.K., Mishra P.K., Mallick B.K., Behura N.C. (2015b). Evaluation of different egg quality traits and interpretation of their mode of inheritance in White Leghorns. Vet. World., 8(4): 449–452. https://doi.org/10.14202/vetworld.2015.449-452
S. A. S. Institute, SAS/OR 9.3 User’s Guide: Mathematical Programming Examples, SAS institute, 2012.
Sreenivas D., Prakash M.G., Mahender M., Chatterjee R.N. (2013). Genetic analysis of egg quality traits in White Leghorn chicken. Vet. World., 6(5): 263.
Tadele A., Berhane G., Esatu W., Wassie T. (2023). Effect of genotype on hatchability, growth, morphometric and carcass traits of Chicken. J. Agricult. Food Res., 100531.
Tadele A., Melesse A., Taye M., A, T., A, M., M T. (2018). Phenotypic and morphological characterizations of indigenous chicken populations in Kaffa Zone, South-Western Ethiopia. Anim. Husband., Dairy Vet. Sci., 2(1): 1–9. https://doi.org/10.15761/ahdvs.1000128
Valavan S.E., Omprakash A.V, Bharatidhasan A. (2016). Prod. Performance Kadaknath Organized Poult. Farm. 125–128.
Vekić M., Savić Đ., Jotanović S. (2022). Phenotypic Correlations Between Egg Quality Traits Amid the Laying Phase of Broiler Breeder Hens. Contemp. Agricult., 71(1–2): 13–19. https://doi.org/10.2478/contagri-2022-0003
Woldegiorgiss W.E., Girma M., Dessie T., Cattle A., Project G. (2015). Comparative study on some egg quality traits of exotic chickens in different production systems in East Shewa, Ethiopia. December. https://doi.org/10.5897/AJAR2014.9373
Yonas K., Sandip B., Mestawet T. (2019). Some internal and external egg quality characteristics of local and exotic chickens reared in Yirgalem and Hawassa towns, Ethiopia. Int. J. Livest. Prod., 10(5): 135–142. https://doi.org/10.5897/ijlp2018.0547
To share on other social networks, click on any share button. What are these?






