Tannic Acid Regulates Autophagy in the Ovary of Female Brandt’s Voles Affecting their Reproduction
Tannic Acid Regulates Autophagy in the Ovary of Female Brandt’s Voles Affecting their Reproduction
Ming-Hao Yu, Ming-Hui Gu, Xin Dai, Dao-Chen Wang and Sheng-Mei Yang*
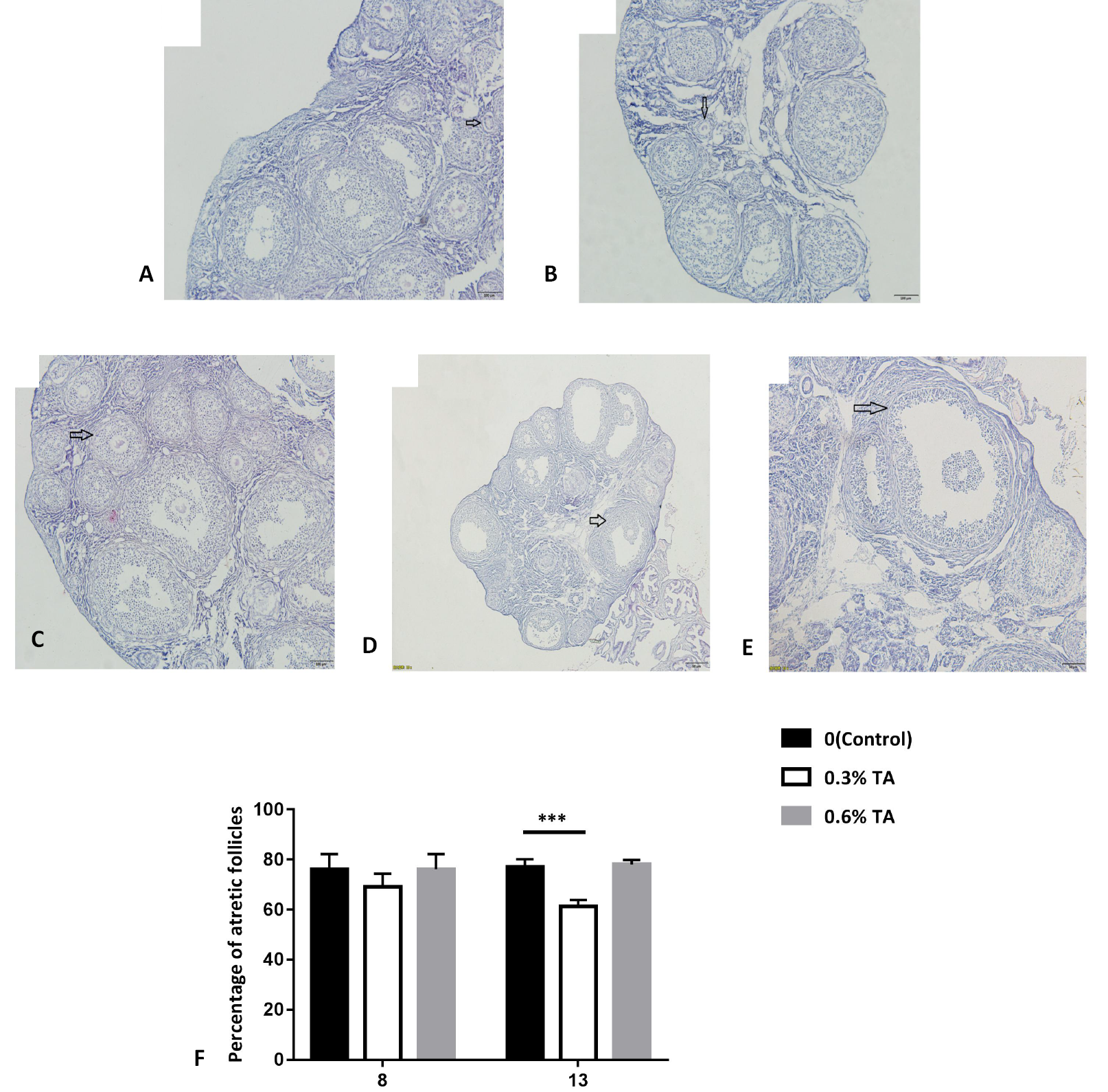
Histology of ovaries treated with different doses of tannic acid. Morphological classification of follicles. (A) primordial follicle (oocyte surrounded by a single layer of flattened granulosa cells), (B) primary follicle (oocyte surrounded by a single layer of cuboidal granulosa cells), (C) secondary follicle (oocyte surrounded by two or more layers of cuboid granulosa cells without a visible antrum) and (D) tertiary follicle (follicles with a clearly defined antral space and multiple layers of granulosa cells around the oocyte). (E) atretic follicle (identified as those follicles that appear to enter the degenerative process without ovulation. In atretic follicles, the oocytes shrink or are missing, and the granulosa cells are replaced by fibrous material). (F) Proportion of ovarian atresia follicles in Brandt’s voles treated with different doses of tannic acids (n=8). Data are expressed as the mean ± sem. ***P < 0.001.
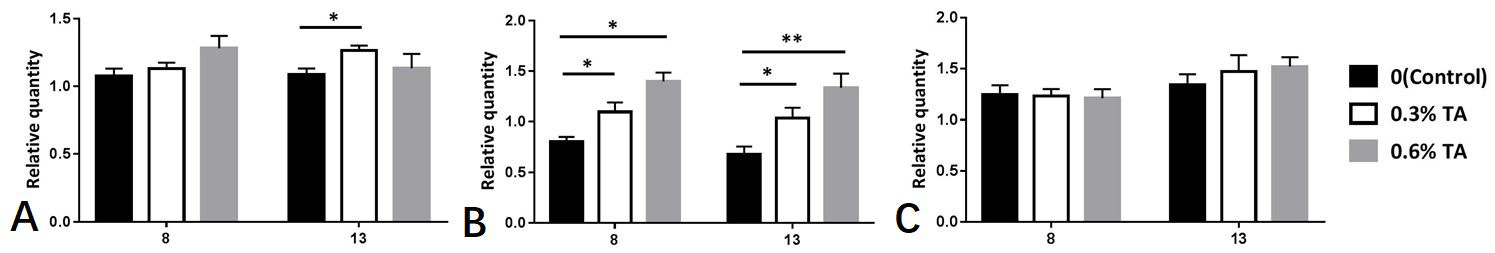
The relative abundance of mRNA of ovarian autophagy-related genes after tannic acid treatment. (A) the effect of different doses of tannic acid on the relative abundance of ovarian Beclinl gene mRNA(n=8), (B) the effect of different doses of tannic acid on the relative abundance of ovarian LC3 gene mRNA (n=8), (C) the effects of different doses of tannic acids on the relative abundance of ovarian P62 gene mRNA (n=8). Data are expressed as the mean ± sem. * P < 0.05, **P < 0.01.
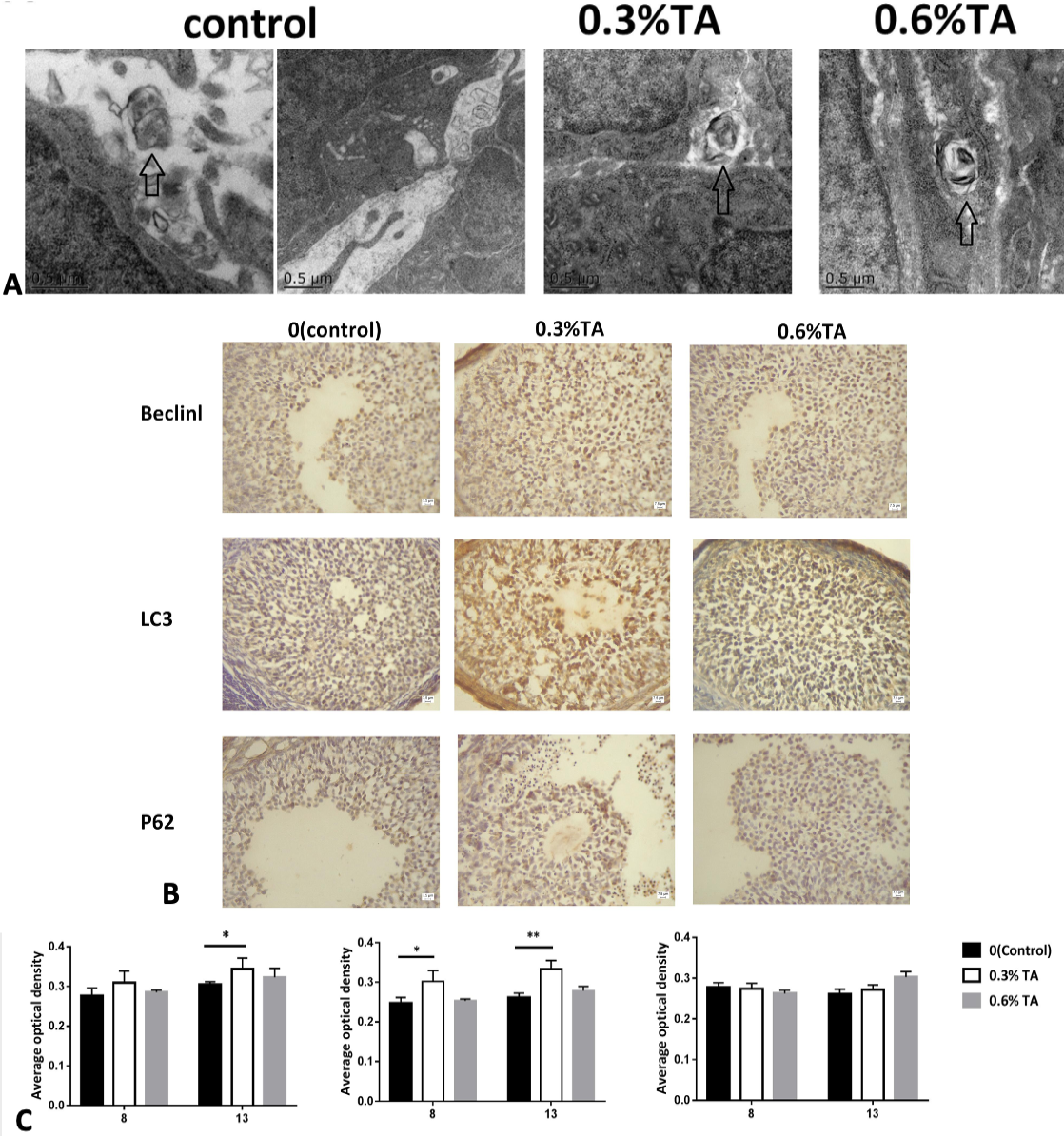
(A) Electron microscopy of the ovaries treated with different doses of tannic acids. The arrows represent autophagosomes. (B) Immunohistochemical staining of Beclin1 protein in the ovary. The immunostaining of the ovaries of the control animals was weaker. The ovaries in the adult low-dose group had stronger immunostaining. Immunohistochemical staining of LC3 protein in the ovary. The immunostaining of the ovaries of the control animals was weaker. Strong immunostaining of the ovaries in the low-dose and high-dose groups. In the ovaries, granulosa cells are strongly stained. Immunohistochemical staining of P62 protein in the ovary. There was no significant difference in immunostaining in each treatment group. (C) The effect of different doses of tannic acid on Beclinl, LC3 and P62 proteins during adolescence and sexual maturity (n=8). Data are expressed as the mean ± sem. * P < 0.05., **P < 0.01
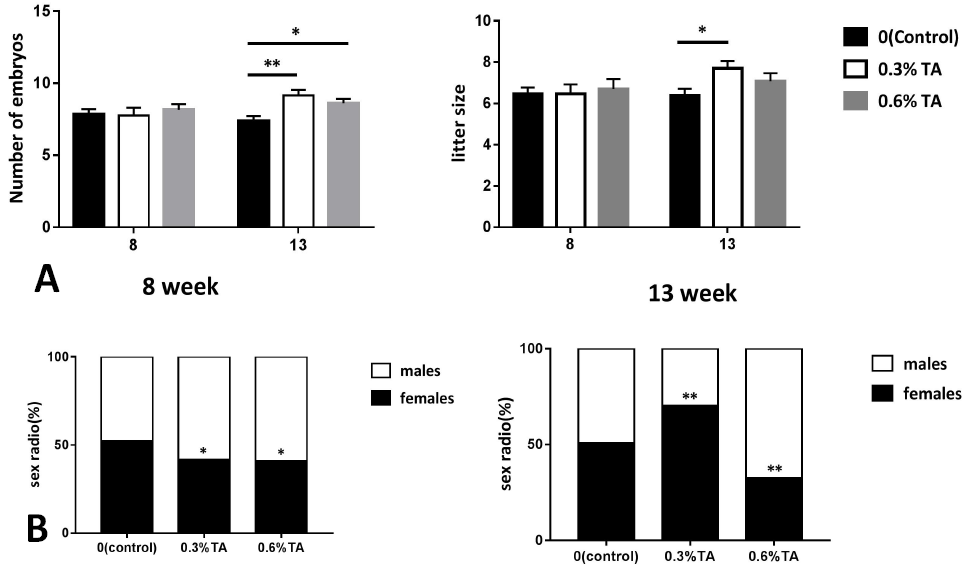
Effects of different doses of tannins on the reproduction of Brandt’s vole (A) The effect of embryo number and litter size of Brandt’s vole treated with different doses of tannin. (B) Female-male ratio of offspring of Brandt’s voles treated with different doses of tannic acid (n=13). Data are expressed as the mean ± sem. * P < 0.05, **P < 0.01.