Sustainable Maize Production by Organic Amendment – A Field Study from Ecuadorian Coast
Sustainable Maize Production by Organic Amendment – A Field Study from Ecuadorian Coast
José J. Cedeño-Díaz1, Caridad A. Torres-García1, Marina García1, Freddy Zambrano-Gavilanes1, Naga Raju Maddela2* and Felipe R. Garcés-Fiallos3*
1Universidad Técnica de Manabí, Facultad de Ingeniería Agronómica, Campus Experimental La Teodomira, Santa Ana EC130105, Ecuador; 2Departamento de Ciencias Biológicas, Facultad de Ciencias de la Salud, Universidad Técnica de Manabí, Portoviejo-130105, Ecuador; 3Laboratory of Phytopathology, Experimental Campus La Teodomira, Faculty of Agronomic Engineering, Technical University of Manabi, Santa Ana EC130105, Ecuador
Abstract | The study aimed to evaluate the effects of the application of organic fertilizers such as caster seed cake (Ricinus communis L.), physic nut seed cake (Jatropha curcas L.), and vermicompost on seedlings and adult plants of INIAP H-603 maize under semi- controlled and field conditions at the Pacific coastal Ecuador. Unfertilized and urea-fertilized plants were used as controls in both environments. Under the greenhouse conditions, different phytometric characteristics were evaluated at 21 days after sowing (DAS). In the field conditions, the incidence (%) and severity (%) of Curvularia leaf spot (Curvularia spp.), Diplodia leaf streak (Diplodia sp.), and leaf blight (Bipolaris sp. or Exserohilum sp.), and various phytometric and chemical parameters were evaluated at 63 DAS. Organic amendments did not show significant effects on maize seedlings under in vitro conditions; however, urea only improved the seedling height by 18% (P ≤ 0.01). Physic nut seed cake amendment resulted in the higher increment of chlorophyll (30%) and grain yield (231%) over the control. With respect to the disease resistance, there was a significant decrease of disease incidence (12.5-48%) and severity (34-46.5%) by physic nut seedcake. In addition, there was a strong positive correlation (r 0.869-0.925; P ≤ 0.01) found among the factors of MFC incidence and severity, and MFD incidence and severity. Above results clearly suggested that physic nut seed cake-based fertilizer has potential in improving the maize productivity through higher grain yield and lower disease susceptibility. Use of organic amendments for a sustainable maize cultivation is greatly warranted for improving the farmers economy and protecting the ecosystem in the Pacific Ecuador.
Received | May 21, 2024; Accepted | July 13, 2024; Published | October 30, 2024
*Correspondence | Dr. Naga Raju Maddela, Ph.D., Professor, Departamento de Ciencias Biológicas, Facultad de Ciencias de la Salud, Universidad Técnica de Manabí, Portoviejo-130105, Ecuador; Dr. Felipe R. Garcés-Fiallos, Ph.D., Professor, Facultad de Ingeniería Agronómica, Universidad Técnica de Manabí, Campus Experimental La Teodomira, Santa Ana EC130105, Ecuador; Email: raju.maddela@utm.edu.ec; felipe.garces@utm.edu.ec
Citation | Díaz, J.J.C., C.A.T. García., M. García., F.Z. Gavilanes., N. R. Maddela. and F.R. Garcés-Fiallos. 2024. Sustainable maize production by organic amendment – A field study from Ecuadorian coast. Sarhad Journal of Agriculture, 39(Special issue 2): 103-117.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39/s2.103.117
Keywords | Zea mays L., Ricinus communis L., Jatropha curcas L., Vermicompost, Organic fertilization, Foliar diseases, Grain yield
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The maize (Zea mays L.) crops have reached approximately 177 million hectares (ha) across the world, with an approximate production of 872 million tons at a yield of 49,194 kg ha–1 (FAOSTAT, 2012). The global corn area (for dry grain) is around 197 million ha, including sub-Saharan Africa, Asia and Latin America (FAOSTAT, 2021). Argentina, Brazil and Paraguay are the main corn producers in Latin America; farmers of these countries have increased the planted area due to strong export demand and high international prices (FAO, 2023). Maize is one of the main multipurpose crops, of human, animal and energy importance, and this crop is extremely susceptible to environmental disturbances and different factors that limit its production (Ramazan et al., 2022).
Diseases are one of the main phytosanitary problems that affect maize cultivation (Garcés-Fiallos et al., 2011). Principal fungal diseases that affect the leaf area of corn are Curvularia leaf spot (Curvularia spp.), Diplodia leaf streak (Stenocarpella maydis (Berk) Sutton syn. Diplodia maydis), northern leaf blight (Exserohilum turcicum (Pass.) KJ Leonard and Suggs), southern leaf blight (Bipolaris maydis (Y. Nisik and C. Miyake)) and rusts (Phakopsora zeae (Mains) Buriticá syn. Physopella zeae, Puccinia sorghi Schwein, P. polysora Underw) (Garcés-Fiallos et al., 2011; Vera-Avilés et al., 2013; Bermúdez-Cardona et al., 2015; Limongi -Andrade et al., 2018; Navarro et al., 2021; Rochi et al., 2018). Other diseases such as gray leaf spot (Cercospora zeae - maydis Tehon and EY Daniels) and tar spot (Phyllachora maydis Maubl.) can also affect the Gramineae family crops (Telenko et al., 2020).
Infection by foliar pathogens can alter the functioning of the photosynthetic system of plants (Bermúdez-Cardona et al., 2015). Some diseases in corn crops can affect the chlorophyll index (Frommer et al., 2019). Another important point related to photosynthetic pigments in plants is that they could depend on the content of nutrients, such as foliar N per unit of leaf area. Thus, N supply significantly prolongs the greening of leaves, even delaying their senescence (Fu et al., 2020). Currently, the management of foliar diseases is mainly based on the application of different fungicides and the cultivation of resistant varieties (Navarro et al., 2021). However, losses caused by diseases in cereal grain yield are more significant in conventional agroecosystems (mean of 25%) than in organic ones (mean of 13%) under both drought and humidity conditions (van Bruggen and Finckh, 2016).
Inorganic or organic plant nutrition are the main management measures in any crop. Inorganic fertilizers are easily absorbed by plants, but their long-term use can reduce the structural stability of the soil (Blanco-Canqui and Schlegel, 2013). On the contrary, organic fertilizers are more slowly absorbed by plants than inorganic ones, improving drainage, structure, air circulation and soil health, and promoting the presence and maintenance of beneficial microorganisms that help with the absorption of nutrients (Li et al., 2017; Ning et al., 2017; Assefa and Tadesse, 2019). Importantly, as a part of trophobiosis (Chaboussou, 1979), nutritionally balanced plants could defend themself against biotic factors such as diseases and pests.
Each nutrient in plants can modulate or activate different metabolic processes associated with the activity of redox enzymes and improve plant vigor indirectly by altering root exudates and changing the dynamics of the microflora, the nutrient content in the rhizosphere, the fluctuation of pH, lignin deposition and phytoalexin biosynthesis (Tripathi et al., 2022). Although balanced nutrition in corn is based on the application of variable doses of nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), sulfur (S) and zinc (Zn), the absence of N significantly reduces the grain yield in this crop (Aliyu et al., 2021). The nitrogen sources most used worldwide are those based mainly on ammonia (NH3), but these can be detrimental to an agroecosystem (Palma-Zambrano et al., 2022). Thus, excessive use of NH3 in a maize agroecosystem causes a hostile situation between its necessity and environmental conservation (Zheng et al. 2018). The use of organic nitrogen sources rich in ammonium (NH4) would be an efficient and environmentally friendly alternative (Palma-Zambrano et al., 2022).
Among the organic nitrogen sources, vermicompost and physic nut seedcake (Jatropha curcas L.) are found to be suitable options (Gomes et al., 2018; Palma-Zambrano et al., 2022). On the one hand, vermicompost is rich in N and P, with high levels of humic acid, has high microbial activity and plant growth regulators, and excellent stability and maturity. In addition to these, once vermicompost is applied to the soil, it biodegrades the organic matter and recovers valuable nutrients from various organic wastes (Joshi et al., 2015; Durán-Lara et al., 2020; Yatoo et al., 2021). Whereas, physic nut seedcake has a high nutrient content and a high percentage of microorganisms, favoring its use as fertilizer (Contran et al., 2013; Mbewe et al., 2018). Recently, it was found that the application of physic nut seedcake in cotton crop resulted in the lowered incidence and severity of Ramularia leaf spot and Cercospora leaf spot caused by Ramulariopsis pseudoglycines Videira Crous and Braun and Cercospora gossypina Cooke, respectively (Palma-Zambrano et al., 2022; Parrales-Rodríguez et al., 2024). Nonetheless, the effect of these nitrogenous or other organic sources on the anatomy of the corn crop, especially on diseases, has been little studied or documented.
The use of organic fertilizers from animal and plant waste is emerging as a sustainable alternative to traditional agriculture (Durán-Lara et al., 2020). Nutrition in corn based on organic nitrogenous sources instead of inorganic fertilizers, could constitute an important tool within sustainable agriculture. Thus, in the present investigation, the effect of the application of organic fertilizers made from castor bean cake (Ricinus communis L.), physic nut seedcake, and vermicompost on the intensity of leaf spots, and the morphoanatomical, physiological and productive characteristics of seedlings and adult plants of maize were investigated. The insights of this investigation have significant importance in improving the economy of maize farmers in the region of Ecuadorian Coast.
Materials and Methods
Location of experiments
The present research was carried out during the rainy season of 2020, at the experimental campus La Teodomira, Faculty of Agricultural Engineering, Technical University of Manabí, Ecuador (80º39’13’’W and 01º17’15’’S,), located at an altitude of 47 meters above sea level. The area has an annual rainfall of 682.5 mm, an annual heliophany of 1,354 light hours and an average temperature of 25.39 ºC.
Experiment in greenhouse conditions
Seeds of the INIAP H-603 hybrid were sown in 12-cell plastic germination trays containing approximately 40 cm3 of sterile peat (Novarbo from Biolan®) as a substrate. The seedlings were fertilized seven DAS during reproductive phenological stage 0.5 (two fully emerged leaves, Ritchie and Hanway, 1982) with different amendments, such as urea (0.10 g seedling–1), castor bean cake (0.5 and 0.9 g seedling–1), physic nut seedcake (0.6 and 1.3 g seedling–1), and vermicompost (0.5 and 0.9 g seedling–1), leaving a control without amendment. The seedlings were irrigated every three days. At 21 DAS during the vegetative phenological stage 1.5 (six fully emerged leaves, Ritchie and Hanway, 1982), emergence (%), height (cm), stem diameter (cm), fresh weight (g) and dry (g) of seedlings were determined. Also at that time, the chlorophyll index (SPAD units) was estimated in the leaf blade of two leaves located in the middle layer of the seedlings using a SPAD-502 chlorophyll meter (Minolta, Japan). In this experiment, a completely randomized design was used, with eight treatments and five repetitions (plants).
Experiment under field conditions
Seeds of the INIAP H-603 hybrid were sown (in February 2020) at 0.20 m between plants and 1.0 m between rows, in plots of 5×5 m (25 m2), totaling an area of 1160 m2 with 50,000 plants ha–1. In this investigation, a completely randomized block design was used, with eight treatments and four repetitions. Each of the treatments (nutritional sources) was prepared based on an average N dose of 200 kg ha–1 distributed in one (sowing) and two applications (during sowing and at 35 DAS during reproductive phenological stage 2.5 (tenth leaf fully emerged, Ritchie and Hanway, 1982) for each treatment as described in the Table 1.
The chemical characterization (concentrations of N, P and K) of different organic sources used in the present investigation were displayed in Figure 1. The weeds were managed throughout the cycle through four manual weeding. No synthetic herbicides or fungicides were used; thus, interference in the response of the evaluated components to the application of nitrogen fertilization was avoided.
Leaf spots evaluation
The incidence (%) and severity (%) of Curvularia leaf spot, Diplodia leaf streak, and leaf blight at 63 DAS during reproductive phenological stage 5 (emerged silks and shedding of pollen in spikes, Ritchie and Hanway, 1982) were evaluated. For this, three leaves in the four plants’ lower, middle, and upper strata were chosen randomly within the two central rows. Incidence percent was calculated using the following equation:
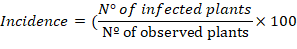
Each disease’s severity (%) was estimated through the percentage of the affected area in the leaves of plants where the incidence was quantified. Lesions larger than 2 mm were analyzed.
Estimation of leaf area and chlorophyll index
Leaf area was estimated by measuring the length and width of all the leaves of each plant, using a flex meter, and was calculated by following formula:

expressed in cm2 plant–1 (Francis et al., 1969). The chlorophyll index (SPAD) was estimated with the SPAD-502 chlorophyll meter. The leaf pigment was evaluated in the leaf opposite the female flower, at 63 DAS during reproductive phenological stage 5 (Ritchie and Hanway, 1982). Both variables were analyzed in eight plants chosen at random within the two central rows.
Anatomical analysis
At 63 DAS during reproductive phenological stage 5 (Ritchie and Hanway, 1982), sections (from the best treatments) of approximately 1 cm2 were obtained in the middle stratum of the lamina of the index leaf of three corn plants located in the central rows for each of the following treatments: urea, pine nut cake, castor cake and vermicompost with a single application and an untreated control. The sheet segments were washed with running water and fixed in FAA (formaldehyde-acetic acid-ethanol 70%), remaining in that solution until processing. To study the leaf epidermis, the sections were immersed in 10% hydrofluoric acid for 24 hours. After that time, the sections were washed with distilled water and kept in 1.75% NaClO for half an hour, finally proceeding to detach both epidermises.
To obtain cross sections, freehand cuts were made with a blade. The segments (four per repetition) were placed in distilled water and then in a Lugol’s solution for 5 min, for the subsequent detection of starch. Both cross sections and epidermis were stained with 0.5% aqueous toluidine blue and mounted on semi-permanent sheets in a water—glycerin (in a 7:3 ratio) solution. All montages were observed under a binocular optical microscope (Nikon E-200, Japan). To determine the density and length of stomata in both epidermis and the thickness of the tissues in the different microscopic sections, a micrometric eyepiece was used. The images obtained were examined and photographed with the help of a camera incorporated into the microscope and processed using the AmScope program.
In the maize sections obtained, the stomatal density (units mm2) and stomatal length (µm) were quantified in the adaxial and abaxial phases, as well as the thickness of the leaf tissue (µm). Chloroplasts and starch were analyzed in chlorophyllous parenchyma cells of the mesophyll and vascular sheath of leaf cross sections, as well as the accumulation of phenolic compounds in epidermal cells, fibers and xylem.
Grain yield quantification
At 150 DAS, when the plants reached reproductive phenological stage 10 (physiological maturity, Ritchie and Hanway, 1982), the ears were collected in the useful area of each plot to determine yield. These tissues were shelled with the help of a maize shelling machine, and subsequently their weight was adjusted to required humidity (i.e. 13%). Finally, the value obtained was used to determine the grain yield (kg ha–1).
Statistical analysis
For each experimental variable, the assumption was tested, verifying the normality of the data distribution and the homogeneity of the variances using the Kolmogorov-Smirnov and Bartlett tests, respectively. Subsequently, the data were subjected to an analysis of variance (ANOVA), and their means were compared by the test of Scott-Knott groupings (P ≤ 0.01). Also, most variables (except for morphoanatomical variables) were correlated using Pearson correlation analysis (P ≤ 0.05; P ≤ 0.01). All analyzes were carried out using the ASSISTAT statistical program version 7.7.
Results and Discussion
Experiment in greenhouse conditions
Only the height of seedlings was affected by fertilization, with a greater height (42.4 cm) observed in seedlings fertilized with urea, compared to the rest of the treated seedlings (Figure 2). The values of emergence, stem diameter, fresh and dry weight, and chlorophyll index in maize seedlings were found to be 71.25%, 35.5 cm, 2.85 mm, 41.37 g, 11.06 g and 29.46 SPAD units, respectively. It is well known that inorganic fertilizers are easily absorbed by plants (Blanco-Canqui and Schlegel, 2013), compared to organic fertilizers (Assefa and Tadesse, 2019). Evidently, the height of the maize seedlings could have been positively affected by the inorganic fertilizer at 21 DAS due to its rapid absorption by the roots.
Experiment under field conditions
Leaf spots intensity: Leaf spots responded differently to the application of nitrogen sources in corn plants (Figure 3). Both the incidence and severity of Curvularia leaf spot were on average 29% times lower in plants treated with physic nut seedcake (Figure 3A and B), compared to those treated or not with the other fertilizers. The incidence of Diplodia leaf streak (55% on average) and leaf blight (69% on average) was similar in plants treated or not with fertilizers (Figure 3C and E). However, Diplodia leaf streak severity was on average 32% negatively affected in all corn plants treated with any of the nitrogen sources compared to untreated plants (Figure 3D).
On the contrary, the severity of leaf blight was 25% on average negatively affected only in plants treated with urea and physic nut seedcake, compared to the other treatments and the untreated control (Figure 3F).
Host susceptibility to diseases depends on the supply and form of N (NO3 or NH4) and the type of phytopathogen (Dordas et al., 2008). On the other hand, the application of nitrogen fertilizers can influence the nutritional quality and/or the defense capacity of plants (Patrice et al., 2021; Tripathi et al., 2022; Virla et al. 2023), but differently as observed in the present investigation. Mineral nutrition can be affected by the plant—pathogen interaction (Patrice et al., 2021; Palma-Zambrano et al., 2022), and even by a tripartite interaction where biocontrol microorganisms also participate (Li et al., 2022). In fact, maize plants fertilized with nitrogen sources present lower intensity of stunting (Spiroplasma kunkelli Whitcomb), without affecting the natural control levels of its vector Dalbulus maidis (Virla et al. 2023). On the other hand, organic sources applied in maize cultivation resulted an increase in the abundance of biocontrollers (such as Aureobasidium and Mortierella) and a decrease of Gibberella (Li et al., 2022), and increased availability of P and K for plants, compared to synthetic sources (Li et al., 2022). There was also inhibition of phytopathogens in soil upon the application of physic nut seedcake (Dordas et al., 2008).
In organic agriculture, root diseases tend to be less aggravating than foliar diseases as observed in this work; this is attributed to the protective role of healthy soil (van Brugge and Finckh, 2016). The soil microbial community and the availability of nitrogen present in organic amendments (such as pine nut cake) play an important role in disease control and grain yield improvement (van Brugge and Finckh, 2016; Gomes et al., 2018). In the case of leaf blight, its severity in maize plants was also reduced by the physic nut seedcake. Conversely, the incidence and severity of Curvularia leaf spot and leaf blight could increase in maize plants fertilized with mineral fertilizers (Patrice et al., 2021). Although the contribution of N, P or K or simply urea could have a direct action on the penetration of pathogens into leaf tissues (Patrice et al., 2021; Tripathi et al., 2022), this was clarified in part by the results found in the histological analysis.
Leaf area, chlorophyll index and grain production: The leaf area did not vary between treatments, with values between 5303.0 and 6730.3 cm2, and an average of 5712.76 cm2 (Figure 4). The chlorophyll index ranged between 33.4 and 48.0 SPAD units. The chlorophyll index range was found to be 22 to 40 SPAD units in the seventh leaf of susceptible sweet maize plants infected or not by Ustilago maydis (DC.) Corda (Frommer et al. 2019), the causal agent of common smut. The quantum efficiency of the leaf per unit of incident radiation may depend on the leaf N content per unit of leaf area, being in turn related to the different levels of chlorophyll (Muchow and Sinclair 1994). Perhaps, the supply of N would significantly prolong leaf greening and delay senescence in maize plants treated with nitrogen sources, due to the efficiency of its remobilization in stem tissues, guaranteeing high levels of N in leaves and grains (Fu et al. al., 2020).
In the present investigation, chlorophyll index increased in the leaves of plants treated with physic nut seedcake 1 (227.25 g) during sowing, showing the maximum value (48.0 SPAD units) compared to the rest of the treatments. Whereas plants fertilized with urea (200 kg N ha–1) presented the second highest average chlorophyll with 42.0 SPAD units (Figure 4). Although chlorophyll levels may vary between susceptible maize plants infected or not by phytopathogens, or even be altered depending on the age of the leaf (Frommer et al., 2019). Nonetheless, in the present field investigation, there was evidence of a positive effect of fertilizers (physic nut seedcake 1 and urea) on the pigment.
Grain yield was higher in plants treated with physic nut seedcake 1 (227.25 g) during sowing, presenting an average of 6929.3 kg ha–1 compared to the rest of the treatments. The second highest yield of 5918.5 kg ha–1 was found in plants fertilized with physic nut seedcake 2 (2,043 g), but it was at 35 DAS (Figure 4). The application of nitrogen fertilizers increased the yield and dry biomass of the maize crop by an average of 20% (Nasar et al., 2021; Patrice et al., 2021). The grain production capacity of maize depends on the crop’s capacity to assimilate CO2 and its relationship with the efficiency of sunlight usage and foliar N content per unit of leaf area (Muchow and Sinclair 1994). As the leaf area was similar in all plants, the increase in grain yield observed in plants fertilized with physic nut seedcake was possibly due to the simultaneous increase in N concentration in plant tissues that include grains (Chen et al., 2015; Fu et al., 2020), the chlorophyll index (Frommer et al., 2019), and metabolites such as phenolic compounds and flavonoids (Fones and Gurr, 2015).
Morphoanatomical alterations: The qualitative anatomical characteristics of the leaf blade of plants treated or not with any of the treatments were similar, observing unistrata epidermis, amphiestomatic leaves with paracytic type stomata (Figure 5) and mesophyll with Kranz anatomy where long and small vascular bundles were observed (data not presented). These results were corroborated with the histological characterization of
maize described by Srivastava and Singh (1972).
However, differences were found in stomatal density (P = 0.001) and leaf tissue thickness (P ≤ 0.001). Only the length of stomata in both leaf phases was similar in all plants treated or not with any of the fertilizers (Table 1). On the other hand, both in the adaxial phase (66 stomata µm–2) and abaxial (93 stomata µm–2) the stomatal density increased in plants treated with physic nut cake, compared to those that received the rest of the organic fertilizers, the synthetic fertilizer and untreated ones (Table 2). The increase in stomatal density in the abaxial epidermis was 37% compared to the adaxial (Table 2). Thickness of leaf tissues was greater in plants fertilized with vermicompost (230.2 µm), followed by those treated with physic nut seedcake (215.2 µm), castor bean cake, urea and the untreated ones (Table 2). These findings suggest that fertilization with organic sources and especially with physic nut seedcake, has a positive impact on stomatal density, which in turn could improve the coordination between stomatal CO2 conductance and photosynthetic efficiency and grain yield (Muchow and Sinclair 1994; Frommer et al., 2019).
Regarding the thickness of the leaf blade, this was greater in plants fertilized with organic sources, with the greatest thickness corresponding to the treatment with vermicompost (230.2 µm), followed by physic nut seedcake and castor bean cake, while in plants treated with urea and not fertilized with any of the sources, the leaf thickness was similar (Table 2). Likewise, some changes were evident in the chlorophyllous parenchyma cells of the mesophyll and the vascular sheath (Figure 5). In the leaf mesophyll of unfertilized plants, there was observation of a lower number of chloroplasts in cells of both tissues and a low presence of starch grains in cells of the vascular sheath (Figures 5A and 5B). While in the maize crop fertilized with urea, both mesophyll cells and vascular sheath cells presented a greater presence of chloroplasts, and a higher content of starch grains was detected (Figure 5C). This response is like that observed in plants fertilized with vermicompost (Figure 5E). In plants fertilized with physic nut seedcake, the greatest number of chloroplasts and starch grains were detected (Figure 5D). Finally, in plants fertilized with castor bean cake, the presence of chloroplasts was scarce in both the mesophyll and vascular sheath cells, and the presence of starch in the latter tissue was also scarce (Figure 5F).
N deficiency caused adverse effect in a crop such as reduced grain yield; this effect is attributed to the movement or efficiency of N, photosynthetic characteristics, carbon-nitrogen balance, and carbohydrate dynamics in plant tissues (Ning et al., 2018; Gong et al., 2019; Fu et al., 2020). The greatest number of chloroplasts and starch grains were observed in the mesophyll of plants fertilized with physic nut seedcake, followed by those treated with urea and vermicompost, in contrast to those treated with castor cake or untreated control. Approximately 75% of the N in leaves is in chloroplasts, and most of this portion is
Table 1. Details of treatments
|
Treatment |
Composition |
|
Control |
Unfertilized plants |
|
Urea |
200 kg N ha–1 distributed on three occasions (20 kg N ha–1 at sowing, 80 kg N ha–1 in the vegetative phenological stage 1, and 100 kg N ha–1 in the reproductive phenological stage 2.5) |
|
Physic nut seedcake 1 |
One application (200 kg N ha–1 at sowing) |
|
Physic nut seedcake 2 |
200 kg N ha–1 distributed in two applications (100 kg N ha–1 at sowing and 100 kg N ha–1 at reproductive phenological stage 2.5). |
|
Castor bean cake 1 |
One application (200 kg N ha–1 at sowing) |
|
Castor bean cake 2 |
200 kg N ha–1 distributed in two applications (100 kg N ha–1 at sowing and 100 kg N ha–1 at reproductive phenological stage 2.5). |
|
Vermicompost 1 |
One application (200 kg N ha–1 at sowing). |
|
Vermicompost 2 |
200 kg N ha–1 distributed in two applications (100 kg N ha–1 at sowing and 100 kg N ha–1 at reproductive phenological stage 2.5). |
Table 2. Density (units mm–2) and stomatal length (µm) in the adaxial and abaxial epidermis, and thickness of the leaf blade (µm) in corn plants fertilized with three organic sources (castor bean cake, physic nut seedcake, and vermicompost), using a control with urea and another without fertilizer application. (CV Coefficient variation).
|
Treatment |
Adaxial epidermis |
Abaxial epidermis |
Leaf blade thickness (µm) |
||
|
Stomal density (units mm–2) |
Stomatal length (µm) |
Stomal density (units mm–2) |
Stomatal length (µm) |
||
|
Control (without treatment) |
44.8 c |
55.6 |
62.4 d |
41.1 |
167.1 d |
|
Urea (200 kg N ha–1) |
42.2 c |
47.8 |
69.3 c |
37.8 |
163.1 d |
|
Castor seed cake 1 (1 application) |
53.8 b |
47.8 |
84.9 b |
41.1 |
190.6 c |
|
Physic nut cake 1 (1 application) |
65.6 a |
48.9 |
93.2 a |
37.8 |
215.2 b |
|
Vermicompost 1 (1 application) |
51.8 b |
48.9 |
91.1 b |
43.3 |
230.2 a |
|
P-value |
0.001 |
0.080 |
0.001 |
0.251 |
≤0.001 |
|
CV |
812 |
13.35 |
8.34 |
15.17 |
6.66 |
used for the synthesis of organic components resulting from photosynthesis (Gong et al., 2019). Organic fertilization can significantly induce an increase in the proportion of chloroplasts and carbon assimilation and transport in leaves and developing reproductive tissues, as well as a higher abundance of transcripts of key genes involved in starch biosynthesis, leading to a higher content of chlorophyll and photosynthetic rate and yield (Wang et al., 2015; Ning et al., 2018). N is essential in plants because it is the main constituent of amino acids, which are the basic components of proteins, nucleotides, chlorophyll and many other metabolites or cellular components (Krapp, 2015; Gong et al., 2019).
Accumulation of phenolic compounds (blue and light blue colors) was observed in epidermal cell walls, fibers and xylem (Figure 5). However, the deposition of these secondary metabolites was more intense in leaf tissues of plants fertilized with physic nut seedcake, followed by those of plants treated with vermicompost (Figure 5E) and urea (Figure 5C). The tissues of plants fertilized with castor bean cake (Figure 5F) and unfertilized (Figure 5A and B) presented a faint intensity in the epidermal cells, fibers and xylem.
Plants exposed to non-optimal levels of N change their metabolism. Among the effects are the lower activity of some key enzymes in the metabolism of phenolic compounds, which therefore reduces the content of phenols and lignin, negatively affecting the plant defense system against infection (Dordas et al., 2008). The results regarding leaf anatomy observed in this research seem to indicate a positive effect on these secondary metabolites in leaf tissues of plants fertilized with physic nut seedcake, followed by those of plants treated with vermicompost and urea. Some organic fertilizers induce chemical and physical barriers in plants such as high concentration of polyphenols in the rhizodermis and silicon deposition in the endodermis (Dordas et al., 2008; Yatoo et al., 2021). In fact, NPK-based inorganic fertilizers reduce the concentration of phenolic compounds and flavonoids in leaves compared to those fertilized with organic sources (Rempelos et al., 2018). Although there is no solid evidence on the association of phenolic compounds and flavonoids with resistance against leaf spots in cereals such as wheat (Fones and Gurr, 2015), in the present investigation a possible association was found between the production of phenolic compounds in maize leaf tissues induced by organic sources, mainly physic nut seedcake.
Pearson correlation analysis showed several significant correlations (P ≤ 0.05 and P ≤ 0.01; Figure 6), described as follows. The incidence and severity of most foliar diseases were positively correlated with each other (R values between 0.763 and 0.925), except for the incidence of leaf blight, which was not correlated with any disease (R values between 0.063 and 0.327), not even with the severity of the same disease (R = 0.288). Though the correlation between the incidence and severity of diseases in plants have been investigated (Garcés-Fiallos and Forcelini, 2011), the correlation of intensity between two or more diseases is a novel approach and is greatly warranted to be investigated in the future.
Although diseases such as Stunting and Anthracnose of the stem are negatively correlated with grain yield in maize cultivation (Cota et al., 2012; da Costa et al., 2019), in the present investigation no correlation was found between any of foliar diseases with the SPAD chlorophyll index or grain yield (Figure 6). However, the SPAD chlorophyll index was positively correlated with grain yield (R=0.871; P ≤ 0.01). Perhaps, the N provided by fertilizers significantly prolonged leaf greening, delaying the senescence of maize plants (Fu et al., 2020). Nonetheless, a greater amount of this pigment in the leaves would result in a higher yield in the maize crop.
A separate discussion deserves the role of physic nut seedcake on the response to leaf spots, and the morphoanatomical, physiological and productive characteristics in maize cultivation. This source has N, P, K, Ca and Mg, as well as a high protein content that allows this residue to be used as fertilizer (Contran et al., 2013; Gomes et al., 2018; Palma-Zambrano et al., 2022; Parrales-Rodríguez et al., 2024). The application of physic nut seedcake could counteract the threat of low grain yield in maize during the dry season and limit the threat of heavy metal contamination in the soil (Hammed et al., 2019). Even the effect generated on grain yield in maize by this organic source may be higher when compared to inorganic nitrogen sources (Mbewe et al., 2018; AdeOluwaa et al., 2021). Physic nut seedcake as organic fertilizer can offer small and medium-sized farmers a better source of organic fertilizer with manageable residue rates compared to other organic sources (Mbewe et al., 2018; Palma-Zambrano et al., 2022). Despite its potential as a fertilizer in maize and other crops such as cotton (Palma-Zambrano et al., 2022; Parrales-Rodríguez et al., 2024) and cucumber (AdeOluwaa et al., 2021), the presence of phorbol esters should be studied in cultivated soils where it is applied physic nut seedcake (Contran et al., 2013).
Secondly, castor bean cake and vermicompost were found as potential organic fertilizers. Although the castor bean plant is a wild species that in recent years has become popular for the production of biodiesel and castor oil extracted from its seeds, the cake of this Euphorbiaceae is rich in microorganisms with biocontrol potential (Cabra-Cendales et al., 2015). The cake has N, P, K, Ca, Mg, Fe, Mn, Zn, Cu and B (Ferreira et al., 2018), and it is even known that castor essential oil has a biocidal effect on larvae and adults of Ulomoides dermestoides Fairmaire, 1893 (Coleoptera: Tenebrionidae ) (de la Cruz-Leytón et al., 2023 ). On the other hand, vermicompost is an excellent soil amendment and a biocontrol agent that makes it a potential organic fertilizer, which improves plant growth, chlorophyll content, qualitative and quantitative productive characteristics, among other parameters in different plant species (Joshi et al., 2015). Vermicompost, like other organic sources, has a diversity of microorganisms that induce different suppression mechanisms against phytopathogens in the soil (Yatoo et al., 2021).
Conclusions and Recommendations
The present study found differential and significant effects of nitrogen sources on different parameters of maize cultivation, both in greenhouse and field conditions. Increased plant height was seen only in seedlings fertilized with urea, compared to those treated or not with any of the organic sources. Physic nut seedcake reduced the incidence and severity of Curvularia leaf spot and the severity of leaf blight. Urea only negatively affected leaf blight. Diplodia leaf streak was decreased with the application of any of the nutritional sources.
Organic fertilization, mainly based on physic nut seedcake, caused an increase in the density of stomata in both epidermises, increased the thickness of the lamina and promoted the accumulation of lignin in the walls of the leaf tissues. Both the SPAD chlorophyll index and grain yield in maize increased with the application of physic nut cake. Therefore, the present study reinforces the importance of the real use of nutritional sources such as physic nut seedcake in the promotion of plant growth, response to foliar diseases, changes in leaf morphoanatomy, and potentializing the chlorophyll index and grain yield in maize plants.
Acknowledgments
Authors are grateful to Technical University of Manabi, for developing this research.
Novelty statement
This study offers new insights into using organic fertilizers, particularly phys-ic nut seedcake, to improve disease re-sistance and maize yield under field conditions in Pacific coastal Ecuador. In contrast to other organic amendments, physic nut seed cake markedly en-hanced chlorophyll content by 30% and increased grain production by 231% while concurrently diminishing the oc-currence and severity of leaf diseases, including Curvularia and Diplodia leaf streak, by 12.5% and 34-46.5%, respec-tively. Organic sources such as physic seedcake caused morphoanatomical changes in maize leaves, which allowed these tissues to defend themselves against pathogens that cause leaf spots. The findings underscore the viability of physic nut seedcake as a sustainable substitute for synthetic fertilizers, en-hancing maize yields and disease man-agement while fostering environmental conservation and economic sustainabil-ity for local farmers.
Authors contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JJC-D, FZG, and FRG-F. Conceptualization and research were worked by CAT-G, FZG, and FRG-F. Histological analysis was realized by MG. The first draft of the manuscript was written by NRM, and FRG-F and all authors reviewed previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict interest statement
There are no competing interests to declare in this work.
References
AdeOluwa, OO, A. Bello., A.A. Olowoake. and H.A. Akintoye. 2021. Potentials of Jatropha seed cake in soil fertility: A case study with cucumber (Cucumis sativa L.). Communications in Soil Science and Plant Analysis, 52: 1197-1206. https://doi.org/10.1080/00103624.2021.1885680
Akanmu, O., A. Sobowale., A. Abiala., J. Olawuyi. and C. Odebode. 2020. Efficacy of biochar in the management of Fusarium verticillioides Sacc. causing ear rot in Zea mays L. Biotechnology Reports, 26, e00474. https://doi.org/10.1016/j.btre.2020.e00474
Aliyu, T., J. Huising., Y. Kamara., M. Jibrin., B.I. Mohammed., G. Nziguheba., A.M. Adam. and B. Vanlauwe. 2021. Understanding nutrient imbalances in maize (Zea mays L.) using the diagnosis and recommendation integrated system (DRIS) approach in the maize belt of Nigeria. Scientific Reports, 11, 16018. https://doi.org/10.1038/s41598-021-95172-7
Assefa, S., S. Tadesse. 2019. The main role of organic fertilizer on soil properties and agricultural productivity-a review. Agric. Res. Technol.: Open Access J., 22 (2): 556192. https://doi.org/10.19080/ARTOAJ.2019.22.556192
Bermúdez-Cardona, M.B, J.M. Wordell Filho., F.A. Rodrigues. 2015. Leaf gas exchange and chlorophyll a fluorescence in maize leaves infected with Stenocarpella macrospora. Phytopatholog, 105 (1): 26–34. https://doi.org/10.1094/PHYTO-04-14-0096-R
Blanco-Canqui, H., and A. Schlegel. 2013. Implications of inorganic fertilization of irrigated corn on soil properties: lessons learned after 50 years. J. Environ. Qual., 42: 861-71. https://doi.org/10.2134/jeq2012.0451
Cabra-Cendales, T., D.C. Meneses-Cabezas. and N.F. Galeano-Vanegas. 2015. Identification of microorganisms associated with castor (Ricinus) Residues communis. Colomb. J. Chem., 44 (2): 10-15. https://doi.org/10.15446/rev.colomb.quim.v44n2.55214
Chaboussou, F. 1979. Recherches sur les facteurs de pullulation des acariens phytophages of the vineyard in the suite of traits pesticides du feuillage. INRA Report, pp. 36
Chen, Y., Xiao, C., Wu, D., Xia, T., Chen, Q., Chen, F., Yuan, L. and G. Mi. 2015. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron., 62: 79-89. https://doi.org/10.1016/j.eja.2014.09.008
Contran, N., L. Chessa., M. Lubino., D. Bellavite., P.P. Roggero. and G. Enne. 2013. State-of-the-art of the Jatropha curcas productive chain: from sowing to biodiesel and by-products. Industrial Crops and Products, 42: 202-215. https://doi.org/10.1016/j.indcrop.2012.05.037
Cota, LV, R.V. da Costa., D.D. Silva., C.R. Casela. And D.F. Parreira. 2012. Quantification of yield losses due to anthracnose stalk rot on corn in Brazilian conditions. J. Phytopathol., 160: 680–684. https://doi.org/10.1111/jph.12008
Da Costa, RV, D.D. da Silva., L.V. Cota., L.J.M. Campos., R.E.M. de Almeida. and F.P. Bernardes. (2019). Incidence of corn stunt disease in off-season corn hybrids in different sowing seasons. Pesquisa Agropecuária Brasileira, 54, e00872. https://doi.org/10.1590/S1678-3921.pab2019.v54.00872
De la Cruz- Leytón, C., C.L. Romero-Cabello., C. Lozano-Lévano. and A. Zavaleta-Rengifo. 2023. Biocidal effect of castor seed extract (Ricinus communis) in Ulomoides dermestoides (Coleoptera : Tenebrionidae). Biotempo, 20 (2). https://doi.org/10.31381/biotempo.v20i2.5965
Dordas, C. 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agronomy for Sustainable Development, 28: 33-46. https://doi.org/10.1051/agro:2007051
Durán-Lara, EF, A. Valderrama. and A. Marican. 2020. Natural organic compounds for application in organic farming. Agriculture, 10 (2): 41. https://doi.org/10.3390/agriculture10020041
FAO. 2023. Crop prospects and food situation. World Quarterly Report No March 1, 2023. Rome. https://doi.org/10.4060/cc4665es
FAOSTAT. 2012. Agricultural Data. FAO, Rome, Italy. http://faostat.fao.org
FAOSTAT. 2021. FAO Stat. FAO, Rome. http://www.fao.org/faostat
Ferreira, L.V., C. Cocco, D. Finkenauer, L. Picolotto and L.E.C. Antunes. 2018. Adubação com torta de mamona sobre o crescimento e produção da amoreira-preta. Revista Cultura Agronômica, 27: 34-43. https://doi.org/10.32929/2446-8355.2018v27n1p34-43
Fones, H. and S. Gurr. 2015. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genetics and Biology, 79: 3-7. https://doi.org/10.1016/j.fgb.2015.04.004
Francis, C.A., J.N. Rutger, and A.F.E. Palmer. 1969. A rapid method for plant leaf area estimation in maize (Zea mays L.)1. Crop Sci., 9: 537-539. https://doi.org/10.2135/cropsci1969.0011183X000900050005x
Frommer, D., L. Radócz. and S. Veres. 2019. Changes of relative chlorophyll content in sweet corn leaves of different ages infected by corn smut. Agriculturae Conspectus Scientificus, 84 (2): 189-192.
Fu, W., Y. Wang., Y. Ye., S. Zhen., B. Zhou., Y. Wang., Y. Hu., Y. Zhao. and Y. Huang. 2020. Grain yields and nitrogen use efficiencies in different types of stay-green maize in response to nitrogen fertilizer. Plants, 9: 474. https://doi.org/10.3390/plants9040474
Garcés-Fiallos, F.R. and C.A. Forcelini. 2011. Relationship between incidence and severity of Asian soybean rust caused by Phakopsora pachyrhizi Sydow and Sydow. Revista Facultad Nacional de Agronomía, 64 (2): 6105-6110.
Garcés-Fiallos, F.R., Á.J. Aguirre-Calderón., G.A. Liuba-Delfini. and J.J. Carbo -Morán. 2012. Curvularia severity in 67 self-fertilized S4 lines of yellow maize. Ciencia y Tecnología, 4 (2): 39-44. https://doi.org/10.18779/cyt.v4i2.107
Gomes, TG, I.I.A.H. Sámed., G.S.C. Alves., S. Mendonça., F.G. De Siqueira. and R.N.G. Miller. 2018. Current strategies for the detoxification of Jatropha curcas seed cake: a review. J. Agric. Food Chem., 66 (11): 2510-2522. https://doi.org/10.1021/acs.jafc.7b05691
Gong, X., J. Li., H. Ma., G. Chen., K. Dang., P. Yang., M. Wang. and B. Feng. 2019. Nitrogen deficiency induced a decrease in grain yield related to photosynthetic characteristics, carbon–nitrogen balance and nitrogen use efficiency in proso millet (Panicum miliaceum L.), Archives of Agronomy and Soil Science, 66, 3: 398-413. https://doi.org/10.1080/03650340.2019.1619077
Guazzelli, M., L. Meirelles., R. Barreto., A. Goncalves., C. Motter. and L. Rupp. 2007. The theory of trophobiosis in pest and disease control. J. Biodyn. Agric. Austral., 76: 51-54.
Hammed, TB, E.O. Oloruntoba. and G.R.E.E. Ana. 2019. Enhancing growth and yield of crops with nutrient-enriched organic fertilizer at wet and dry seasons in ensuring climate-smart agriculture. Inter. J. Recyc. Org. Waste Agric., 8 (Suppl 1): 81-92. https://doi.org/10.1007/s40093-019-0274-6
Joshi, R., J. Singh. and A.P. Vig. 2015. Vermicompost as an effective organic fertilizer and biocontrol agent: effect on growth, yield and quality of plants. Rev. Environ. Sci. Bio/Technol., 14: 137-159. https://doi.org/10.1007/s11157-014-9347-1
Krapp, A. 2015. Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Current Opinion in Plant Biology, 25: 115-22. https://doi.org/10.1016/j.pbi.2015.05.010
Li, F., Chen, L., J. Zhang., J. Yin. and S. Huang. 2017. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Frontiers in Microbiology, 8: 187. https://doi.org/10.3389/fmicb.2017.00187
Li, X., Q. Lu., D. Li., D. Wang., X. Ren., J. Yan., T. Ahmed. and B. Li. 2022. Effects of two kinds of commercial organic fertilizers on growth and rhizosphere soil properties of corn on new reclamation land. Plants, 11: 2553. https://doi.org/10.3390/plants11192553
Limongi -Andrade, R., D. Alarcón-Cobeña., E. Zambrano-Zambrano., M. Caicedo., P. Villavicencio- Linzan., J. Eguez., B. Navarrete., C. Yanez. and J.L. Zambrano. 2018. Development of a new maize hybrid for the Ecuadorian lowland. Agronomía Colombiana, 36 (2): 174-179. https://doi.org/10.15446/agron.colomb.v36n2.68782
Mbewe, E.C., P.C. Nalivata., W. Makumba. and V.H. Kabambe. 2018. The efficacy of jatropha (Jatropha curcas L.) seed cake as an organic fertilizer. Afric. J. Agric. Res., 13 (51): 2889-2897. https://doi.org/10.5897/AJAR2018.13657
Muchow, RC. and T.R. Sinclair. 1994. Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum. Crop Science, 34: 721-727. https://doi.org/10.2135/cropsci1994.0011183X003400030022x
Mur, A., C. Simpson., A. Kumari., K. Gupta. and J. Gupta. 2017. Moving nitrogen to the center of plant defense against pathogens. Annals of Botany, 119: 703-709. https://doi.org/10.1093/aob/mcw179
Nasar, J., W. Khan., M.Z. Khan., H.I. Gitari., J.F. Gbolayori., A.A. Moussa., A. Mandozai., N. Rizwan., G. Anwari. and S.M. Maroof. 2021. Photosynthetic activities and photosynthetic nitrogen use efficiency of maize crop under different planting patterns and nitrogen fertilization. J. Soil Sci. S.M. Plant Nutr, 21: 2274-2284. https://doi.org/10.1007/s42729-021-00520-1
Navarro, BL, L. Ramos Romero., M.B. Kistner. J. Iglesias. and A. von Tiedemann. 2021. Assessment of physiological roots of Exserohilum turcicum isolates from maize in Argentina and Brazil. Tropical Plant Pathology, 46: 371-380. https://doi.org/10.1007/s40858-020-00417-x
Ning, C., P. Gao., B. Wang., W. Lin., N. Jiang. and K. Cai. 2017. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric., 16: 1819-1831. https://doi.org/10.1016/S2095-3119(16)61476-4
Ning, P., P. Yunfeng. and F.B. Fritschi. 2018. Carbohydrate dynamics in maize leaves and developing ears in response to nitrogen application. Agronomy, 8 (12): 302. https://doi.org/10.3390/agronomy8120302
Palma -Zambrano, OJ, F. Zambrano-Gavilanes., D. Portalanza. and F.R. Garcés-Fiallos. 2022. Ramularia leaf spot and Boll rot are affected differently by organic and inorganic nitrogen fertilization in cotton plants. Biosci. J., 38: e38077. https://doi.org/10.14393/BJ-v38n0a2022-61479
Parrales-Rodríguez VF, F. Zambrano-Gavilanes., D. Portalanza. and F.R. Garcés-Fiallos. 2024. Cercospora leaf spot management with nitrogenous fertilizers in cotton is dependent on the disease amount in the plant canopies. Scientia Agropecuaria, 15 (2): 279-288. https://doi.org/10.17268/sci.agropecu.2024.021
Patrice N.D.J, B. Sabine., D. Bouba., G.N. Ntsefong., G. Lorandine., T.T.D. Brice., B. Taoga., P. Kosma. and A. Zachee. 2021. Impact of crop rotation and fertilizers (chemical, organic and bio) on diseases and yield of maize (Zea mays) in far North Cameroon. J. Agric. Crop Res., 9 (2): 34-39. https://doi.org/10.33495/jacr_v9i2.20.215
Ramazan, S., I. Nazir., W. Yousuf. and R. John. 2022. Tolerance to environmental stress in maize (Zea mays): role of polyamine metabolism. Functional Plant Biology, 50 (2): 85-96. https://doi.org/10.1071/FP21324
Rempelos, L., A.M. Almuayrifi., M. Baranski., C. Tetard -Jones., M. Eyre., P. Shotton., I. Cakmak., L. Ozturk., J. Cooper., N. Volakakis., C. Schmidt., E. Sufar., J. Wang., A. Wilkinson., E.A.S. Rosa., B. Zhao., T.J. Rose., C. Leifert. and P. Bilsborrow. 2018. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem., 66 (40): 10369-10379. https://doi.org/10.1021/acs.jafc.8b02626
Ritchie, S.W. and J.J. Hanway. 1982. How a Corn Plant Develops. Special Report No. 48, Iowa State University of Science and Technology, Cooperative Extension Service.
Rochi, L, M.J. Diéguez., G. Burguener., M.A. Darino., M.F. Pergolesi., L.R. Ingala., A.R. Cuyeu., A. Turjanski., E.D. Kreff.and F.Sacco. 2018. Characterization and comparative analysis of the genome of Puccinia sorghi Schwein, the causal agent of maize common rust. Fung. Genet. Biol, 112: 31-39. Fung. Genet. Biol. 2019. Jul; 128:74. https://doi.org/10.1016/j.fgb.2018.12.009
Srivastava, L.M. and S.S. Singh. 1972. Stomatal structure in the corn leaves. J. Ultrastruc. Res., 39: 345-363. https://doi.org/10.1016/S0022-5320(72)90028-7
Telenko, E., D. Ravellette. and A. Wise. 2020. Assessing late vegetative and tasseling fungicide application timings on foliar disease and yield in Indiana corn. Plant Health Progress, 21 (4): 224-229. https://doi.org/10.1094/PHP-03-20-0022-RS
Tripathi, R., R. Tewari., K.P. Singh., C. Keswani., T. Minkina., A.K. Srivastava., U. De Corato. and E. Sansinenea. 2022. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Frontiers in Plant Science, 13: 883970. https://doi.org/10.3389/fpls.2022.883970
van Bruggen, A.H.C. and M.R. Finckh. 2016. Plant diseases and management approaches in organic farming systems. Annual Review of Phytopathology, 54: 25-54. https://doi.org/10.1146/annurev-phyto-080615-100123
Vera-Avilés, D., G. Liuba-Delfini., L. Godoy-Montiel., E. Díaz-Ocampo., F. Sabando-Ávila., F.R. Garcés-Fiallos. and G. Meza- Bone. 2013. Stability analysis for the yield of corn hybrids (Zea mays) in the Central Region of the Ecuadorian Coast. Scientia Agropecuaria, 4 (3): 211 - 218. https://doi.org/10.17268/sci.agropecu.2013.03.07
Virla, EG, E.B.L. Albarracín., C. Díaz., G.A. Van Nieuwenhove., F.D. Fernández., M.V.Coll Aráoz., N. Melchert., L.R. Conci. and M.P. Giménez Pecci. 2023. Bottom-up effect of nitrogen fertilization on the density of the corn leafhopper and its impact on both disease incidence and natural parasitism. J. Pest Sci., 96: 93-104. https://doi.org/10.1007/s10340-022-01500-9
Xuan, W., T. Beeckman. and G. Xu. 2017. Plant nitrogen nutrition: Sensing and signaling. Current Opinion in Plant Biology, 39: 57-65. https://doi.org/10.1016/j.pbi.2017.05.010
Yatoo, AM, M.N. Ali., Z.A. Baba. and B. Hassan. 2021. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agronomy for Sustainable Development, 41: 7. https://doi.org/10.1007/s13593-020-00657-w
Wang, TM., W. Chen., W.J. Pan., J. Yao., K.S. Wei., B. Chen., C. Liu., B. Dong., X.F. Zong. and S.G. Wang. 2015. Differences of leaf gas exchange traits, chlorophyll fluorescence characteristics and chloroplast ultrastructure of Nicotiana tabacum L.K326 under organic fertilization and chemical fertilization. J. Plant Nutr. Fert., 21 (2): 517-526. https://doi.org/10.11674/zwyf.2015.0228
Zheng, J., M.M. Kilasara., W.N. Mmari. and S. Funakawa. 2018. Ammonia volatilization following urea application at maize fields in the East African highlands with different soil properties. Biology and Fertility of Soils, 54: 411-422. https://doi.org/10.1007/s00374-018-1270-0
To share on other social networks, click on any share button. What are these?







