Sulfate Reducing Bacterial Corrosion of Mild Steel in Liquid and Solid Media
Sulfate Reducing Bacterial Corrosion of Mild Steel in Liquid and Solid Media
Sanaullah Sattar1, Ali Hussain1,2*, Javed Iqbal Qazi2, Arshad Javid1 and
Shahid Mehmood1
1Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Outfall Road, Lahore, Pakistan
2Institute of Zoology, University of the Punjab, Quaid-i-Azam Campus, Lahore, Pakistan
3Department of Poultry Production, University of Veterinary and Animal Sciences, Outfall Road, Lahore, Pakistan
ABSTRACT
This study was carried out to check the corrosive impact of sulfate-reducing bacteria (SRB) on mild steel coupons in solid and liquid media under two variable nutritional conditions. The bacterial species for the experimental trials was isolated from buried corroded metallic installment and found motile, Gram-negative, non-spore former and identified by 16S rRNA gene sequencing as Desulfovibrio desulfuricans. The corrosive impact of SRB on steel coupons was performed in water as liquid medium and the processed clay as the solid medium without and with the provision of Postgate B medium. After 60 days of anaerobic incubation, corrosion rate (CR) and average percent weight loss (APWL) of the steel pieces were calculated. Higher CR and APWL were observed in the liquid medium (water) than in the solid medium (clay). The CR and APWL in water were 249 ± 2 µg dm−2 d−1 and 4.16 ± 0.25 %, respectively, while the corresponding figures for CR and APWL in clay were 108 ± 2 and 1.88 ± 0.29, respectively. In addition, the CR and APWL were higher in the presence of Postgate B medium compared to the medium without it. X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopic analyses reveals the presence of FeS in the corrosion product. Our findings of this study will be helpful for devising corrosion-protecting strategies.
Article Information
Received 13 July 2022
Revised 06 September 2022
Accepted 08 October 2022
Available online 01 March 2023
(early access)
Published 26 April 2024
Authors’ Contribution
SS methodology. AH supervision. JIQ co-supervision. AJ data analysis. SM data compilation.
Key words
Anaerobic bacteria, Biodeterioration, Corrosive metabolites, Metal corrosion, Sulfidogenic bacteria
DOI: https://dx.doi.org/10.17582/journal.pjz/20220713160735
* Corresponding author: ali.zool@pu.edu.pk, ali.hussain@uvas.edu.pk
0030-9923/2024/0003-1351 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Metals and their alloys are very important for the construction of buildings and manufacturing of different useful equipment but varying environmental conditions are posing big challenges to metals’ stability and becoming the major cause of metallic corrosion (Videla and Herrera, 2005; Ferreira et al., 2007). Microbiologically influenced corrosion (MIC), in this context, is a very complex phenomenon involving interaction between metals, microorganisms, and their environmental conditions. In different environments, MIC deteriorates metals with the involvement of oxidation and reduction reactions (Beech and Sunner, 2004; Chugh et al., 2022). In oxidation and reduction reactions, one metal donates electrons, and the other metal accepts electrons and drives the corrosion process. The flow of electrons between these two metal sites determines the CR and severity of metals’ damage (Kaesche, 2003; Enning and Garrelfs, 2014).
The estimated damage cost due to MIC is in billion dollars per year (Thompon et al., 2007). In many industries including the gas and oil industry, MIC is a major challenge (Li et al., 2016; Okoro et al., 2016; Liu et al., 2017). A wide variety of microorganisms are involved in the corrosion of metals including bacteria and fungi (Liu et al., 2015). Fungi are the abundantly available eukaryotic microorganisms causing metallic corrosion (Qu et al., 2017; Kadaifçiler et al., 2022; Zhang et al., 2022b). Fungi have the potential to produce organic acids and other corrosive metabolites. These metabolites and organic acids play a crucial role in the corrosion of metals and their alloys (Little and Lee, 2007). Among prokaryotic microbes, acid-producing, iron-oxidizing, sulfate and nitrate-reducing bacterial groups are considered the major contributors causing MIC by colonization on buried gas and oil pipelines (Dang and Lovell, 2015; Qian and Cheng, 2017; Lv et al., 2019). Acid-producing bacteria (APB) produce predominately organic acids that cause corrosion of metallic installations (Juzeliunas et al., 2007; Qu et al., 2017; Madirisha et al., 2022). APB can survive under anaerobic environments and initiate the corrosion process (Gu et al., 2018). Nitrate-reducing bacteria such as Bacillus licheniformis and Pseudomonas aeruinosa are also considered the causative agents of MIC (Xu et al., 2013). Iron-oxidizing and sulfate-reducing bacteria (SRB) are the key bacterial groups causing corrosion of materials under aerobic and anaerobic conditions, respectively (Lv et al., 2019). However, among all the corrosion-causing bacterial groups, SRB are considered the key culprits due to their widespread distribution in anoxic environments of aquatic ecosystems and the frequent availability of sulfate there (Hussain et al., 2016; Lv and Du, 2018). Anoxic environments may contain sulfate concentrations up to 28 mM (Pester et al., 2012). SRB consume sulfate as their terminal electron acceptors and generate corrosive sulfide as well as organic acids that cause localized pitting corrosion of metals (Hussain et al., 2016; Zhang et al., 2022a). Various SRB genera (Desulfovibrio, Desulfobulbus, Desulfococcus, Desulfonema and Desulfotomaculum) have been reported to play a vital role in corrosion (Lv and Du, 2018). Keeping in view the wide-ranged environmental distribution and corrosion-causing potential of SRB, the present study was designed for studying the corrosive impact of pure cultured Desulfovibrio desulfuricans on mild steel in water and clay.
MATERIALS AND METHODS
Isolation and molecular identification of SRB
The SRB species was isolated from a wastewater stream (Hudiara Drain) flowing through Mohlanwal, Lahore, Pakistan metallic installations. For the purpose, water (about 100 mL) was collected in a sterile glass container and transported to the laboratory for the isolation of SRB. The bacteria were isolated in deep agar by the dilution to extinction method as described by Postgate (1984) using the Postgate B medium (concentration in g L−1: CaSO4, 1.0; FeSO4.7H2O, 0.5; KH2PO4, 0.5; MgSO4.7H2SO4, 2.0; NH4Cl, 1.0; Ascorbic acid, 0.1; Sodium lactate, 3.5; Thioglycolic acid, 0.1; yeast extract, 1.0).
The bacterial pure culture was characterized phenotypically by motility detection, Gram’s, and endospore staining. The bacterial strain was then identified at the molecular level by 16S rRNA gene sequencing. For the purpose, SRB growth was revived in the Postgate B medium. Total genomic DNA from freshly grown bacterial cells was extracted and amplified following Hussain et al. (2014) using universal primers [27f (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492r (5′-GGTTACCTTGTTACGACTT-3′)]. The amplified gene was then got sequenced through Macrogen, Korea. Neighbor-joining phylogenetic tree of the bacteria isolated in the present study was constructed along with reference sequences recovered from the NCBI GenBank database.
Collection and processing of clay
Clay was collected from the vicinity of UVAS, Ravi Campus, Pattoki and processed in the laboratory for the experimental trial. The collected clay fraction was dried completely in an electric oven (Bioevopeak, DON-18E) at 105 oC till the achievement of constant weight and then sieved to obtain its fine powder (particle size ~ 1 mm). The sieved clay was stored in clean, dry, and air-tightened glass containers till the start of the experimental trial.
Mild-steel coupons
The coupons (1 × 1 × 0.1 cm) were procured from the Department of Metallurgy and Materials Engineering, University of the Punjab, Lahore, Pakistan, and degreased with analytical grade acetone (Cat No. 10014, Merck, Germany) as previously described by Bano (2008). The coupons were then polished with polishing paper (Cat No. WD240, Caswell, Taiwan). After cleaning, the coupons were washed with ethanol (Cat No. 100983, Merck, Germany) followed by cleansing with distilled water, and dried completely at 80 oC for 10 min in an electric oven. The initial weights (g) of coupons were measured by using an electric balance after cooling (Zuo and Wood, 2004). The coupons were then autoclaved and buried under different experimental conditions.
Experimental trial
The experimental trial for studying the corrosive impact of SRB on steel coupons was conducted in moist clay and water with and without the provision of Postgate B medium. For studying corrosion in clay in the presence of nutrients, 30 g clay was mixed with 10 mL of Postgate B medium. Similarly, for studying corrosion in clay in the absence of nutrients, 30 g clay was mixed with 10 mL of distilled water. In other experimental sets, only water (30 mL) was used instead of clay with and without the provision of Postgate B medium. All the experiments were conducted in sterile serum bottles (SSB) of 30-mL capacity. Initially, in all the experiments, pH was set between 7.0 and 7.5. The processed and autoclaved coupons were then inserted in the solid media vertically and left horizontally in the liquid media. Half vials of both sets (A and B) were inoculated with revived SRB cultures [5 % (v/v) ⁓ 1.8×106 C.F.U. mL−1], while the remaining half vials were kept un-inoculated (control). Oxygen diffusion in the vials was stopped by sealing SSB with butyl rubber stoppers (Cat No. 32-A2, Sunshine, China) and aluminum crimp seals (Cat No. SU27227-U, Supelco, Germany). The sealed SSB were then incubated at 30 oC for 60 days.
Measurement of CR and APWL
At end of the experimental period, coupons were recovered from the clay under aseptic conditions. Corrosion products and other contaminants were removed from the coupons using Clark’s solution (Sb2O3, 20 g; SnCl2, 50 g; HCl, 774 mL) following Angeles-Chavez et al. (2001). Contaminant-free coupons were rinsed with distilled water, dried completely, and weighed to check the weight loss. The rate of corrosion (µg dm−2 d−1) was then estimated by following the given formula (Majumdar et al., 1999):
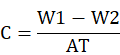
Where, C represents CR (µg dm−2 d−1), while W1 and W2 are weights of coupons before and after the harvesting, respectively. A represents the surface area of the coupons and the experimental period (days) is represented by T.
XRD and FTIR spectroscopy analyses of the corrosion product
Fine powder of the corrosion product collected after crushing was processed for XRD analysis for checking the existence and nature of oxides and sulfides. A semi-computer-controlled XRD (RIGAKU, Model DMAX/A) analysis was performed to determine the composition of the corroded material.
The corroded product was then observed with FTIR (Nicolet AVATAR360, Nicolet, Madison, AL, USA) for the quantitative analysis of organic groups produced by SRB during growth and consequent corrosion of the coupons.
Statistical analysis
All the experiments were carried out in triplicates. The data were analyzed by one-way ANOVA using Minitab 19. Mean differences were considered significant at P < 0.05.
RESULTS
The present study reported SRB-induced CR and APWL in clay and water in the absence (A) and presence (B) of some mineral nutrients. For this purpose, SRB species were isolated from a wastewater stream and found motile, Gram-negative, non-spore former and identified by 16S rRNA gene sequencing as Desulfovibrio desulfuricans. A comparison of sequence homology with known sequences is shown in Figure 1 as basis for identification. On average, CR and APWL appeared maximally in the experimental coupons of both experimental sets A and B. The CR and APWL were significantly higher in water as compared to clay (Fig. 2). The CR and APWL in sets A and B appeared as:
CR and APWL in the experimental set A
CR and APWL in clay
The CR and APWL in clay appeared almost the same in the control vials as compared to the experimental vials. The CR and APWL in the experimental vials were 43 ± 2 µg dm−2 d−1 and 0.75±0.25 %, respectively, while the corresponding figures for CR and APWL in the control vials appeared as 46±2 and 0.74±0.11, respectively (Table I). The impact of the SRB remained masked in this set of experiments.
Table I. Corrosion rate (CR, µg dm−2 d−1) and average percent weight loss (APWL, %) of mild steel coupons in clay and water under different experimental conditions.
|
Index |
With postgate B medium |
Without postgate B medium |
||||||
|
Clay |
Water |
Clay |
Water |
|||||
|
Ino-culated n= 3 |
Unino-culated n= 3 |
Ino-culated n= 3 |
Unino-culated n= 3 |
Ino-culated n= 3 |
Unino-culated n= 3 |
Ino-culated n= 3 |
Unino-culated n= 3 |
|
|
CR |
108 ± 3 |
62 ± 2 |
249 ± 2 |
166 ± 2 |
43 ± 2 |
46 ± 2 |
68 ± 2 |
55 ± 2 |
|
APWL |
1.88 ± 0.29 |
1.68 ± 0.11 |
4.16 ± 0.25 |
2.83 ± 0.14 |
0.75 ± 0.25 |
0.76 ± 0.11 |
1.22 ± 0.45 |
0.98 ± 0.19 |
CR and APWL in water
The CR and APWL in water appeared amazingly higher in the experimental vials as compared to the control vials. The CR and APWL in the experimental vials were 68 ± 2 µg dm−2 d−1 and 1.22 ± 0.45 %, respectively, while the corresponding figures for CR and APWL in the control vials appeared as 55 ± 2 and 0.98 ± 0.19, respectively (Table I). The significant impact of SRB on the experimental coupons was observed in this set of experiments.
CR and APWL in the experimental set B
CR and APWL in clay
The CR and APWL in clay appeared slightly higher in the experimental vials as compared to the control vials. The CR and APWL in the experimental vials were 108±3 µg dm−2 d−1 and 1.88±0.29 %, respectively, while the corresponding figures for CR and APWL in the control vials appeared as 62±2 and 1.68±0.11, respectively (Table I). The impact of SRB remained masked in this set of experiments.
CR and APWL in water
The CR and APWL in water were significantly higher in the experimental vials as compared to the control vials. The CR and APWL in the experimental vials were 249 ± 2 µg dm−2 d−1 and 4.16 ± 0.25 %, respectively, while the corresponding figures for CR and APWL in the control vials appeared as 166 ± 2 and 2.83 ± 0.14, respectively (Table I). The significant impact of SRB was observed in this set of experiments.
XRD and FTIR spectroscopy analyses of the corrosion product
Analyses of the corrosion product depicted the presence of FeS in all the inoculated groups, while different iron oxidation forms were appeared in the uninoculated groups (Figs. 3 and 4).
DISCUSSION
In the current study, the corrosive impact of SRB on mild steel coupons was studied in fluid and solid media. For this purpose, a motile, Gram-negative, and non-spore former SRB species was isolated from a wastewater stream and identified by 16S rRNA gene sequencing as D. desulfuricans. SRB have been studied extensively and considered one of the key causative agents of corrosion of buried metals and their alloys (Muyzer and Stams, 2008; Yuan et al., 2013; Guan et al., 2016; Xu et al., 2016).
In this study, two major sets of experiments were performed with and without the addition of nutrients of the Postgate B medium. In experimental set B, the addition of nutrients manifested higher CR and APWL values. Numerous SRB genera (Desulfobulbus, Desulfococcus, Desulfonema, Desulfotomaculum, and Desulfovibrio) have been reported to play a vital role in corrosion due to the abundant availability of sulfate in anoxic environments (Lv and Du, 2018; Tran et al., 2021). In the presence of nutrients, SRB grew well and generate more H2S and thus caused more corrosion. It is well known that nascent H2S acidifies water and causes pitting corrosion of metals. Furthermore, it enhances the corrosive action when combines with O2 and CO2 (Asmara, 2018).
Higher CR and APWL values were observed in water when compared with that of clay. The CR and APWL in water were 249±2 µg dm−2 d−1 and 4.16±0.25 %, respectively, while the corresponding figures for CR and APWL in clay were 108 ± 3 and 1.88 ± 0.27, respectively. The isolated SRB species for the current study appeared motile. The motile bacteria are more likely to show their impact in liquid media. Motile bacteria may penetrate the pores present on the metal surface and thus result in more severe corrosion. Similar findings have been reported by Jimenez-Lopez et al. (2008) while studying the advantageous motility of Myxococcus xanthus in porous material thus increasing the consolidation efficiency.
XRD and FTIR spectra showed the presence of FeS in all the inoculated groups. It has been reported that SRB use sulfate as their terminal electron acceptors and a variety of organic substrates as their electron donors and generate H2S (Hussain et al., 2016). The biogenic H2S acidifies the moisture contents that cause the pitting corrosion of metals (Butt and Mohsin, 2022; Qin et al., 2022; Xu et al., 2022). In our study, the presence of FeS in the inoculated groups confirmed the production of H2S and consequent corrosion of the steel coupons.
CONCLUSION
The present study reveals the corrosive impact of SRB on mild steel coupons in clay and water in the absence (A) and presence (B) of some mineral nutrients. On average, CR and APWL appeared maximally in the experimental coupons of both experimental sets A and B. The CR and APWL were significantly higher in water as compared to clay while depicting the corrosive potential of SRB. Future studies on the corrosion behaviour of SRB while utilizing various other electron acceptors and donors are needed to explore more facts on SRB-based corrosion compatible with various geochemical conditions.
Acknowledgements
The institutional support of the Department of Metallurgy and Materials Engineering, University of the Punjab, Lahore, Pakistan, for providing mild steel coupons is highly acknowledged.
Funding
The study received no external funds.
IRB approval
The current research work was approved by Departmental Board of Studies of Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Angeles-Chavez, C., Romero, J.M., Amaya, M., Martinez, L. and Perez, R., 2001. New strain of anaerobic bacteria and its association with corrosion pitting of X52 pipeline steel. Br. Corros. J., 36: 292-296. https://doi.org/10.1179/000705901101501631
Asmara, Y.P., 2018. The roles of H2S gas in behavior of carbon steel corrosion in oil and gas environment: A review. J. Tek. Mesin Mercu Buana, 7: 37-43. https://doi.org/10.22441/jtm.v7i1.2279
Bano, A.S., 2008. Microbiologically influenced corrosion of buried mild steel pipes and its prevention through bacterial antibiosis. PhD thesis, University of the Punjab, Lahore, Pakistan.
Beech, I.B. and Sunner, J., 2004. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol., 15: 181-186. https://doi.org/10.1016/j.copbio.2004.05.001
Butt, N.Z. and Mohsin, F., 2022. Determination of sulphide in Arabian seawater using UV-visible spectrophotometer. Nucleus, 58: 37-41.
Chugh, B., Sheetal, Singh, M., Thakur, S., Pani, B., Singh, A.K. and Saji, V.S., 2022. Extracellular electron transfer by Pseudomonas aeruginosa in biocorrosion: A review. ACS Biomater. Sci. Eng., 8: 1049-1059. https://doi.org/10.1021/acsbiomaterials.1c01645
Dang, H. and Lovell, C.R., 2015. Microbial surface colonization and biofilm development in marine environments. Microbiol. mol. Biol. Rev., 80: 91-138. https://doi.org/10.1128/MMBR.00037-15
Enning, D. and Garrelfs, J., 2014. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. environ. Microbiol., 80: 1226-1236. https://doi.org/10.1128/AEM.02848-13
Ferreira, C.A.M., Ponciano, J.A.C., Vaitsman, D.S. and Pérez, D.V., 2007. Evaluation of the corrosivity of the soil through its chemical composition. Sci. Total Environ., 388: 250-255. https://doi.org/10.1016/j.scitotenv.2007.07.062
Gu, T., Rastegar, S.O., Mousavi, S.M., Li, M. and Zhou, M., 2018. Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour. Technol., 261: 428-440. https://doi.org/10.1016/j.biortech.2018.04.033
Guan, F., Zhai, X., Duan, J., Zhang, M. and Hou, B., 2016. Influence of sulfate-reducing bacteria on the corrosion behavior of high strength steel EQ70 under cathodic polarization. PLoS One, 11: 0162315. https://doi.org/10.1371/journal.pone.0162315
Hussain, A., Hasan, A., Javid, A. and Qazi, J.I., 2016. Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: A mini review. 3 Biotech, 6: 119. https://doi.org/10.1007/s13205-016-0437-3
Hussain, A., Shakir, H.A. and Qazi, J.I., 2014. Anaerobic biodegradation of sulphate employing animal manure as a cost-effective growth substrate. J. Anim. Pl. Sci., 24: 913-918.
Ia, R., Li, Y., Al-Mahamedh, H.H. and Gu, T., 2017. Enhanced biocide treatments with D-amino acid mixtures against a biofilm consortium from a water cooling tower. Front. Microbiol., 8: 1538. https://doi.org/10.3389/fmicb.2017.01538
Jimenez Lopez, C., Jroundi, F., Pascolini, C., Rodriguez Navarro, C., Pinar Larrubia, G., Rodriguez Gallego, M. and González Muñoz, M.T., 2008. Consolidation of quarry calcarenite by calcium carbonate precipitation induced by bacteria activated among the microbiota inhabiting the stone. Int. Biodeterior. Biodegradation, 62: 352-363. https://doi.org/10.1016/j.ibiod.2008.03.002
Juzeliunas, E., Ramanauskas, R., Lugauskas, A., Leinartas, K., Samulevicienė, M., Sudavicius, A. and Juskenas, R., 2007. Microbially influenced corrosion of zinc and aluminium two-year subjection to influence of Aspergillus niger. Corros. Sci., 49: 4098-4112. https://doi.org/10.1016/j.corsci.2007.05.004
Kadaifçiler, D., Danışman, M., Arslan-Vatansever, D., Cansever, N. and Ilhan-Sungur, E., 2022. Corrosion behavior of galvanized steel exposed to Fusarium oxysporum f. sp. cumini isolated from a natural biofilm. Microbiology, 91: 445-453. https://doi.org/10.1134/S0026261722300221
Kaesche, H., 2003. Corrosion of metals: Physicochemical principles and current problems. 1st edn, Springer, Berlin, GM.
Li, H., Lin, D., Peng, Y. and Zheng, J., 2016. Oxidative bio activation of nitrofurantoin in rat liver microsomes. Xenobiotica, 47: 103-111. https://doi.org/10.3109/00498254.2016.1164913
Little, B. and Lee, J.S., 2007. Microbiologically influenced corrosion. 1st edn. Wiley-Interscience, New York. https://doi.org/10.1002/047011245X
Liu, H., Fu, C., Gu, T., Zhang, G., Lv, Y., Wang, H. and Liu, H., 2015. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci., 100: 484-495. https://doi.org/10.1016/j.corsci.2015.08.023
Liu, H., Gu, T., Lv, Y., Asif, M., Xiong, F., Zhang, G. and Liu, H., 2017. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO2-saturated oilfield produced water. Corros. Sci., 117: 24-34. https://doi.org/10.1016/j.corsci.2017.01.006
Lv, M. and Du, M., 2018. A review: Microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria. Rev. environ. Sci. Biotechnol., 17: 431-446. https://doi.org/10.1007/s11157-018-9473-2
Lv, M., Du, M., Li, X., Yue, Y. and Chen, X., 2019. Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol., 8: 4066-4078. https://doi.org/10.1016/j.jmrt.2019.07.016
Madirisha, M., Hack, R. and Van der Meer, F., 2022. The role of organic acid metabolites in geo-energy pipeline corrosion in a sulfate reducing bacteria environment. Heliyon, 20: e09420. https://doi.org/10.1016/j.heliyon.2022.e09420
Majumdar, I., D’Souza, F. and Bhosle, N.B., 1999. Microbial exopolysaccharides, effect on corrosion and partial chemical characterization. J. Indian Inst. Sci., 79: 539-550.
Muyzer, G. and Stams, A.J.M., 2008. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol., 6: 441-454. https://doi.org/10.1038/nrmicro1892
Okoro, C., Ekun, O.A., Nwume, M.I. and Lin, J., 2016. Molecular analysis of microbial community structures in Nigerian oil production and processing facilities in order to access souring corrosion and methanogenesis. Corros. Sci., 103: 242-254. https://doi.org/10.1016/j.corsci.2015.11.024
Pester, M., Knorr, K.H., Friedrich, M.W., Wagner, M. and Loy, A., 2012. Sulfate-reducing microorganisms in wetlands–fameless actors in carbon cycling and climate change. Front. Microbiol., 3: 72. https://doi.org/10.3389/fmicb.2012.00072
Postgate, J.R., 1984. The sulfate-reducing bacteria. Cambridge University Press, Cambridge.
Qian, S. and Cheng, Y.F., 2017. Accelerated corrosion of pipeline steel and reduced cathodic protection effectiveness under direct current interference. Constr. Build. Mater., 148: 675-685. https://doi.org/10.1016/j.conbuildmat.2017.05.024
Qin, Q., Xu, J., Wei, B., Fu, Q., Yu, C., Sun, C. and Wang, Z., 2022. Biotic enhancement of Desulfovibrio desulfuricans on multi-factor influenced corrosion of X80 steel in saline soil. Corros. Sci., 200: 110228. https://doi.org/10.1016/j.corsci.2022.110228
Qu, Q., Li, S., Li, L., Zuo, L., Ran, X., Qu, Y. and Zhu, B., 2017. Adsorption and corrosion behaviour of Trichoderma harzianum for AZ31B magnesium alloy in artificial seawater. Corros. Sci., 118: 12-23. https://doi.org/10.1016/j.corsci.2017.01.005
Thompon, N.G., Yunocivh, M. and Dunmiret, D., 2007. Cost of corrosion and corrosion maintenance strategies. Corros. Rev., 25: 247-262. https://doi.org/10.1515/CORRREV.2007.25.3-4.247
Tran, T.T.T., Kannoorpatti, K., Padovan, A. and Thennadil, S., 2021. Sulphate-reducing bacteria’s response to extreme pH environments and the effect of their activities on microbial corrosion. Appl. Sci., 11: 2201. https://doi.org/10.3390/app11052201
Videla, H.A. and Herrera, L.K., 2005. Understanding microbial inhibition of corrosion: A comprehensive overview. Int. Biodeterior. Biodegradation, 63: 896-900. https://doi.org/10.1016/j.ibiod.2009.02.002
Xu, D., Li, Y. and Gu, T., 2016. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry, 110: 52-58. https://doi.org/10.1016/j.bioelechem.2016.03.003
Xu, D., Li, Y., Song, F. and Gu, T., 2013. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci., 77: 385-390. https://doi.org/10.1016/j.corsci.2013.07.044
Xu, L., Guan, F., Ma, Y., Zhang, R., Zhang, Y., Zhai, X., Dong, X., Wang, Y., Duan, J. and Hou, B., 2022. Inadequate dosing of THPS treatment increases microbially influenced corrosion of pipeline steel by inducing biofilm growth of Desulfovibrio hontreensis SY-21. Bioelectrochemistry, 145: 108048. https://doi.org/10.1016/j.bioelechem.2021.108048
Yuan, S., Liang, B., Zhao, Y. and Pehkonen, S.O., 2013. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci., 74: 353-366. https://doi.org/10.1016/j.corsci.2013.04.058
Zhang, Y., He, J., Zheng, L., Jin, Z., Liu, H., Liu, L., Gao, Z., Meng, G., Liu, H. and Liu, H., 2022a. Corrosion of aluminum alloy 7075 induced by marine Aspergillus terreus with continued organic carbon starvation. NPJ Mater. Degrad., 6: 27. https://doi.org/10.1038/s41529-022-00236-2
Zhang, Y., Zhai, X., Guan, F., Dong, X., Sun, J., Zhang, R., Duan, J., Zhang, B. and Hou B., 2022b. Microbiologically influenced corrosion of steel in coastal surface seawater contaminated by crude oil. NPJ Mater. Degrad., 6: 35. https://doi.org/10.1038/s41529-022-00242-4
Zuo, R. and Wood, T.K., 2004. Inhibiting mild steel corrosion from sulfate-reducing and iron-oxidizing bacteria using gramicidin-S-producing biofilms. Appl. Microbiol. Biotechnol., 65: 747-753. https://doi.org/10.1007/s00253-004-1651-1
To share on other social networks, click on any share button. What are these?










