Study of Heavy Metals in Soil and Wheat Crop and their Transfer to Food Chain
Research Article
Study of Heavy Metals in Soil and Wheat Crop and their Transfer to Food Chain
Nazish Huma Khan*, Mohammad Nafees and Adila Bashir
Department of Environmental Sciences, University of Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | The present study was conducted in orchard field of peach and comparative non-orchard field with an attempt to know about the soil and wheat crop contamination caused by heavy metals (Cd, Cr and Ni). Soil samples were collected from both orchard and non-orchard fields and were extracted with Aqua-regia and Mehlich-3 extracting solutions for total and available metals respectively. Wheat crops from both fields (orchard and non-orchard) were collected and analyzed with aqua-regia for heavy metals contents. Results showed that the average concentrations of metals in soils of orchard and non-orchard fields were as chromium (Cr) 56.9 and 52.2 mg kg-1, Ni 29.8 and 27.9 mg kg-1 and Cd 2.46 and 1.11 mg kg-1, respectively. The risk contribution from Cd, Cr and Ni were significant in crop seeds of orchard field (THQ>1). The substantial load of heavy metals in studied fields showed the anthropogenic source of pollution. Therefore regular monitoring of heavy metals in soil and crops is necessary.
Received | June 03, 2015; Accepted | May 15, 2016; Published | June 05, 2016
*Correspondence | Nazish Huma Khan, Department of Environmental Sciences, University of Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: khuma10@yahoo.com
Citation | Huma-Khan, N., M. Nafees and A. Bashir. 2016. Study of heavy metals in soil and wheat crop and their transfer to food chain. Sarhad Journal of Agriculture, 32(2): 70-79.
DOI | http://dx.doi.org/10.17582/journal.sja/2016/32.2.70.79
Keywords | Bio-accumulative factor, Health risk, Irrigation water, Non-orchard, Orchard, Target hazard quotient
Introduction
Soil serves as a sink for some noxious elements known as heavy metals like chromium, cadmium, nickel and lead where they remain in soil for long times effecting the quality of agricultural soil and crops (Nicholsona et al., 2003). The anthropogenic activities contribute these dangerous metals in soil which indirectly attack on human’s health through food chain (Simeonov et al., 2003). The upper 25cm surface region of the soil is mostly affected by the toxic metals where roots of the plants/crops are located (Freitas et al., 2004). The anthropogenic activities which contribute pollution to water bodies are industrial and sewage effluents, surface washing, domestic sewage, organic matter of plants and animals, agrochemicals and treatment work’s wastes (Lokeshwari et al., 2006).
The progressive increase in industrialization, urbanization and improper environmental planning leads to shortage of irrigation water. To cope with this issue of water shortage in irrigation sector, waste water has been used as a resource from past few decades (Chary et al., 2007). To long-term use of waste water for irrigation is not only causing heavy metals accumulation in soil but it also contaminating crops and vegetables (Boularbah et al., 2006). Soil receives heavy metals from wastewater irrigation, in case of continuous irrigation with waste water. Soil losses to hold the capacity of heavy metals and therefore releases these metals to ground or the soil solution available for plant uptake. This uptake affects the food quality and safety (Zhao et al., 2010). In Pakistan the availability of fresh water is not enough to fulfill the irrigation water for agriculture therefore, farmers use wastewater as the alternative source for irrigation purposes (Sadiq et al., 2005). In the Middle East, Pakistan, Oman, Saudi Arabia, Morocco and Jorden are the countries which use waste water for irrigation purposes (Arabi et al., 1996).
Food chain contamination is one of the important pathway which contributes 90% of heavy metals in comparison of other sources like inhalation and dermal contact (Loutfy et al., 2006). The use of contaminated water not only harms soil quality but also affects agricultural products and results the potential threats for human’s health (Iqbal et al., 2011). Cadmium is present in earth crust about 0.5 ppm and is toxic for human’s health (Yang et al., 2012). The common sources of Cd in agricultural fields are phosphate fertilizers, zinc fertilizers, sewage sludge and animal manure (Nicholson et al., 2003). Food is considered as one of the major source of Cadmium exposure (Rahman et al., 2014). Cd has no important function in the body but it accumulates in kidney and cause several disorders being carcinogenic in nature (Cheli et al., 2010). Chromium is a toxic element naturally occurring in soil in the range of 10-50 mg kg-1 and is carcinogenic in nature (Lendinez et al., 2001). Nickel is a trace element in the earth crust, acts as heavy metal which comes into the environment from both natural and anthropogenic sources (Iyaka, 2011). In agricultural soils nickel is present at levels of 8.5 to 15 mg/kg. It is mostly present in crops at youngest stage (early one month).
Contamination of soil, water and food systems by heavy metals is progressive issue due to its potential threats to human, animal’s health and environmental quality degradation. The accumulation of heavy metals in soil and plants is of major concern because of its toxicity to food chain contamination. Contamination level arises when heavy metals are present above their permissible limits which is considered a threat for food chain (Ivezic et al., 2013). The important sources of this contamination is the amendments in soil by heavy applications of fertilizers, pesticides, use of low quality water for irrigation and some natural process such as weathering and erosion (Shirisha et al., 2014). Plants when grow on such type of soil take up these metals and then find their way to animals and humans (Westfall et al., 2005). The consumption of toxic metals in food causes incidence of cancer (Arora et al., 2008). Due to all these reasons it is quite important to monitor these heavy metals for safety assessment of human’s health and environment. The purpose of this study was to give an overview of accumulation of potentially toxic elements in edible parts of wheat plants and their transfer to food chain.
Material and Methods
The research work was carried out on peach orchards and adjacent non-orchard fields in Dab-Banda, District Charsadda. The study area is situated on road side near the junction point of River Swat and River Kabul (KP). The village lies between latitude of 34o– 03’, 34o–38’ N and longitude of 71o–28’, 71o–53’ E (Afridi et al., 2014). This area is famous for horticulture having the orchards of peach, plum, pear, strawberry and guava. As the area covered by orchards is increasing in KP, the increased trend of horticulture in study area encourages the use of waste water for irrigation purposes. By using contaminated source of water the chances for toxic metals accumulation will increase (Khan et al., 2010). To cope with the problems of accumulation, soil, water and crops need to be monitored.
Sampling and preparation
The composite soil samples were collected randomly from 24 selected sites of orchard and non-orchard fields with 0-25 cm depth with the help of a spade. Total area was 202343 m2 (20.2 hectare). A field of 10x5 meters was selected at 24 different sites. 4 points were selected randomly, two for orchard and 2 for non-orchard, after dividing the selected field into grids of 1 m2. Each composite sample consists of 10 randomly selected points by covering 10% of each site. Each sample was taken in labeled polythene bag and brought to the laboratory of Environmental Sciences, University of Peshawar. The collected samples were mixed thoroughly, air dried, ground to pass through 2 mm sieve and stored in labeled plastic jars for analysis. The representative seven fresh water samples were collected from irrigation channel of respective fields in 2 liter labeled plastic bottles. As per standard method each water sample was acidified with 1ml of HNO3, the idea to stop bacteriological activity if any and the metal adsorption on inner surface of bottle. The samples were stored in laboratory for analysis at 4oC in refrigerator (Sheldrick and Wang, 1993). Wheat crop was collected randomly after harvesting in month of May. Plant was oven dried, divided into four parts (seed, leaf, stem and root) and each part was ground and crushed in powdered form through a grinding mill and stored for further analysis.
Laboratory analysis
The soil pH and electrical conductivity (EC) were measured using pH meter and conductivity meter with a suspension of 1:5, soil and water (Richard, 1954). Soil texture was determined by pipette method as 20 g sample of each soil sample was dissolved in little amount of distilled water in shaker cup and added 5 ml of sodium hexa-metaphosphate and stirred for 5 minutes. Then poured the extract into 500 ml graduated cylinder and filled with water, covered its top with parafilm, inverted it several times to re-suspend the soil and then left to stand the cylinder for 48 seconds. Removed the parafilm, took the first aliquot from upper 10 cm of suspension after 48 seconds and marked it as 10 cm pipette. Transfer this aliquot in weighted china dish and placed it in oven at 105oC. Another aliquot of 25 ml was taken from upper 5 cm after 40 minutes in a separate pre-weighted china dish and put it in oven at 105oC. After overnight, removed china dishes from oven and find their weights which gave the 2nd readings. In last the final readings were subtracted from initial readings and the percentage values of sand, silt and clay were calculated by given formulas (Gee and Bauder, 1986).
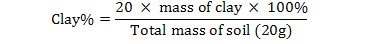
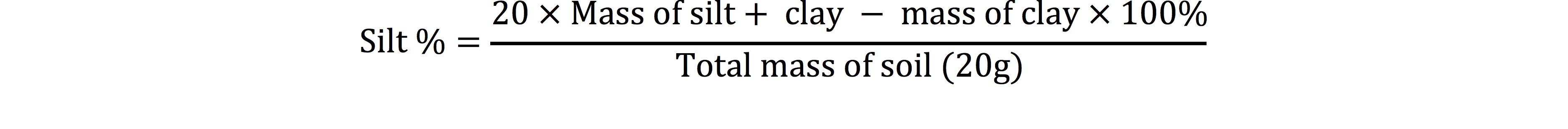
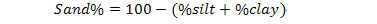
Soil organic content was determined by Walkely Black method according the standard method of Nelson and Sommers (1982). A total of 1g soil was taken in 500 ml flask then added 10 ml of K2Cr2O7 solution, 20 ml of H2SO4 and shacked for a minute then heated it for 5 minutes on hotplate and then allowed to stand for 30 minutes. After 30 minutes it was diluted up to 200 ml with distilled water then added 10 ml of H3PO4, 0.2 g of NaF and 3-4 drops of o-phenanthroline indicator and titrated against Fe(NH4)2 (SO4)2 solution (0.5 M). Blank without soil was also run and noted the reading till the color changed from greenish blue to reddish brown. Organic Carbon was calculated with the help of formula:
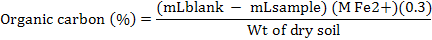
For heavy metals analysis two digestion methods were used i) aqua-regia extraction method (for total nutrients and is efficient, safe and rapid digestion which prevent the loss of volatile metals, reported by Shirdama et al. (2008)). ii) Mehlich-3 extraction method was used for total available metals to plant (Mehlich, 1984). In aqua-regia method 1 g of soil was digested in 15 ml of aqua-regia (5:1:1 HNO3: H2SO4: HCLO4) in digestion block at temperature range of 80oC to 180oC until the solution become transparent (Chen and Ma, 1998). The solution was filtered and diluted to 50 ml with distilled water and subjected to atomic absorption spectrophotometer AAS (700) Acetylene flame for analysis.
To determine the available content of metals, soil samples were extracted with Mehlich-3 solution (0.2N CH3CHOOH + 0.25N NH4NO3 + 0.015N NH4F + 0.013N HNO3+ 0.001M ETDA). In brief, 2 g of each sample was agitated with 20 ml of Mehlich-3 solution for 5 minutes and after filtration, samples were analyzed through atomic absorption spectrophotometer (Mehlich, 1984). The water samples were also analyzed through atomic absorption spectrophotometer (Arnold, 1992). While for metals extraction in crop, 0.5 g of crop sample was digested with 15 ml of aqua-regia and then analyzed on spectrophotometer (Chen and Ma, 2001). The detection limits for these metals were 22.8, 357.9 and 232ppb for Cd, Cr and Ni respectively.
Statistical analysis
Descriptive statistical analysis like average, standard deviation and correlation of metals were done using MS Excel 2007. An independent t-test was employed to find the significant level of comparative fields using SPSS software at 0.05 significance level and 95% of confidence interval.
Bio-Accumulative Factor (BAF): was calculated by using the following equation.
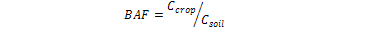
Where;
Ccrop and Csoil, show the metal concentrations in crop and soil samples respectively (Yu et al., 2006).
Target Hazard Quotient (THQ)
Target hazard quotient is the ratio between exposure and oral reference dose. The significance value for THQ is 1. It was calculated by the given formula (Chien et al., 2002).
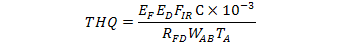
Where;
EF represents exposure frequency (365 days/year), ED is the exposure duration (70 years)(Bennett et al., 1999), FIR is the rate of food intake (g/person/day), C is the concentrations of metals in crop seed, RFD is oral reference dose (mg kg-1 day-1) which are 1 x 10-3, 1.5and 0.02 mg kg-1 day-1 for Cd, Cr and Ni, respectively WAB is average body weight and TA is the average exposure time (365 days year-1x number of exposure years (average life expectancy70 years). The average adult and children weight were considered as 55.9 and 32.7 kg, respectively (USEPA, 2000).
Results and Discussion
Soil properties
The pH of orchard and non-orchard soils ranged as 6.8 and 7.3 respectively (Table 1). Low pH of orchard field was due to its good organic content. The organic contents ranged as 1.7% and 0.5% in orchard and non-orchard fields respectively. Electrical conductivity (EC) was 139 µS/cm in orchard field and 135 µS/cm in non-orchard field. Although orchard field showed high electrical conductivity but collectively both type of fields were below the permissible limit (2000 µS/cm) (Ryan et al., 2001). Soil texture was loamy sand type.
Heavy metals in soil
The average concentrations of cadmium (Cd), chromium (Cr) and nickel (Ni) were found above the permissible limits in aqua-regia extracted soils of orchard fields. In non-orchard the levels were within the safe limits (Table 2 and 3). The aqua-regia extracted soils found rich in heavy metals as the values were high, showing an evidence of soil contamination. The variances of comparative study showed insignificant results for Cr (p> 0.05) and highly significant values for Cd and Ni contents as p< 0.05, given in Table 2.
Tests results of mehlich-3 extracted soils showed the available contents of metals in order of Cr>Ni>Cd in both orchard and non-orchard fields (Table 2). In orchard field low pH contributes the accumulation of metals in soil solution as compared to non-orchard field (Table 1). In research area the inputs of composts/animal manure and pesticide is very common and these inputs contribute the risk of heavy metals in soil and then environmental risks (Khan et al., 2012). The use of pesticides and fertilizers increase economic value but on other hand it also increases the risk of human health. Furthermore, high level of heavy metals in analyzed soils is related to anthropogenic sources like the progressive uses of agrochemicals (regular sprays of fungicides and insecticide on orchards), use of animal manures/compost, and contaminated source of irrigation water as reported by Nafees et al. (2009). Our findings showed water as one of the major contributor of heavy metals to soil and their transfer to plants (Table 5). These metals have long life time therefore they remain in soil for long periods and easily available to plants grown over there (Camelo et al., 1997). The excessive concentrations of heavy metals in soil reduce microbial activities and thus lower
Table 1: Parameters of soil samples
|
Sample |
pH |
EC uS/cm |
Organic Matter % |
Sand |
Silt |
Clay |
Texture |
||||||
|
Min |
Max |
Avg |
Min |
Max |
Avg |
Min |
Max |
Avg |
% |
% |
% |
||
|
Orchard |
6.8 |
6.9 |
6.8 |
137 |
141 |
139 |
1.6 |
1.8 |
1.7 |
62 |
11 |
27 |
Loamy sand |
|
Non-orchard |
7.2 |
7.3 |
7.3 |
134 |
136 |
135 |
0.4 |
0.5 |
0.5 |
41 |
28 |
31 |
Loamy sand |
Table 2: Total and available contents of heavy metals in soil (mg kg-1)
|
Orchard |
Non-Orchard |
P(2-tail) |
||||||||
|
Element |
Min |
Max |
Avg |
S.D |
Min |
Max |
Avg |
S.D |
||
|
Total contents of H.M |
Cd |
1.92 |
3.04 |
2.46 |
1.1143 |
0.77 |
1.39 |
1.11 |
0.391 |
0.00 |
|
Cr |
54.9 |
59.1 |
56.9 |
1.631 |
49.3 |
54.03 |
52.2 |
16.12 |
0.03 |
|
|
Ni |
26.65 |
33.2 |
29.8 |
9.416 |
18.45 |
24.74 |
21.83 |
7.036 |
0.00 |
|
|
Available Contents of H.M |
Cd |
1.29 |
2.19 |
1.71 |
0.604 |
0.87 |
1.37 |
1.07 |
0.557 |
0.00 |
|
Cr |
5.89 |
8.86 |
7.62 |
2.591 |
3.28 |
6.23 |
5.14 |
1.801 |
0.01 |
|
|
Ni |
3.72 |
7.59 |
5.81 |
2.199 |
3.52 |
6.04 |
4.91 |
1.704 |
0.27 |
|
Table 3: Permissible limits of heavy metals in soil and crop
|
Metal |
Crops |
Soil |
|
Cd |
0.1-0.2 mg kg-1 (Khan et al., 2008) |
0.58 mg kg-1 (Khanlariet al., 2008) |
|
Cr |
1.30 mg kg-1 (Iqbal et al., 2011) |
50 mg kg-1 (Shankeret al., 2005) |
|
Ni |
10 mg kg-1 (Iqbal et al., 2011) |
19 mg kg-1 (Khanlariet al., 2008) |
Table 4: Concentrations of heavy metals in wheat crops
|
Orchard |
Non-Orchard |
P (2-tail) |
||||||||
|
Element |
Sample |
Min |
Max |
Avg |
S.D |
Min |
Max |
Avg |
S.D |
|
|
Cd (mg kg-1) |
Seed |
0.58 |
0.69 |
0.62 |
0.232 |
0.22 |
0.44 |
0.32 |
0.098 |
0.00 |
|
Leaf |
0.14 |
0.19 |
0.17 |
0.056 |
0.08 |
0.17 |
0.11 |
0.045 |
0.49 |
|
|
Stem |
0.18 |
0.26 |
0.22 |
0.067 |
0.01 |
0.3 |
0.1 |
0.11 |
0.16 |
|
|
Root |
0.01 |
0.06 |
0.04 |
0.020 |
0.001 |
0.04 |
0.02 |
0.013 |
0.22 |
|
|
Cr (mg kg-1) |
Seed |
1.7 |
3.01 |
2.3 |
0.719 |
1 |
1.04 |
1.02 |
0.316 |
0.00 |
|
Leaf |
0.62 |
0.8 |
0.72 |
0.229 |
0.32 |
0.64 |
0.46 |
0.172 |
0.00 |
|
|
Stem |
1.04 |
1.08 |
1.06 |
0.326 |
0.38 |
0.7 |
0.55 |
0.200 |
0.00 |
|
|
Root |
0.51 |
0.9 |
0.7 |
0.244 |
0.19 |
0.56 |
0.38 |
0.161 |
0.00 |
|
|
Ni (mg kg-1) |
Seed |
1.92 |
3.04 |
2.6 |
0.826 |
0.32 |
1.67 |
1.01 |
0.316 |
0.09 |
|
Leaf |
0.77 |
1.39 |
1.11 |
0.391 |
0.04 |
1.02 |
0.4 |
0.158 |
0.01 |
|
|
Stem |
0.87 |
2.65 |
1.9 |
0.846 |
0.46 |
1.23 |
0.8 |
0.402 |
0.45 |
|
|
Root |
0.87 |
1.35 |
1.05 |
0.326 |
0.68 |
1.22 |
0.91 |
0.327 |
0.24 |
|
down the process of recycling of important nutrients, control of pests and maintenance of soil structure (Wang et al., 2007).
Heavy metals in wheat crops
In orchard field, wheat grain showed significantly higher Cd content compared to its permissible limit (Table 3 and 4). It was further noted that Cd content in wheat grain in orchard field was also significantly (p<0.05) higher over the non-orchard field (Table 4). Higher in the wheat grain of orchard field over non-orchard might be due to contaminated water, use for irrigation (Table 4). It was further noted that both Cd concentrations in leaves, stem and roots of orchard and non-orchard wheat crops were non-significant (Table 4). The Cr content in crop seed/grain of orchard field showed high limit and the comparative study showed significant results (<0.05) for all parts of wheat crop in both orchard and non-orchard fields as in Table 3. Therefore Cr uptake by crop is high, crossing the permissible limit (Table 3). This increase is related to the source of irrigation water, carrying Cr contents to soil. While Ni was observed within the permissible limit, showing insignificant results in all parts of wheat crops (<0.05) as shown in Table 3 and 4. The comparative study showed high contents of metals in crop of orchard field (especially in seed parts). In this study the concentrations of studied heavy metals in crop of orchard field were significantly higher over their concentration in reference field crop (non-orchard). This variation is caused by the differences of pH, organic matter, physiology of plant, temperature and humidity, as Oluyemi et al. (2009) reported that these physiological properties of soil affect the uptake of heavy metals. Low pH of soils encourages the metals availability to plant as reported by Dudka and Miller, (1999). Therefore, this study also favors the author’s statement. The excessive concentrations of toxic metals affect plant growth and they get enter into humans by different possible pathways in which the most important one is food chain (Bratakos et al., 2002).
Findings of the study cleared that consumption of crop or vegetable grown on contaminated soils allow an open pathway for heavy metals to human/animal’s bodies causing the potentials of health risks. Therefore prevention is necessary which can be possible by lowering the use of agrochemicals and use the non-polluted source of water for irrigation purposes.
Table 5: Heavy metals concentrations in water samples (mg/L)
|
Samples |
Cd |
Cr |
Ni |
|
W1 |
0.02 |
0.12 |
1.05 |
|
W2 |
0.19 |
0.03 |
0.03 |
|
W3 |
0.14 |
0.05 |
0.10 |
|
W4 |
0.01 |
0.34 |
0.53 |
|
W5 |
0.22 |
0.17 |
0.42 |
|
W6 |
1.07 |
1.14 |
0.39 |
|
W7 |
0.46 |
0.85 |
1.01 |
|
Min |
0.01 |
0.03 |
0.03 |
|
Max |
1.07 |
1.14 |
1.39 |
|
Average |
0.30 |
0.43 |
0.45 |
|
S.D |
0.370 |
0.764 |
0.514 |
|
WHO standard 1996 |
0.003 |
0.05 |
0.02 |
|
USEPA 1986 |
0.005 |
0.1 |
0.1 |
Heavy metals in water
To find the source of contamination irrigation water was tested for heavy metals. The results were compared with WHO and USEPA standards for surface water which showed high concentrations of all heavy metals except zinc thatwas observed within safe limits (Table 5). The associated sources involed in water contamination are erosion of mountainous areas, release of tannery waste, sugar mill, ghee and textile effluents which introduce these toxic metals to soils and cause their accumulation in agricultural products (IUCN, 1994). The lack of waste treatment plants lead the discharge of highly polluted waste to the Kabul River and thus detoriating the water quality for agricultural sector (Khan et al., 2011). The qualitative results of water samples showed that both erosion of mountains (River Swat water channel) and effluents form industries (River Kabul) carry a load of heavy metals to irrigation channels. Water quality use for irrigation has an important role in crop yield. It is therefore cleared that irrigation water for the fields under study is one of the main contributory factor of heavy metals to soil and subsequent contamination of wheat crop.
Metals corelation
The Cd, Cr and Ni in aqua-regia extracted soils showed strong correlations with crop leaf, seed and root of orchard field (R2 = 0.63, 0.91, 0.72, 0.63, respectively). In non-orchard soils close correlations of Cd and Cr were observed with crop stem and leaf as R2 = 0.69, and 0.88 respectively (Table 6). In mehlich-3 extracted soils Cr wasfound in strong correlations with crop root asR2 = 0.64. While Ni showed strong correltion with crop root of non-orchard field only (R2 = 0.92) given in Table 6. The aqua-regia extraction method is considered to be more efficient by showing the strong corelations of metals between soil and crop.In water samples Cd, Ni and Cr showed close correlations with crop root, seed and leaf of orchard fileds (R2=0.73, 0.95 and 0.63respectively) . In non-orchard fields close correlations of Cd and Cr were observed with crop stemand leaf (R2= 0.69 and 0.88 respectively) as shown in Table 6. Overall the results showed close associations of crop metals with water and soil samples which cleared that both of these sources are responsible for availability of metals to crop.
Bio-accumulative factor (BAF)
The bio accumulative factor of analyzed metals showed high values for cadmium as 0.25 and 0.24 in crop seeds of orchard and non-orchard fields, respectively (Table 7). The whole trend of BAF was Cd>Ni>Cr. The results showed high uptakes of metals in orchard crops as compared to non-orchard.
Table 6: Correlation (R2) of crop metals with soil and water samples
|
Field |
Sample |
R2 with Aqua-regia Extracted soil |
R2 with Mehlich-3 Extracted soil |
R2 with Water |
||||||
|
Cd |
Cr |
Ni |
Cd |
Cr |
Ni |
Cd |
Cr |
Ni |
||
|
Orchard |
Seed |
0.18 |
0.73 |
0.02 |
0.07 |
0.02 |
0.02 |
0.43 |
0.63 |
0.21 |
|
Leaf |
0.63 |
0.01 |
0.32 |
0.52 |
0.32 |
0.43 |
0.07 |
0.05 |
0.21 |
|
|
Stem |
0.00 |
0.01 |
0.17 |
0.01 |
0.17 |
0.26 |
0.6 |
0.01 |
0.05 |
|
|
Root |
0.01 |
0.41 |
0.64 |
0.04 |
0.64 |
0.42 |
0.73 |
0.35 |
0.95 |
|
|
Non-Orchard |
Seed |
0.02 |
0.21 |
0.11 |
0.45 |
0.43 |
0.05 |
0.47 |
0.93 |
0.02 |
|
Leaf |
0.02 |
0.88 |
0.16 |
0.15 |
0.03 |
0.15 |
0.05 |
0.63 |
0.07 |
|
|
Stem |
0.69 |
0.03 |
0.12 |
0.31 |
0.09 |
0.01 |
0.01 |
0.01 |
0.12 |
|
|
Root |
0.03 |
0.41 |
0.17 |
0.57 |
0.01 |
0.92 |
0.5 |
0.34 |
0.47 |
|
Table 7: The bio-accumulative factor and target health quotient of metals
|
Sample |
Orchard |
Non-Orchard |
|||||
|
Cd |
Cr |
Ni |
Cd |
Cr |
Ni |
||
|
BAF for Metals |
Seed |
0.25 |
0.04 |
0.08 |
0.24 |
0.01 |
0.05 |
|
Leaf |
0.06 |
0.01 |
0.04 |
0.09 |
0.01 |
0.01 |
|
|
Stem |
0.08 |
0.01 |
0.08 |
0.09 |
0.01 |
0.07 |
|
|
Root |
0.01 |
0.01 |
0.04 |
0.02 |
0.01 |
0.03 |
|
|
THQ |
Adult |
1.81 |
1.34 |
1.13 |
0.93 |
0.59 |
0.42 |
|
Children |
2.11 |
1.64 |
1.39 |
0.91 |
0.72 |
0.30 |
|
Target Hazard Quotient
The target hazard quotient for Cd, Cr and Ni was calculated in edible part of wheat crops (seed/grain). The results showed high THQ for Cd, Cr and Ni in orchard field (>1). In crop seeds of non-orchard field THQ for studied metals were below the contamination level and safe in both, adults and children (THQ<1) given in Table 7. Therefore, the results of heavy metals in orchard fields showed that consumption of wheat crops may have a risk to the locality.
Conclusion
The present study revealed that use of contaminated water for irrigation and overuses of agrochemicals led to accumulation of heavy metals in soils and their uptake by the crops grown over there. The uptakes of heavy metals by wheat crops varied in different parts. The edible parts especially crop seed/grain showed high accumulation of heavy metals. The target hazard quotient showed health risk to local population associated with Cd, Cr and Ni contamination in wheat crops of orchard field (THQ>1). Good agricultural practices and regular monitoring of soil, crop and water quality with prevention of metals entering into agricultural products is a way to decrease the potential health hazards for inhabitants.
Acknowledgment
The authors are thankful to Centralized Resource Laboratory Physics department and department of Environmental Sciences University of Peshawar for lab experimentations.
Authors’ Contribution
Nazish Huma Khan is the original author who worked on this study as M. Phil researcher, now enrolled in PhD. This work was done under the supervision of Dr. Muhammad Nafees. Adila Bashir helped in field survey and laboratory analysis.
References
Afridi, J.H., M. Sajjad, S. Ali, M. Nazir and N. Bacha. 2014. Comparing the profitability of bakar and other varieties of wheat in District Charsadda. Int. J. Food Agri. Econ. 2(1):177-190.
Arabi, E l., M. Rashed and A. Vermeulen. 1996. Environmental impacts of sewage water irrigation on ground water, Int. managing environmental changes due to irrigation and drainage. Proceedings of special workshop, International commission, Cairo, Egypt.
Arnold, E. 1992. Standard methods for the examination of water and wastewater. 18th edition, American public health association Washington, DC, Method 4500-H+B. pH value/electrometric method. P. 209-269.
Arora, M., B. Kiran, S. Rani, A. Rani, B. Kaurand and N. Mittal. 2008. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem1. 11(4):811-815. http://dx.doi.org/10.1016/j.foodchem.2008.04.049
Bennett, D.H., W.E. Kastenberg and T.E. McKone. 1999. A multimedia, multiple pathway risk assessment of atrazine: the impact of age differentiated exposure including joint uncertainty and variability. Reliability Engineering and System Safety. 63(2):185-198. http://dx.doi.org/10.1016/S0951-8320(98)00046-5
Bratakos, M.S., E.S. Lazos and S.M. Bratakos. 2002. Chromium content of selected Greek foods. Sci. Total Environ. 290(1-3):47-58. http://dx.doi.org/10.1016/S0048-9697(01)01057-9
Boularbah, A., C. Schwartz, G. Bitton, W. Aboudrar, A. Ouhammou and J.L. Morel. 2006. Heavy metal contamination from mining sits in south Morocco: Assessment of metal accumulation and toxicity in plants. Chemosphere. 63(5):811-817. http://dx.doi.org/10.1016/j.chemosphere.2005.07.076
Camelo, L.G., S. R. Miguez and L. Marbh. 1997. Heavy metals input with phosphate fertilizers used in Argentina. Sci. Total Environ. 204(1):245-250.
Chary, N.S., C.T. Kamala and D.S.S. Raj. 2007. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Eco. Environ. Safety. 69(3):513-524. http://dx.doi.org/10.1016/j.ecoenv.2007.04.013
Chen, M., and L.Q. Ma. 1998. Comparison of four USEPA digestion methods for metal analysis using certified and Florida soils. J. Environ. Qual. 27:1294–1300. http://dx.doi.org/10.2134/jeq1998.00472425002700060004x
Chen, M., and L.Q. Ma. 2001. Comparison of aqua-regia methods for 20 Florida soils. Soil Sci. Soc. Am. J. 65(2):491-499. http://dx.doi.org/10.2136/sssaj2001.652491x
Cheli, F., A. Campagnoli, V. Ventura, C. Brera, C. Berdini, E. Palmaccio and V. DellOrto. 2010. Effects of industrial processing on the distributions of deoxynivalenol, cadmium and lead in durum wheat milling fractions. Food Sci. Technol. 43(7):1050-1057.
Chien, L.C., T.C. Hung, K.Y. Choang, C.Y. Yeh and P.J. Meng. 2002. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 285:177–85. http://dx.doi.org/10.1016/S0048-9697(01)00916-0
Dudka, S. and W. P. Miller. 1999. Accumulation of potentially toxic elements in plants and their transfer to human food chain. J. Environ. Sci. Health. 34(4):681-708. http://dx.doi.org/10.1080/03601239909373221
Freitas, H., M.N.V. Prasad and Pratas, J. 2004. Plant community tolerant to trace elements growing on the degraded soils of Sao Domingos mine in the South East of Portugal. Environ. Int. 30(1):65-72. http://dx.doi.org/10.1016/S0160-4120(03)00149-1
Gee, G.W., and J.W. Bauder. 1986. Particle size analysis. In: Klute, A. (Ed.), Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods, 2nd ed. Agronomy, vol. 9. p. 383-409. American Society of Agronomy and Soil Science Society of America, Madison.
IUCN (The World Conservation Union), 1994, Pakistan. Planning, Environment & Development. Department Civil Secretariat, Peshawar. Department of Environmental Planning and Management Peshawar University Peshawar. p.14-28
Iqbal, M.A., M.N. Chaudhary, S. Zaib, M. Imran, K. Ali and A. Iqbal. 2011. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb) in agricultural soils and spring seasonal plants, irrigated by industrial waste water. J. Environ. Technol. Manag. 2(1):1-9.
Iyaka, Y.A. 2011. Nickel in Soils: A review of its distribution and impacts. Sci. Res. Essays. 6(33):6774-6777.
Ivezic, V., Z. Loncaric, M. Engler and B.R. Kerovec. Singh. 2013. Singh comparison of different extraction methods representing available and total concentrations of Cd, Cu, Fe, Mn and Zn in soil. Poljoprivreda. 19(1):53-58.
Khan, S., Q. Cao, Y.M. Zheng, Y.Z. Huang and Y.G. Zhu. 2008. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 152(3):686-692. http://dx.doi.org/10.1016/j.envpol.2007.06.056
Khan, S., S. Rehman, K. Anwar-Zeb, K.M. Amjad and S.M. Tahir. 2010. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, Northern Pakistan. Ecotox. Environ. Safe. 73(7):1820-1827. http://dx.doi.org/10.1016/j.ecoenv.2010.08.016
Khan, T., S. Muhammad, B. Khan and H. Khan. 2011. Investigating the levels of selected heavy metals in surface water of Shah Alam River (A Tributary of River Kabul, Khyber Pakhtunkhwa). J. Himal. Earth Sci. 44(2):71-79.
Khan, S., M. Shahnaz, N. Jehan, S. Rehman, M.T. Shah and I. Din. 2012. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 30(1):1-9.
Khanlari, Z.V., and M. Jalali. 2008. Concentrations and chemical speciation of five heavy metals (Zn, Cd, Ni, Cu, and Pb) in selected agricultural calcareous soils of Hamadan Province, Western Iran. Arch. Agron. Soil Sci. 54(1):19-32. http://dx.doi.org/10.1080/03650340701697317
Lendinez, E., M.L. Lorenzo, C. Cabrera and M.C. López. 2001. Chromium in basic foods of the Spanish diet: Seafood, cereals, vegetables, olive oils and dairy products. Sci. Total Environ. 278(1–3): 183-189.
Lokeshwari, H. and G.T. Chandrappa. 2006. Impact of heavy metal contamination of Bellandur Lake on soil and cultivated vegetation. Curr. Sci. 91(5):623- 628.
Loutfy, N., M. Fuerhacker, P. Tundo, S. Raccanelli, A.G. Dien and M.T. Ahmed. 2006. Intake of dioxins and dioxin-like PCBs, due to the consumption of dairy products, fish/seafood and meat from Ismailia city, Egypt. Sci. Total Environ. 370(1):1-8. http://dx.doi.org/10.1016/j.scitotenv.2006.05.012
Mehlich, A. 1984. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15:1409-1416. http://dx.doi.org/10.1080/00103628409367568
Nafees, M., H. Khan, M.R. Jan, N. Rashid and F. Khan. 2009. Soil contamination in Swat valley caused by cadmium and copper. Sarhad J. Agri. 25(1):37-43.
Nelson D.W., and L.E. Sommers. 1982. Total carbon, organic carbon and organic matter. In: methods of soil analysis (Ed. A.L. Page). Part 2. Agronomy Monographs 9.ASA and SSSA, Madison. WI. p. 539-579.
Nicholsona, F.A., S.R. Smith, B.J. Alloway, C.C. Smith and B.J. Chambersa. 2003. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 311(1-3):205-219. http://dx.doi.org/10.1016/S0048-9697(03)00139-6
Oluyemi, E.A., G. Feuyit, J.A.O. Oyekunle and A.O. Ogunfowokan. 2009. Seasonal variations in heavy metal concentrations in soil and some selected crops at a landfill in Nigeria. Afr. J. Environ. Sci. 2(5):89-96.
Parveen, S., N. Wajahat M.F. Ahmad, A. Khan and I.A. Khattak. 2006. Nutritional status of different orchards irrigated with wastewater in district Peshawar. J. Agri. Biol. Sci. 1(1):1990-6145.
Quevauviller, P., J. Imbert and M. Olle. 1993. Evaluation of the use of microwave oven systems for the digestion of environmental samples. Mikrochim. Acta. 112:147–154. http://dx.doi.org/10.1007/BF01243331
Rahman, M.A., M.M. Rahman, S.M. Reichman, R.P. Lim and R. Naidu. 2014. Heavy metals in Australian grown and imported rice and vegetables on salein Australia: Health hazard. Ecotoxicol. Environ. Safety. 100:53-60. http://dx.doi.org/10.1016/j.ecoenv.2013.11.024
Richard, L.A. 1954. Diagnosis and improvement of Saline and Alkali soils. USDA Handbook 60. Washington DC.
Ryan, J., G. Estefan and A. Rashid. 2001. Soil and Plant Analysis Laboratory Manual. 2nd ed. Int. Center for Agric. Res. in the Dry Areas (ICARDA), Aleppo, Syria. p.172.
Sadiq, M.S., S. Haider and G. Abbas. 2005. Genetic parameters for economic traits in exotic germplasm of mungbean (Vignaradiata (L.) Wilczek). Agri. Res. 43:103-109.
Simeonov, V., J.A. Stratis, C. Samara, G. Zachariadis, D. Voutsa, A. Anthemidis, M. Sofoniou and T. Kouimtzis. 2003. Assessment of the surface water quality in northern Greece. Water Res. 37(17):4119-4124. http://dx.doi.org/10.1016/S0043-1354(03)00398-1
Shanker, A.K., C. Cervantes, H. Loza-Tavera and S. Avudainayagam. 2005. Chromium toxicity in plants. Environ. Int. 31(5):739-753. http://dx.doi.org/10.1016/j.envint.2005.02.003
Shirdama, R., Z.M. Tehrani and F. Dastgoshade. 2008. Microwave assisted digestion of soil, sludge and sediment for determination of heavy metals with ICP-OES and FAAS.Rasayan. J. Chem. 1(4):757-765.
Sheldrick, B.H. and C. Wang. 1993. Particle size distribution, soil sampling and methods of analysis. Canadian Society of Soil Science. Lewis Publishers. Ann Arbor. p. 499-511.
Shirisha, K., L. Kanwar, B. Sahrawat, D. Prathibha, P. Suhas and Wani. 2014. Simple and accurate method for routine analysis of heavy metals in soil, plant, and fertilizer. Commun. Soil Sci. Plant Anal. 45(16):2201-2206. http://dx.doi.org/10.1080/00103624.2014.911303
USEPA (United States Environmental Protection Agency), 2002. Risk-based concentration table. Philadelphia PA: United States Environmental Protection Agency, Washington Agency, Washington DC.
Wang, Y-P., J-Y. Shi, H. Wang, Q. Lin, X-C. Chenand and Y-X. Chen. 2007. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxol. Environ. Safe. 67(1):75-81. http://dx.doi.org/10.1016/j.ecoenv.2006.03.007
Westfall, D.G., J.J. Mortvedt, G.A. Peterson and W.J. Gangloff. 2005. Efficient and environmentally safe use of micronutrients in agriculture. Commun. Soil Sci. Plant Anal. 36(1-3):169-182. http://dx.doi.org/10.1081/CSS-200043024
WHO (World Health Organization), 1993. Evaluation of certain food additives and contaminants (41st Report of the Joint FAO/WHO Expert Committee on Food Additives), WHO Technical Report Series, No. 837.
WHO (World Health Organization), 1996. Permissible limits of heavy metals in soil and plants, Genava Switzerland.
Yang, L.S., X.W. Zhang, Y.H. Li, H.R. Li, Y. Wang andY. Wang. 2012. Bioaccessability and risk assessment of cadmium from uncooked rice using an in vitro digestion model. Biol. Trace Elem. Res. 145(1):81-86. http://dx.doi.org/10.1007/s12011-011-9159-x
Yu, L., W. Yan-bin, G. Xi, S. Yi-bing, W. Gang. 2006. Risk assessment of heavy metals in soils and vegetable around non-ferrous metals mining and smelting sites, Baiyin China. J. Environ. Sci. 18(6):1124-1134. http://dx.doi.org/10.1016/S1001-0742(06)60050-8
Zhao, K., X. Liu, J. Xu and H.M. Selim. 2010. Heavy metals contaminations in a soil-rice system: Identification of spatial dependence in relation to soil properties of paddy fields. J. Hard Mater. 181(1-3):778-787. http://dx.doi.org/10.1016/j.jhazmat.2010.05.081
To share on other social networks, click on any share button. What are these?








