Response of Mid and Short Season Promising Genotypes of Cotton Crop to Earias vittella (Fab.) Infestation in Punjab, Pakistan
Response of Mid and Short Season Promising Genotypes of Cotton Crop to Earias vittella (Fab.) Infestation in Punjab, Pakistan
Muhammad Tahir Jan1,2, Mushtaq Ahmad Saleem3,*, Muhammad Binyameen1,* and Sarfraz Ali Shad1
1 Department of Entomology, Faculty of Agricultural Sciences & Technology, Bahauddin Zakariya University, 60800 Multan, Pakistan
2 Cotton Research Station, Regional Agriculture Research Institute, Model Town A, Gulberge Road, Bahawalpur
3Al Ghazi Institute of Technology, Dera Ghazi Khan
ABSTRACT
Spotted bollworm, Earias vittella Fab (Lepidoptera: Noctuidae) is serious Asiatic pest, particularly associated with cotton in Pakistan. The study was planned to determine response of long and short duration cotton genotypes to E. vittella infestation. Field experiment was conducted at the Central Cotton Research Institute, Multan for two years. Long and short duration genotypes were sown during the 3rd week of May to investigate their response to E. vittella. E. vittella infestation (damage in fruits and larval population) was almost similar during both the years of experimentation. However, the fruiting parts were higher during second year as compared to the first year. The response of the genotypes CIM-496 and CIM-506 was variable to E. vittella infestation in fruiting parts and damage in mature bolls. Treatments showed significant impact on fruiting parts, number of damage fruits and larval population. However, no significant interaction was observed between the varieties and treatments. The most critical months were August and September in which the rate of IMF damaged by single larva was 4.5 and 4.1 and MB at the rate of 1.5 and 1.6 in CIM-496 while 3.6 and 4.1 IMF and 1.7 MB damage per larva in CIM-506, respectively. By controlling the pest infestation up to70-80% damage or 66% larval population, the yield was increased by 22-24% in long or short duration genotypes. Inevitable losses due to E. vittella were 0.8% in IMF or 0.3-0.4% in MB even with 10 applications in both the genotypes. The critical tolerance period for the long duration genotype CIM 496 was the last week of August and for short duration genotype CIM-506 was the second week of August. Similarly losses compensation was 4.5 and 9.1% higher in long duration genotypes as compared to short duration genotype in 1%TL and TL2, respectively. It was concluded that E. vittella can cause 22-24% fruiting losses if the pest is not controlled. Plant protection measures should therefore be taken before the critical tolerance period.
Article Information
Received 06 May 2019
Revised 30 June 2020
Accepted 13 February 2021
Available online 04 August 2021
(early access)
Published 20 April 2022
Authors’ Contribution
MTJ and MAS conceived and designed the experiments. MTJ and MB analyzed the data, prepared the figures and tables, and wrote the manuscript. SAS and MAS reviewed the manuscript and read and approved the final manuscript.
Key words
E. vittella, Mid-season cotton variety, Short season cotton variety, Critical period, Loss compensation.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190506110521
* Corresponding author: [email protected]; [email protected]
0030-9923/2022/0004-1719 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Earias vittella is a serious Asiatic pest of Malvaceae and particularly associated with cotton (Abro et al., 2003). The larvae of E. vittella feed on growing vegetative parts, developing seed in the cotton bolls, shoots of the main stem, tops of side branches and flower buds (Kumar and Urs, 1988; Abhilash and Patil, 2008). This pest causes upto 14, 51, 3 and 69 % damage to seedlings, buds, flowers and bolls (Arif and Attique, 1990; Kamaluddin, 1994; Dhillon and Sharma, 2004) and as a result of high infestation, 20% seed cotton yield is lost (Khan, 2011). It remains active throughout the year, produces 6-8 generations and reduces the seed cotton yield from 66 to 91%, when this pest infests the bolls during August to September (Sidhu and Sandhu, 1977). E. vittella showed significant effects on cotton species Gossypium hirsutum L. for larval survival, pupation, adult emergence, egg hatchability and growth index over G. arboreum (Dhillon and Sharma, 2004). In Pakistan, most of the commercial non Bt-cotton varieties belong to G. hirsutum and were found susceptible to E. vittella (Abro et al., 2003; Razaq et al., 2004). In any cropping system, genotypes with varied crop maturity duration play vital role in development of crop management strategies (Raper and Gwathmey, 2014). For instance cultivation of short season varieties of cotton instead of conventional full season varieties offer several potential benefits to cotton growers (Silvertooth and Farr, 2001). One benefit of earliness in cotton was thought to reduce late season pest infestations and diseases and increase at economic return by reducing input cost (Anderson et al., 1976). In Pakistan, infestation by the late season cotton pests; i.e. Pectinophora gossypiella (Saunders) and Bemisia tabaci (Gennadius), during September and first week of October and high population of Helicoverpa armigera (Hübner) in 3rd week of September to 4th week of October cause severe problems to cotton (Baloch et al., 2014). In case of these serious insect pests, entomologists stress the importance of utilizing a short season cotton production pattern on a wide-spread basis to extend the host-free period (Silvertooth and Farr, 2001). On other hand, the short season cotton varieties offer no compensation time for early season square loss in case of early infestation of E. vittella, which needs more protection of these squares than those formed late in the season (Ahmad and Malik, 1996). Therefore the exact combination of conditions leading to a definite advantage of short season variety over mid or full season is not clear (Silvertooth and Farr, 2001).
Considering the importance the integrated pest management (IPM) in the cotton cropping system, the present study was planned to evaluate two commercial and promising non-Bt genotypes having distinct growth periods (long and short duration) to determine response of these genotypes to E. vittella infestation under sprayed and unsprayed conditions. This study in future, will lead to determine the critical period of tolerance to E. vittella infestation and to develop management strategies for the control of this pest for each genotype.
Materials and methods
Experimental design
Field experiments were conducted at the Central Cotton Research Institute (30°12’N, 71°26’E) Multan, Pakistan over two crop seasons. Two commercial genotypes, CIM-496 and CIM-506, were sown during the 3rd week of May, in a split plot design. The genotypes were the main plots with two conditions, (1%TL = Sprayed at approximately 1% damage level and TL2 =Unsprayed) as sub plots and three replications. Plot size, row to row and plant to plant distance was 6.9 x 12.2 m, 0.76m and 22.9 cm, respectively. All other agronomic requisites were provided when required.
Sampling of E. vittella infestation
Fruiting parts (healthy and damaged) along with larval population in both immature fruits and mature bolls and total fruits were recorded. For analysis, various abbreviations were used as: IMF (immature fruits), MB (mature bolls), TFP (total fruiting parts), IMF-dm, MB-da, TFP-da (damage in the respective fruiting parts) and IMF-lar, MB-lar, TFP-lar (larval population in the respective fruiting parts). For regular weekly pest scouting, five randomly selected plants in a row were examined for healthy and freshly damaged fruits with larval population. However, to ascertain the insecticidal impact on E. vittella infestation data was recorded after three days of each insecticide application. Observation was initiated from 1st July till mid October and last week of October in the respective years of the study. Before the crop was harvested in the 3rd week of October in 1st crop season and 3rd week of November in 2nd crop season, unopened bolls (UOB), open bolls (OB) and total bolls (TB) were also recorded, to determine recovery of losses due to E. vittella infestation.
General characteristics of CIM-496 and CIM-506
Khan et al. (2011) classified CIM-496 as mid-season genotype and CIM-506 as short season genotype on the basis of crop duration. The former genotype completes its cycle till November (140-160 days) whereas the later one terminates within 100-120 days (upto mid October).
Insecticides and spray applications
Three commercial insecticides were used in rotation including spinosad (Tracer) 480 EC (Dow Agro Sciences) @197.6 ml hec-1, deltamethrin (Decis) 10EC (Bayer) @ 815.1 ml hec-1 and cypermethrin (Arrivo) 10EC (Bayer) @ 815.1 ml hec-1. Spray application was made at or before availing 1% immature fruit damage using simple mathematical equation as following:

Number of applications during the crop season
In CIM-496, ten applications of cypermethrin, deltamethrin and spinosad were given during each crop season from mid July to mid October. During the first crop season, one application in July (cypermethrin), four applications in August (two deltamethrin, one each cypermethrin and spinosad), in September, (two deltamethrin, one each cypermethrin and spinosad) and one application in October (spinosad) were given. During the second cop season, one application in July (cypermethrin), four applications in August (two deltamethrin, one each cypermethrin and spinosad) and in September three applications (one each deltamethrin, spinosad and cypermethrin) and two in October (deltamethrin and spinosad) were given.
Similarly in CIM-506, during 1st crop season ten applications, four applications in the month of August i.e. two application of deltamethrin, one each of cypermethrin and spinosad) and six applications in month of September i.e. three of cypermethrin, two of spinosad and one of deltamethrin were given for the control of E. vittella keeping below 1% TL. During the 2nd crop season nine applications, five applications in the month of August i.e. two each deltamethrin and spinosad and one cypermethrin) and four applications in the month of September i.e. two applications of cypermethrin and one each application of spinosad and deltamethrin were given.
Data analysis
Before subjected to analysis, the entire replicated data for fruiting parts only was transformed to running averages to synchronize the data with the help of following equation:
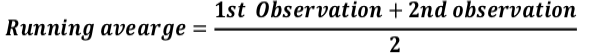
Statistix 8.1v Software (Statistix, 2008) was used for statistical analysis. Split Plot ANOVA was used to determine the combined effect of the genotypes for number of fruits, damage, larval population and their interaction presented in Table I. Data for fruiting parts along with damages and larval population during study period was pooled and the mean of two years was analyzed with General ANOVA for working out the phenology i.e. the seasonal variation and means of damage and larval population for each genotype shown in Table II. Differences in percentages among damages as well as the larval populations were estimated by common mathematical equations to determine the larval survival rate or escapism from the insecticide applications, inevitable losses in total fruits and bolls. Damage compensation or tolerance was determined through the number of leftover bolls when the crop was harvested in October during 1st crop season and in November during 2nd season. For critical period of tolerance and rate of fruit consumption of E. vittella were graphically interpreted.
For calculation of percentage differences between the variables:

RESULTS
Phenology of E. vittella infestation
ANOVA analyzed with split plot design is presented in Table I. E. vittella infestation (damage in fruits and larval population) was almost similar during both the crop seasons. However, the fruiting parts were significantly higher during 2nd season as compared to the 1st season. Both the genotypes i.e. CIM-496 and CIM-506 responded to E. vittella infestation in similar pattern except the fruiting parts as well as damage in mature fruits which were significantly different between both the genotypes. Treatments revealed significant differences in pest infestation and fruiting parts as well. However, no significant interaction was observed between the varieties and treatments.
Pest damage
The pest was active during the entire crop growth period. However, its damage and larval population responded significantly different in different months of the growing period. The most critical months were August and September in which the reproductive growth of the plant and pest infestation intensity were significantly higher. Figures 1 and 2 represent fruit damage caused by larvae. In immature fruits (IMF) including squares, flowers and small bolls, rate of fruit damage was higher during these two months of crop growth period as compared to mature bolls (MB). From Table II it was estimated at 4.5 and 4.1 IMF damaged by single larva in CIM-496 while 3.6 and 4.1 IMF in CIM-506 during August and September, respectively. Mature fruits (MB) were damaged at the rate of 1.5 and 1.6 per larva in the former genotype and 1.7 per larva in the later genotype. Overall damage per larva was ranged from 3 to 4 in total fruiting parts (TF) in both the genotypes. In October both, the rate of food consumption and infestation (the damage as well as larval population) were significantly reduced.
Table I.- Split plot ANOVA for varieties, treatments and varieties-treatment interaction.
|
Sources of variances and ANOVA |
Immature fruits |
Mature fruits |
Total fruits |
|||||||
|
FP |
Da |
Lar |
FP |
Da |
Lar |
FP |
Da |
Lar |
||
|
Years |
F (1,2) |
2.26 |
2.75 |
0.19 |
505.41 |
1.27 |
0.05 |
227.58 |
0.00 |
0.33 |
|
P- values |
0.27 |
0.24 |
0.71 |
0.002* |
0.34 |
0.84 |
0.004* |
1.00 |
0.62 |
|
|
Varieties |
F (1,2) |
61.78 |
0.69, |
0.42 |
473.74 |
27.92 |
0.51 |
136.33 |
3.47 |
2.31 |
|
P- values |
0.016* |
0.49 |
0.58 |
0.002* |
0.034* |
0.55 |
0.007* |
0.20 |
0.27 |
|
|
Treatment |
F (1,88) |
2.27 |
48.51 |
38.53 |
7.13 |
43.32 |
44.05 |
8.52 |
59.89 |
60.21 |
|
P- values |
0.102 |
0.00* |
0.00* |
0.009* |
0.00* |
0.00* |
0.004* |
0.00* |
0.00* |
|
|
Var x Tret |
F (1,88) |
0.218 |
0.08 |
0.23 |
0.00 |
0.13 |
0.00 |
0.09 |
0.12 |
0.09 |
|
P- values |
0.67 |
0.77 |
0.63 |
0.97 |
0.72 |
0.95 |
0.76 |
0.73 |
0.77 |
|
FP, fruiting parts; Da, damage fruits; Lar, larval population.
The results further revealed that the rate of food consumption by E. vittella was slightly higher in August in CIM-496 while in September in CIM-506. Similarly IMF was more preferred by the larvae as compared to MB.
Impact of control measures on E. vittella infestation
The results in Table II reveal that 10 applications in CIM-496 and 9-10 applications in CIM-506 kept E. vittella infestation below 1% damage levels with significant increase in fruiting parts while reduction in E. vittella infestation when compared with untreated plots. In CIM-496, 22.1 % MB and 18.2 % TF were increased due to plant protection measures when 73.3% damage and 66.3% larval population was controlled. Similarly in CIM-506, 24.5% MB and 16.8% TF increased whereas 80.4% damage and 66% larval population were reduced. It is concluded that controlling the pest infestation up to 70-80% damage or 66% larval population, the yield might be increased by 22-24% in long or short duration genotypes.
Larval survival / escapism from the insecticide / inevitable losses
From Table II it was estimated that allowing upto 1% damage level, E. vittella caused 1.6-1.41 (0.8%) damage in IMF or 0.6-0.7 (0.3-0.4%) damage in MB that could not be controlled even with 10 applications in both the genotypes and were considered as inevitable losses. These losses were mostly ignored or not considered and cause slight miscalculation. These losses should be taken as correction factors at the time of estimation of ETL for E. vittella.
Table II.- Treatment and seasonal mean number of fruiting parts (FP), damage fruits (Da) and larval population (Lar) of E. vittella in immature (IMF), mature bolls (MB) for the genotypes (CIM-496) and (CIM-506) under unsprayed plots.
|
Source of variance |
Treatments |
Immature fruits (IMF) |
Mature fruits (MB) |
Total fruits (TF) |
||||||
|
FP |
Da |
Lar |
FP |
Da |
Lar |
FP |
Da |
Lar |
||
|
CIM-496 |
||||||||||
|
Treatment |
1%TL |
200.9 a |
1.6 b |
0.8 b |
189.3 a |
0.6 b |
0.4 b |
390.2 a |
2.13 b |
1.15 b |
|
TL2 |
149.0 b |
14.0 a |
3.2 a |
120.8 b |
3.9 a |
2.5 a |
269.8 b |
17.90 a |
5.67 a |
|
|
% difference in T1 over T2 |
22.1 |
18.2 |
-73.3 |
-66.3 |
||||||
|
ANOVA |
F (1,19) |
53.10 |
23.94 |
6.91 |
19.56 |
28.12 |
31.55 |
36.75 |
0.88 |
40.27 |
|
P-values |
0.00 |
0.001 |
0.025 |
0.003 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
|
Months |
July |
83.9 c |
2.0 b |
0.7 b |
5.7 c |
0.05b |
0.0 b |
89.55 d |
2.02 b |
0.7 b |
|
August |
308.9 a |
15.4 a 4.5 / lar |
3.4 a |
104.4 b |
2.2 a 1.5/ lar |
1.5 a |
413.3 b |
17.62 a 3.7/ lar |
4.8 a |
|
|
September |
247.7 b |
11.93 a 4.1/ lar |
2.9a |
250.2 a |
3.9 a 1.6/ lar |
2.4 a |
497.9 a |
15.78 a 3.0/ lar |
5.3 a |
|
|
October |
83.9 c |
1.8 b 2.0/ lar |
0.9 b |
260.1a |
2.9 a 1.5/ lar |
1.9 a |
319.4 c |
4.65 b 1.7/ lar |
2.7 b |
|
|
ANOVA |
F (3,19) |
294.81 |
7.58 |
20.51 |
62.45 |
6.37 |
7.31 |
78.58 |
7.61 |
8.78 |
|
P-values |
0.00 |
0.016 |
0.02 |
0.00 |
0.003 |
0.002 |
0.000 |
0.002 |
0.007 |
|
|
CIM-506 |
||||||||||
|
Treatment |
1%TL |
168.44 a |
1.41 b |
0.80 b |
169.88 a |
0.70 b |
0.47 b |
338.30 a |
2.10b |
1.23 b |
|
TL2 |
137.82 b |
14.87 a |
3.58 a |
102.97 b |
4.50 a |
2.58 a |
240.76 b |
19.34a |
6.14 a |
|
|
% difference in T1 over T2 |
24.5 |
16.8 |
-80.4 |
-66.0 |
||||||
|
ANOVA |
F (1,19) |
9.42 |
26.18 |
30.11 |
16.91 |
31.01 |
30.84 |
20.74 |
33.48 |
45.77 |
|
P-values |
0.0063 |
0.001 |
0.00 |
0.006 |
0.00 |
0.00 |
0.002 |
0.00 |
0.00 |
|
|
Months |
July |
36.48 c |
0.60 b |
0.30 b |
0.43 c |
0.00 c |
0.00 b |
36.92 d |
0.60 b |
0.30 c |
|
August |
299.03 a |
16.27 a 3.6/ lar |
4.52 a |
249.17a |
2.45 b 1.7/ lar |
1.47 a |
371.07 b |
18.70 a 3.13/ lar |
5.98 a |
|
|
September |
249.43 b |
13.75 a 4.2/lar |
3.27 a |
224.02 a |
4.65 a 1.7/ lar |
2.57 a |
498.57 a |
18.37 a 3.17/ lar |
5.80 a |
|
|
October |
36.48 c |
1.93 b 2.8/ lar |
0.68 b |
72.07 b |
3.30 ab 1.6/ lar |
2.05 a |
251.57 c |
5.22 b 1.9/ lar |
2.72 b |
|
|
ANOVA |
F (3,19) |
200.63 |
9.29 |
16.09 |
54.17 |
8.21 |
8.53 |
84.01 |
9.57 |
14.17 |
|
P-values |
0.00 |
0.005 |
0.00 |
0.00 |
0.001 |
0.009 |
0.00 |
0.005 |
0.00 |
|
Means followed by the same letter in a column are not significantly different at p=0.05.
Table III.- Mean number of unopened bolls (UOB open bolls (OB) and total bolls (TB) and % increase differences in treatments, years and varieties.
|
Genotypes, ANOVA and differences |
1st year |
2nd year |
||||||
|
UOB |
OB |
UOB |
OB |
|||||
|
1%TL |
TL2 |
1%TL |
TL2 |
1%TL |
TL2 |
1%TL |
TL2 |
|
|
CIM-496 |
43.3 a |
24.0 b |
81.0 a |
36.0 b |
22.3 a |
8.0 a |
108.3 a |
58.0 b |
|
F(1,2) P-values |
841.0 (0.0012) |
141.28 (0.01) |
16.51(0.056) |
75.75 (0.013) |
||||
|
1%TL % over TL2 |
28.7 |
38.5 |
47.2 |
30.2 |
||||
|
% Inc in Y2 over Y1 |
-32.0 |
-50.0 |
14.4 |
23.4 |
||||
|
CIM-506 |
13.0 a |
12.7 a |
83.0 a |
37.0 b |
0.3 |
0.3 |
99.0 a |
48.3 b |
|
F(1,2) P-values |
0.02 (0.90) |
396.75 (0.003) |
0.01 (0.93) |
3300.57 (0.003) |
||||
|
1%TL % over TL2 |
1.2 |
38.3 |
0.0 |
34.4 |
||||
|
% Inc in Y2 over Y1 |
-95.5 |
-95.4 |
8.8 |
13.2 |
||||
|
% Inc. in V1 over V2 |
53.8% |
30.8% |
-1.2% |
-1.4% |
97.3% |
92.8% |
4.5% |
9.1% |
Means followed by the same letter in a column are not significantly different at p=0.05.
Similarly some of the larvae at 1% threshold level i.e. on an average of 0.8 larvae in IMF and 0.4-0.5 in MB were recorded during each observation. These larvae were alive damaging the crop. These larvae could therefore be considered as escaped from the insecticide applications.
Response of the cotton genotypes to E. vittella infestation
The responses of both the genotypes to E. vittella infestation were observed as critical period for tolerance during the crop growth periods and shown in Table III. Compensation at the time of harvest in total bolls (open (OB) and unopened bolls (UOB) is given in Table III.
Table III shows number of total fruiting parts per 5 plants per week plotted against 1% threshold level (1%TL) and unsprayed plots (TL2). The continuous lines represent fruiting pattern in 1%TL and dotted line for fruiting pattern in TL2.
In long duration genotype, CIM-496, both the continuous and doted lines run together from the month of July till the last week of August and started separating from each other in 1st week of September and the gap increased to a significant level till the time of harvest of the crop during both the year of study. The gap between these lines reveals the impact of insecticides as well as the response of the genotype to pest infestation. This long duration genotype CIM-496 tolerated the pest infestation till the last week of August and then the losses could not recover when E. vittella remained unchecked thereafter. The last week of August is the critical tolerance period for the long duration genotype CIM-496.
In short duration genotype of CIM-506, both the lines run together upto 2nd week of August and got separated afterword that continued till harvest. Similar situation was observed during second year of the study. The critical tolerance period of the short duration genotypes could be the second week of August.
The crop was harvested on 23rd October during 1st crop season and a month later on 23rd November during 2nd season, to determine year wise, treatment wise and varietal wise response of the short and long duration genotypes to E. vittella infestation. Table III reveals significant impact of control measures in percentage opening, and compensation percentage. In CIM-496, the impact of control measures could be observed on both the unopened (left over) and open bolls. In 1st crop season, 28.7 % UOB and 38.5 % OB were recorded whereas 47.2 % UOB and 30.2% OB in 2nd crop season were higher in 1%TL than in TL2. In CIM-506, only OB was taken into account and 38.5 % in 2009 and 34.4% in 2nd year were higher in 1%TL when compared with TL2. Very few leftover bolls (UOB) were observed with the negligible impact.
UOB was reduced by 32 and 50% while OB was increased by 14.4 and 23.4 % in CIM-496. OB was slightly increased by 8.8 and 13.2% in CIM-506 in 2nd crop season when compared with 1st crop season, respectively in both the treatments. The results further revealed that during 2009 UOB were higher by 53.8 and 30.8% but OB were lower by 1.2 and 1.4% in CIM-496 from CIM-506 when the crop was harvested in October. When the crop was harvested a month later in November during 2nd year, both UOB (97. 3 and 92.8%) and OB (4.5 and 9.1%) were increased in CIM-496 in both the treatments. Hence losses compensation was 4.5 and 9.1% higher in long duration genotypes as compared to short duration genotype in 1%TL (controlled) and TL2 (unsprayed conditions), respectively.
Discussion
Impact of E. vittella on cotton genotypes
Both the genotypes i.e. CIM-496 and CIM-506 were peer susceptible to E. vittella by showing similar trend of fruits damaged and larval population. It might be due to the availability of nutrients and preference to both the genotypes. It is a published fact that preference and non preference to a crop species is estimated on the basis of quality of the crop and the availability of the nutrients (Sharma et al., 1982; Dhillon and Sharma, 2004). Moreover, E. vittella larvae had always showed higher tendency to G. hirsutum with trivial variation over the other cotton species like G. arboreum (Dhillon and Sharma, 2004; Razaq et al., 2004; Khan et al., 2007). So the unchecked pest in the present study was found to be the most serious to both genotypes and caused maximum of 79% of total fruits and 74% mature bolls in CIM-496 and 78.0% of total fruits and 83% mature bolls in CIM-506.
Infestation of the unchecked E. vittella was continued either as longer as with the plant life from 1st week of July till 4th week of October on CIM-496 or as shorter as from 3rd week of July till 1st week of October on CIM-506. However, the peak infestation period was between August and September. Broadly speaking, E. vittella incidence commenced when the crop was three weeks old, mostly in the month of July if sown in the month of May. Infestation of E. vittella mostly initiates with the development of reproductive parts of the plants and its population builds up with the effective boll development phase of crop (Baloch et al., 1990; Vennila et al., 2005).
August and September are the peak months for the fruit bearing (fructification) and E. vittella infestation. Significantly higher damage in immature fruits (IMF) as compared to damage in mature bolls (MB) was due to frequent attack by young larvae (1st to 3rd instars) in immature fruits. MB were damaged by full grown larvae (Srinivasan, 2001). It was further observed that once full grown larvae entered the bolls, these remained there till pre-pupation period. The field observation revealed that larvae of all stages were recorded in immature fruits, while full grown larvae were in the bolls only. These findings follow the observations made by Johnson and Zalucki (2007) who anticipated that young larvae have relatively higher growth rate and feed at more sites unlike the mature and full-grown larvae that fed at the selected sites (bolls). Early studies attributed for larval shifting to the change in abundance of fruit in each age class as the season progressed (Wilson and Waite, 1982; Raubenheimer and Browne, 2000; Vennila et al., 2007). In addition the degree of food utilization depends on the digestibility of food and the efficiency with which digested food is converted into biomass resulting higher growth rate in young larvae (Batista-Pereira et al., 2002). Such a larval distribution indicated the intra- specific competition among E. vittella larvae and the behavioral adjustment by frequent movements for their survival. This disproportionate of fruiting structure damage substantiated the behavioral adaptations in response to competition among E. vittella larval stages (Reed, 1994).
The spray regime in the present study was used as management tactics against E. vittella for both mid and short duration genotypes. In the former case, insecticide applications covered the period from mid July to mid October whereas in later case the crop period was August to September. Cypermethrin, deltamethrin and spinosad were found to be the most effective combination of insecticides with 72-86% larval control at 1% TL supporting the previous results of Pardeshi et al. (2009) and Dhaka and Prajapati (2013) with similar performance.
The least damaged fruits by few E. vittella larvae in 1% TL were inevitable / unavoidable losses as was termed by Mi et al. (1998), El-Heneidy et al. (2003) and Abhilash and Patil (2008). Similarly survival of larvae as full grown in the green bolls in 1% TL was considered as escaped from the insecticide applications and were assumed as resistant larvae because Ahmad and Arif (2009) and Jan et al. (2015) had already reported resistance in E. vittella against cypermethrin (42-123 fold), deltamethrin (17-31 fold) and spinosad (15-20 fold). However, there could also be some other reasons to larval survival in the present study. For instance expiration of the insecticide residual activity, timing of application, pest-dose-response phenomenon called Hormesis, occur in the pest populations exposed to sub lethal doses of pesticides (Hedin et al., 1988; Dutcher, 2007). The pattern of insecticide combinations and estimation of post application survival of the larvae in the current study suggested that the insecticides should be used in combination (on alternate basis) for effective control otherwise resistance may develop using these insecticides separately with repeated applications.
Higher number of larvae survived as full-grown in the bolls under unsprayed condition supports favorable environment and minimum number of natural enemies in the field for E. vittella population build up. Fye and Surber (1971) were of the opinion that natural mortality of bollworm eggs and early- stage larvae were caused due to unfavorable weather conditions such as wind, rain, and high temperatures, while King and Coleman (1989) and Kogan et al. (1989) also suggested the low level of actions of natural enemies in E. vittella larval mortality in the field condition.
Response of the cotton varieties to E. vittella infestation
CIM-496 expressed tolerance to E. vittella infestation in unsprayed condition till last week of August. Afterward significant decrease in the number of fruiting parts was observed in the month of September. Similarly delaying in harvest had a positive impact on this genotype. The crop retained and compensated the damaged fruits due to E. vittella infestation in terms of leftover or UOP bolls at the time of harvest. Ahmad and Malik (1996) reported that full season cotton varieties have primary and secondary fruiting cycles with the full season production scheme. The secondary fruiting cycle or top crop would begin in late August and continue to early October. Ahmad and Malik (1996) and Silvertooth (2015) also determined the potential of fruit retention for loss of early and late season fruit in full season cotton varieties resulting from poor management, bad weather and poor insect control. Management tactics for the genotypes like CIM-496 having the potential to tolerate the E. vittella infestation for longer period and can retain the damage fruits in late season of the crop must be initiated prior to the critical period of the crop. The crop protection measures could be started a week before the start of critical period. Third week of August should be the threshold period in which plant protection measures become mandatory. Control measures through chemicals in the late August to mid October are suggested to manipulate E. vittella infestation on such genotypes.
On the contrary, CIM-506 showed the tolerance to pest infestation till 1st week of August with significant reduction in the number of fruiting parts thereafter under unsprayed condition. Moreover early termination of the crop by mid October and negligible number of UOB at harvest time show limited potential of boll retention or compensation by the genotype for early fruit losses due to E. vittella infestation. Ahmad and Malik (1996) were of the opinion that short season and early maturing varieties mature before 1st week of October and cannot utilize the secondary flowering cycle to produce high yields of quality cotton at reduced cost. However, these varieties have little capacity to recuperate from biotic and abiotic stresses experienced during the season. Hence, Reed (1994) considered Earias spp. such as E. insulana, E. vittella and E. biplaga threat to short season cottons where significant populations are present early in the season of India and Pakistan and for short season genotypes like CIM-506 the critical fruiting period is August and September. Mandatory period for plant protection measures in short duration genotype should be the first week of August. Control measures through chemicals are suggested in the months of August and September for successful E. vittella management on short duration genotypes with lower management cost and higher quality cotton with broader options to the growers in terms of double-cropping possibilities (Silvertooth and Farr, 2001).
Conclusions
In the country like Pakistan where diverse climatic environment exists, cotton varieties can easily be grown with clearly known varietal characters and pest protection cost. Based on the results of current study, it is suggested that mid or full season varieties should be adopted where the late season pests and following crop are not the issue and the growers are satisfied with more yields of cotton seed. Similarly short season cotton will be adopted where late season pests like P. gossypiella and H. armigera are the serious problems and the growers are in a hurry to plant the next crops. Further E. vittella can be managed sensibly if the first and successive applications are given well in time and at proper intervals at levels other than 1% threshold levels depending upon the nature of the genotypes.
Acknowledgments
We thank and acknowledg Dr. Zahid Mehmood, Director Central Cotton Research Institute, Multan and Mr. Muhammad Rafiq, Head Entomology, CCRI, Multan for their financial and technical support to conduct the trials. Dr. Muhammad Razaq from BZU Multan is appreciated for providing technical and moral support. This research was supported financially by the CCRI, Multan.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abhilash, C. and Patil, R., 2008. Determination of economic injury level for the soybean pod borer, Cydia ptychora (Meyrick). Karnataka J. agric. Sci., 21: 446-447.
Abro, G.H., Syed, T.S. and Dayo, Z.A., 2003. Varietal resistance of cotton against Earias spp. Pak. J. biol. Sci., 6: 1837-1839. https://doi.org/10.3923/pjbs.2003.1837.1839
Ahmad, M. and Arif, M.I., 2009. Resistance of Pakistani field populations of spotted bollworm Earias vittella (Lepidoptera: Noctuidae) to pyrethroid, organophosphorus and new chemical insecticides. Pest Manage. Sci., 65: 433-439. https://doi.org/10.1002/ps.1702
Ahmad, Z. and Malik, M.N., 1996. Short season cotton: how far can it go? Proceedings of the Beltwide Cotton Conferences, National Cotton Council of America, Memphis, Tennessee, USA. Papers Presented at a Technical Seminar at the 55th Plenary Meeting of the International Cotton Advisory Committee, Tashkent, Uzbekistan, pp. 32.
Anderson, J.M., Bridge, R.R., Heagler, A.M. and Tupper, G.R., 1976. The economic impact of recently developed early-season cotton strains on firm and regional cropping systems and income. Proceed. Belt wide Cott. Prod. Res. Conf. National Cotton Council of America, Memphis,T.N. USA, pp. 98-100.
Arif, M.I. and Attique, M.R., 1990. Alternative host in carry over of Earias insulana (Boisd.) and Earias vittella (F.) (Lepidoptera: Noctuidae) in Punjab, Pakistan. Pakistan Cottons, 34: 91-96.
Baloch, A.A., Soomro, B.A., Leghari, M.A. and Sanjrani, M.W., 1990. Studies on economic injury levels of insect pests of cotton. Turkish J. Ent., 14: 131-148.
Baloch, M., Khan, N., Rajput, M., Jatoi, W., Gul, G., Rind, I. and Veesar, N., 2014. Yield related morphological measures of short duration cotton genotypes. J. Anim. Pl. Sci., 24: 1198-1211.
Batista-Pereira, L.G., Petacci, F., Fernandes, J.B., Correˆa, A.G., Vieira, P.C., da Silva, M.F.G.F. and Malaspina, O., 2002. Biological activity of astilbin from Dimorphandra mollis against Anticarsia gemmatalis and Spodoptera frugiperda. Pest Manage. Sci., 58: 503-507. https://doi.org/10.1002/ps.478
Dhaka, S.S. and Prajapati, C.R., 2013. Management of okra fruit borer, Earias vittella (Fab.) with chemicals and biopesticides. Progr. Res., 8: 186-189.
Dhillon, M.K. and Sharma, P.D., 2004. Studies on biology and behavior of Earias vittella (Lepidoptera: Noctuidae) for mechanisms of resistance in different cotton genotypes. Crop Protect., 23: 235-241. https://doi.org/10.1016/j.cropro.2003.08.012
Dutcher, J.D., 2007. A review of resurgence and replacement causing pest outbreaks in IPM, General concepts in integrated pest and disease management. Springer, pp. 27-43. https://doi.org/10.1007/978-1-4020-6061-8_2
El-Heneidy, A.H., Ibraheem, M.M., Megahed, H.E., Attia, A.A., Magdy, A.A., Abdel-Awal, W.M. and Hassan, M.M., 2003. Assessment of economic injury and threshold levels for key cereal aphid species in Egyptian wheat regions. Bull. entomol. Soc. Egypt (Econ. Ser.), 29: 43-56.
Fye, R.E. and Surber, D.E., 1971. Effects of several temperature and humidity regimens on eggs of six species of lepidopterous pests of cotton in Arizona. J. econ. Ent., 64: 1138-1142. https://doi.org/10.1093/jee/64.5.1138
Hedin, P.A., Parrott, W.L., Jenkins, J.N., Mulrooney, J.E. and Menn, J.J., 1988. Elucidating mechanisms of tobacco budworm resistance to allelochemicals by dietary tests with insecticide synergists. Pesticide Biochem. Physiol., 32: 55-61. https://doi.org/10.1016/0048-3575(88)90121-6
Jan, M.T., Abbas, N., Shad, S.A., Rafiq, M. and Saleem, M.A., 2015. Baseline susceptibility and resistance stability of Earias vittella Fabricius (Lepidoptera: Noctuidae) to cypermethrin, deltamethrin and spinosad. Phytoparasitica, 43: 577-582. https://doi.org/10.1007/s12600-015-0477-y
Johnson, M.L. and Zalucki, M.P., 2007. Feeding and foraging behaviour of a generalist caterpillar: are third instars just bigger versions of firsts? Bull. entomol. Res., 97: 81-88. https://doi.org/10.1017/S0007485307004750
Kamaluddin, S., 1994. Redescription of spotted bollworm Earias fabia Stoll (Lepidoptera: Arctiidae) from Pakistan with special reference to its genitalia, life cycle, nature of damage and control. Proc. Pakistan Congr. Zool., 14: 321-325.
Khan, R.R., Ahmed, S., Saleem, M.W. and Nadeem, M., 2007. Field evaluation of different insecticides against spotted bollworms Earais spp. at district Sahiwal. Pak. Entomol., 29: 129-133.
Khan, S.A., Khan, N.U., Mohammad, F., Ahmad, M., Khan, I.A., Bibi, Z. and Khan, I.U., 2011. Combining ability analysis in intraspecific F1 diallel cross of upland cotton. Pak. J. Bot., 43: 1719-1723.
Khan, S.M., 2011. Varietal performance and chemical control used as tactics against sucking insect pests of cotton. Sarhad J. Agric., 27: 255-261.
King, E.G., and Coleman, R.J., 1989. Potential for biological control of Heliothis species. Annu. Rev. Ent., 34: 53-75. https://doi.org/10.1146/annurev.en.34.010189.000413
Kogan, M., Helm, C., Kogan, J. and Brewer, E., 1989. Distribution and economic importance of Heliothis virescens and Heliothis zea in North, Central, and South America and of their natural enemies and host plants. Proceedings of the Workshop on Biological Control of Heliothis: Increasing the Effectiveness of Natural Enemies, New Delhi, India, pp. 11-15.
Kumar, K.K. and Urs, K.C.D., 1988. Population fluctuation of Earias vittella (Fab.) on okra in relation to abiotic factors. Indian J. Pl. Protect., 16: 137-142.
Mi, S., Danforth, D.M., Tugwell, N.P. and Cochran, M.J., 1998. Plant-based economic injury level for assessing economic thresholds in early-season cotton. J. Cotton Sci., 2: 35-52.
Pardeshi, A.M., Bharodia, R.K., Joshi, M.D., Makadia, R.R. and Kate, A.O., 2009. Chemical control of Earias vittella (Fabricius) on okra. Int. J. Pl. Protect., 2: 231-233.
Raper, T.B. and Gwathmey, C.O., 2014. Guide to earliness management in short-season cotton production. Department of Agriculture, Institute of Agriculture, University of Tennessee, U.S.A., pp. 1-8.
Raubenheimer, D. and Browne, L.B., 2000. Developmental changes in the patterns of feeding in fourth and fifth instar of Helicoverpa armigera caterpillars. Physiol. Ent., 25: 390-399. https://doi.org/10.1046/j.1365-3032.2000.00212.x
Razaq, M., Aslam, M., Shad, S.A. and Naeem, M., 2004. Evaluation of some new promising cotton strains against bollworm complex. J. Res. (Sci.), 15: 313-318.
Reed, W., 1994. Earias spp.(Lepidoptera: Noctuidae). Insect pests of cotton. CAB International, Ascot, United Kingdom, pp. 151-176.
Sharma, H.G., Aarwal, R.A. and Singh, M., 1982. Effect of some antibiotic compounds in cotton on post-embryonic development of spotted bollworm (Earias vittella F.) and the mechanism of resistance in Gossypium arboreum. Proc. Anim. Sci., 91: 67-77. https://doi.org/10.1007/BF03186061
Sidhu, A.S. and Sandhu, S.S., 1977. Damage due to the spotted bollworm (Earias vittella Fabr.) in relation to the age of bolls of hirsutum variety j-34. J. Res. Punjab Agric. Univ., 14: 184-187.
Silvertooth, J.C., 2015. General maturity groups for cotton varieties. College of Agriculture, University of Arizona, Tucson, AZ.
Silvertooth, J.C., and Farr, C.R., 2001. Management considerations for short season cotton in Arizona. College of Agriculture and Life Sciences, University of Arizona, Tucson, AZ.
Srinivasan, G., 2001. Response of summer irrigated cotton (Gossypium hirsutum) to sowing of dates and nutrient-management practices. Indian J. Agron., 46: 552-556.
Statistix, 2008. Statistix 8.1. analytical software, Tallahassee. McGraw-Hill, Co., Maurice/Thomas text, Florida, USA.
Vennila, S., Biradar, V.K., Sabesh, M. and Bambawale, O.M., 2007. Know your cotton insect pest spotted and spiny bollworms. Crop Protection Folder Series, pp. 5 of 11.
Vennila, S., Biradar, V.K., Panchbhai, P.R., Gadpayle, J.G., Deshmukh, A.Y., Nemade, P.W., and Karanjkar, P.P., 2005. Seasonal dynamics, survival and feeding preference of Earias vittella (Fab.) larval instars on cotton. Annls. Pl. Protect. Sci., 13: 60-64.
Wilson, L.T. and Waite, G.K., 1982. Feeding pattern of Australian Heliothis on cotton. Environ. Ent., 11: 297-300. https://doi.org/10.1093/ee/11.2.297
To share on other social networks, click on any share button. What are these?









