Rapid and Simple Detection of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) by Reverse Transcription Loop-Mediated Isothermal Amplification
Rapid and Simple Detection of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) by Reverse Transcription Loop-Mediated Isothermal Amplification
Xi-wen Chen1,2, Qian Wang1,3, Miao Yin1, Zhong-hui Pu1,2, Ai-wei Guo3, Lian Li 1,3, Wen-tao Luo1,3 and Xiong-qing Wang1,*
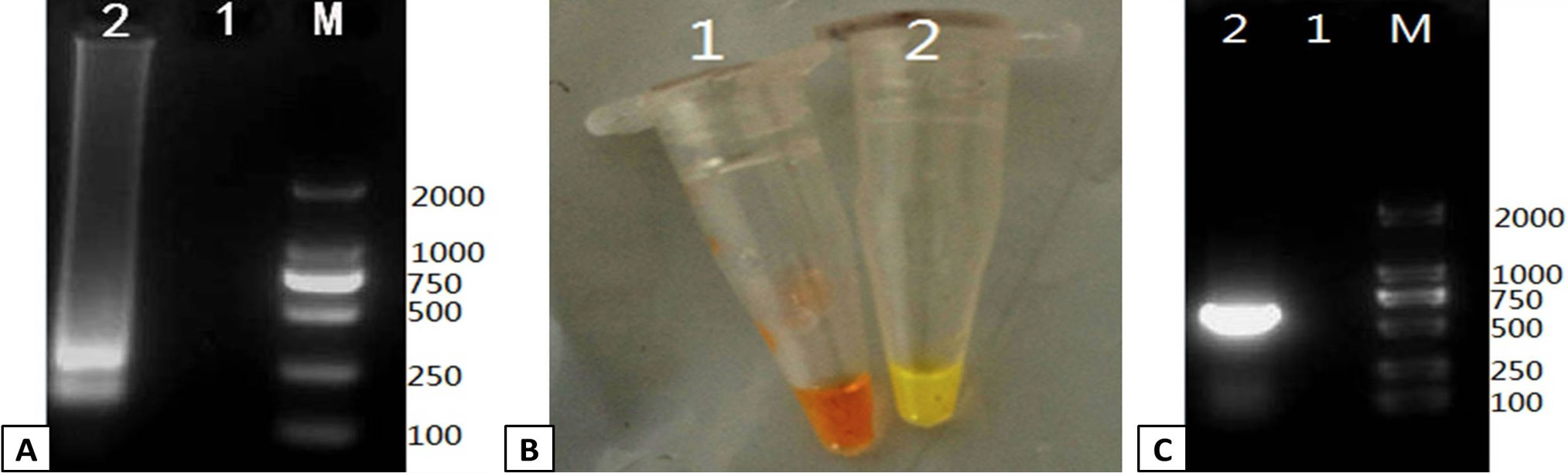
Verification of the PRRSV RT-LAMP assay. RT-LAMP was used to amplify a positive control template and compared to a negative control. The output of the RT-LAMP assay was monitored by gel electrophoresis and color change. (A) 1% agarose gel electrophoresis of the RT-LAMP products. Lanes: M; DL2000 Marker, 1; negative control, 2; positive control. (B) Color change in the RT-LAMP reaction was monitored followig addition of SYBR Green I dye. Tube 1: negative control, Tube 2: positive control. (C) The positive and negative controls were verified by RT-PCR 1% agarose gel electrophoresis of the RT-PCR products. Lanes: M; DL2000 Marker, 1; negative control, 2; positive control.
Optimizing the duration of the RT-LAMP reaction. RT-LAMP was used to amplify PRRSV containing samples. The products of the RT-LAMP reactions were visualized by (A) 1% agarose gel electrophoresis, (B) UV transillumination, and (C) color change using SYBR Green I dye. Lanes M; DL2000 Marker (A only), 1-5; 45, 50. 55, 60, and 65 min, respectively.
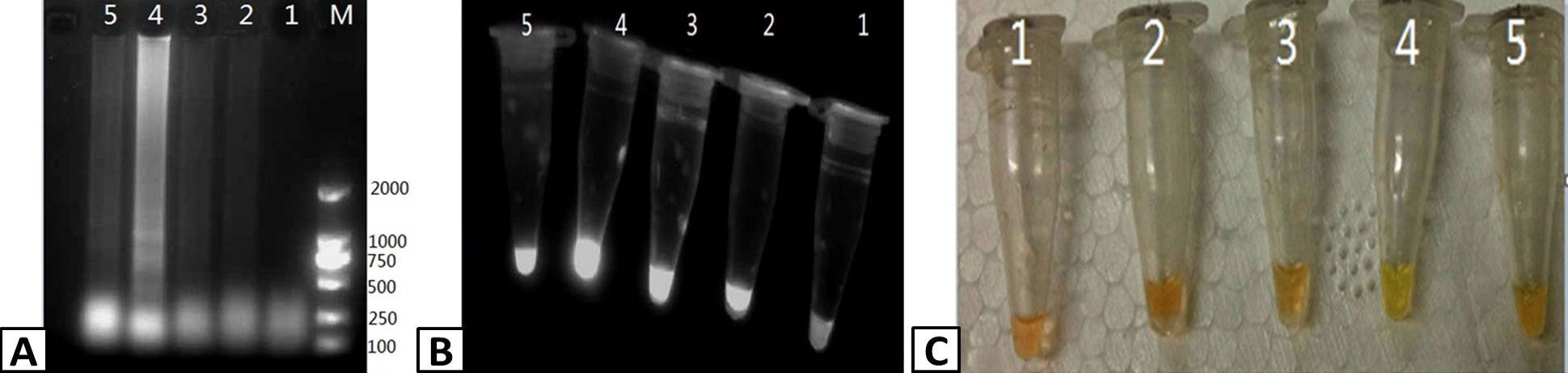
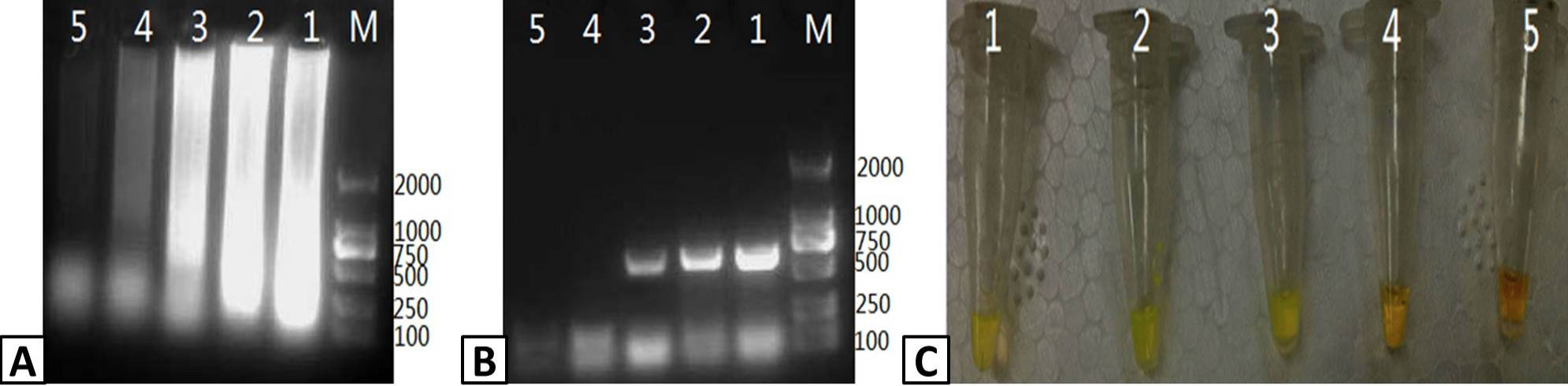
Optimizing the temperature of the RT-LAMP reaction. RT-LAMP was used to amplify PRRSV containing samples. The products of the RT-LAMP reactions were visualized by (A) 1% agarose gel electrophoresis. (B) UV transillumination, and (C) color change using SYBR Green I dye. Lanes: M; DL2000 Marker (A only), 1-5; 59, 61, 63, 65, and 67 C, respectively.
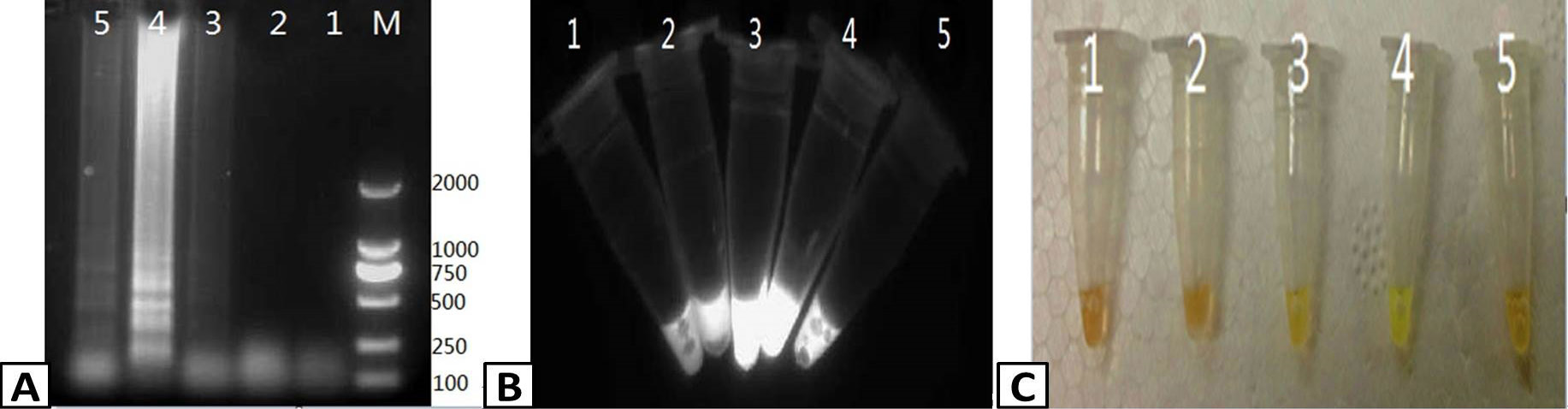
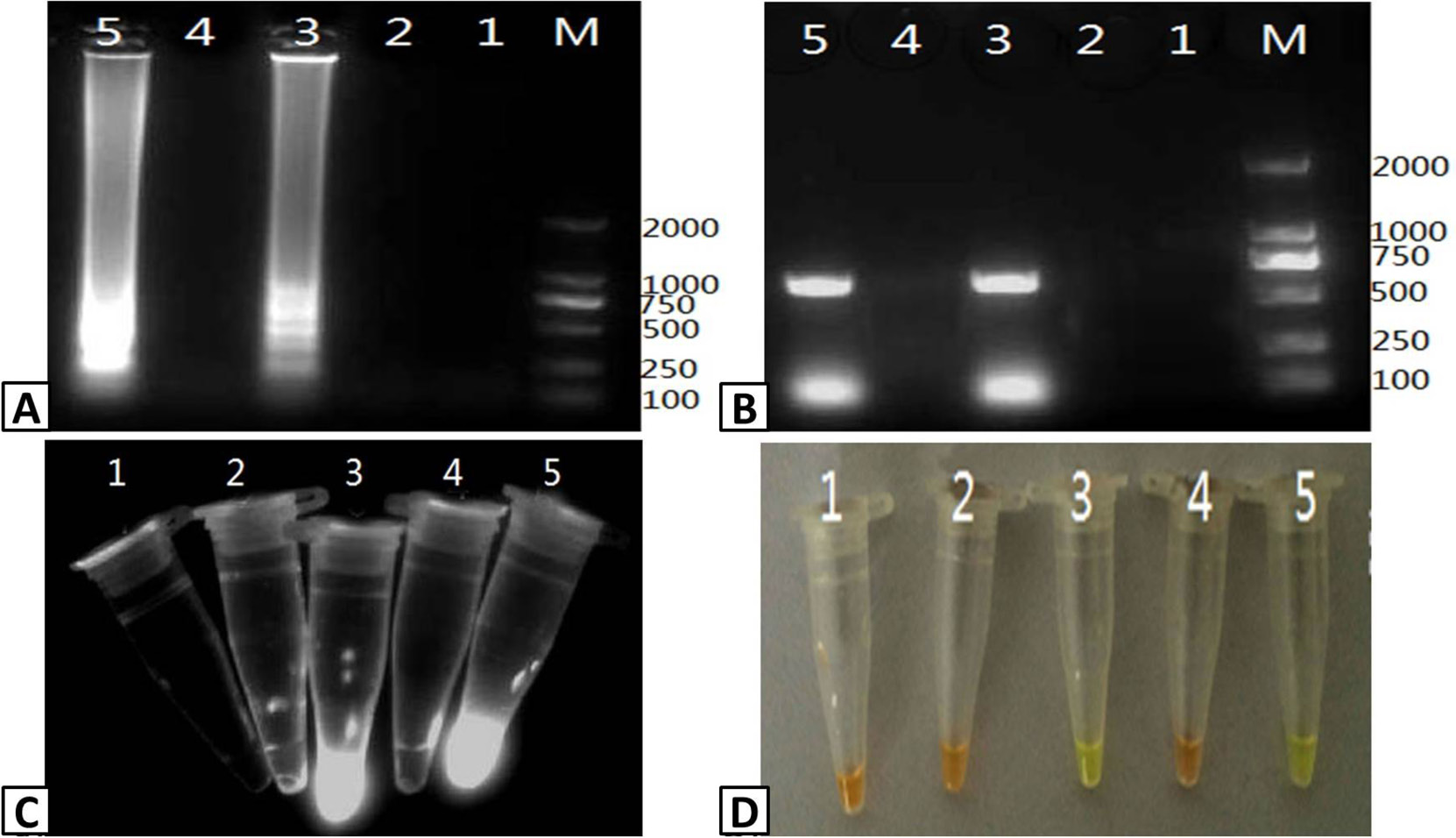
Assessing the specificity of RT-LAMP for PRRSV. Samples were isolated from pigs infected with PRRSV, CSFV, PCV-2, PPV, and PRV and tested usig the RT-LAMP assay. The output of the assay was visualized using (A) agarose gel electrophoresis and (C) color change with SYBR Green I dye. (B) The presence of PRRSV in the samples was confirmed by RT-PCR and visualized using agarose gel electrophoresis. Lanes: M; DL2000 Marker (A and B only). 1; CSFV, 2; PCV-2, 3; PPV, 4; PRRSV, 5; PRV.
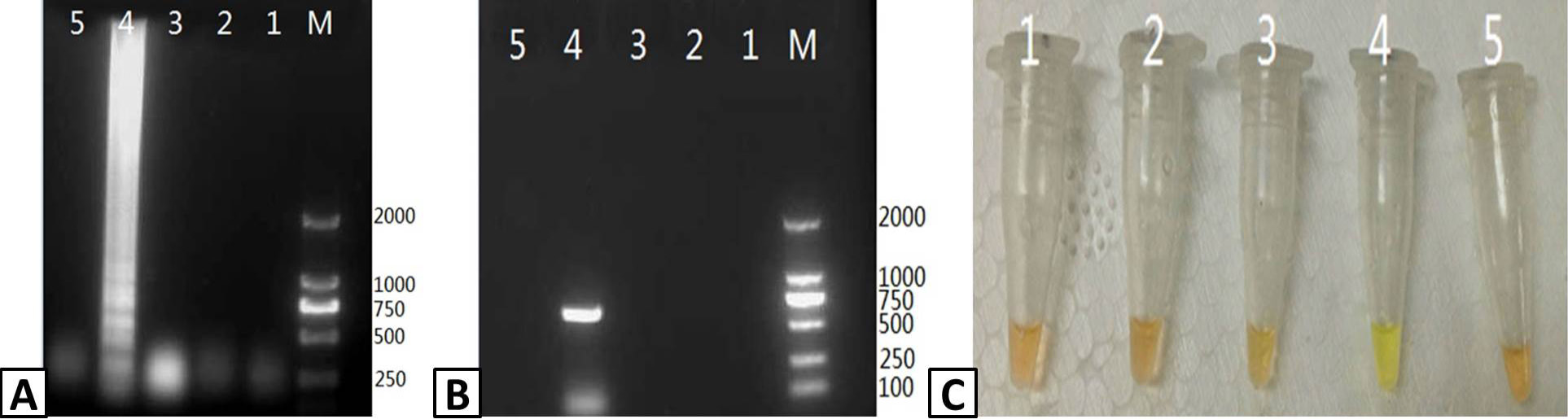
Assessing the sensitivity of the PRRSV RT-LAMP assay. 10-fold serial dilutions of a stock PRRSV RNA template (10ng/μL) were used to assess the sensitivity of the RT-LAMP assay compared to RT-PCR. Output from the RT-LAMP assay was visualized by (A) agarose gel electrophoresis and (C) color change with SYBR Green I dye. (B) RT-PCR amplification of PRRSV RNA was visualized by agarose gel electrophoresis. Lanes: M; DL2000 Marker (A and B only); 1-5: 10-1, 10-2, 10-3, 10-4, and 10-5 dilutions of the stocktemplate.
Detection of PRRSV infection in serum samples. Five (5) serum samples were tested for PRRSV infection using RT-LAMP and RT-PCR. The output of the RT-LAMP assay was visualized using (A) agarose gel electrophoresis, (C) UV transillumination, and (D) color change with SYBR Green I dye. (B) RT-PCR products were visualized using agarose gel electrophoresis of RT-PCR products. Lanes M; DL2000 Marker (A and B only); 1-5; serum samples.