Raphanus sativus L. Var. caudatus as an Analgesic and Antipyretic Agent in Animal Models
Raphanus sativus L. Var. caudatus as an Analgesic and Antipyretic Agent in Animal Models
Ishrat Younus1,2 and Afshan Siddiq1*
1Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, University of Karachi, Karachi
2Department of Pharmacology, Faculty of Pharmacy, Hamdard University, Karachi.
ABSTRACT
Raphanus sativus L. Var. caudatus belongs to Radish plant has been reported for analgesic potential thus it might have antipyretic potential. The present investigation was undertaken to evaluate analgesic and antipyretic activities of ethanol extract of Raphanus sativus var. caudatus in albino mice. The analgesic and antipyretic activities were determined at three different doses (50, 100 and 200 mg/kg) using different pain models (writhing induced, tail flick) and yeast induced pyrexia tests, respectively. The extract exhibited significant (p < 0.05) in vivo analgesic and antipyretic potential in term of % writhes inhibition, increased latency time and reduced rectal temperature when compared with control. The extract displayed noteworthy analgesic and antipyretic capabilities especially at the dose of 200 mg/kg that are almost comparable with standard drug (aspirin). Although the finding of present investigation highlighted the medicinal importance of the plant but there is dire need to carry out the fractional extraction of crude extract with different solvents based on polarity to identify the active constituents with exact analgesic and antipyretic mechanistic action of Raphanus caudatus as well as clinical trials are required in future for medicinal use of the plant in humans.
Article Information
Received 21 August 2020
Revised 26 October 2020
Accepted 19 November 2020
Available online 29 July 2021
(early access)
Published 18 April 2022
Authors’ Contribution
IY carried out research, compiled the data and wrote manuscript. AS designed the study, supervised and reviewed the manuscript.
Key words
Raphanus caudatus, Ethanol extract, Yeast induced pyrexia, Writhing induced test, Tail flick test, Anti-pyretic, Analgesic
DOI: https://dx.doi.org/10.17582/journal.pjz/20200812110845
* Corresponding author: afshanpharma@gmail.com
0030-9923/2022/0004-1643 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Raphanis sativus var. caudatus, commonly known as rat-tailed radish is an important healthy food crop belongs to Brassicaceae family. Member of this family are enriched in isothiocyanates, polyphenols and vitamins and considered to be chemopreventive and health promoting agents (Sangthong et al., 2014).
R. sativus commonly called radish have been known since long for prevention and treatment of various diseases. All the parts of plant have been reported to possess different pharmacological activities (Gutiérrez and Perez, 2004). The fresh extract of leaves possesses diuretic and purgative properties, roots are utilized for urinary ailments, gastric problems, hemorrhoids and different gastric illnesses. The seeds of this herb have been reported for expectorant, purgative, digestive, diuretic, and carminative effects (Magesh et al., 2016).
Pain is basic factor that influences individuals worldwide consistently. Pain can emerge from damage or regular events like sprain or acidity (Pinky et al., 2015). Pain is an inclination activated in the sensory system.
Over the counter medications such as ibuprofen or paracetamol have unfavorable symptoms like gastric ulceration and hepatotoxicity. Thus there is dire need to identify new medicines of enhanced efficacy and minimum side effects (Sultana et al., 2014).
The physiological action for analgesics was proposed by a few scientists that they restrain prostaglandin combination in the central nervous system to interfere with nociceptive receptors in the cerebrum; others have suggested that they trigger specifically on nociceptive neural pathways to develop analgesic effect. This class of medications additionally has antipyretic action (Shimada et al., 1994).
Fever is a condition of raised core temperature, which is usually, part of the defensive mechanism of living beings. The agents used to decrease the body temperature are called as antipyretic agents. People have been using such compounds for more than two centuries. Antiquated Assyrian, Egyptian, and Greek doctors all evidently worked for assessing medicinal plants and their antipyretic properties (Plaisance and Mackowiak, 2000).
Radish has been reported for analgesic potential (Mikaili et al., 2010). Historically, it was used for treating the pain of migraine (Wu et al., 2014). A study evaluated that the crude extract of Raphanus sativus leaves showed actions to relieve spasm in a dose dependent manner. They have significantly produced evidence for its activity against constipation and relieving spasm for having analgesic activity (Gilani and Ghayur, 2004).
Raphanus was found to be rich in saturated fatty acids and eicosenoic acids, thus, supposed to have some correlation towards analgesic and antipyretic activity (Mandal et al., 2002).
Hence the present study was undertaken to evaluate the analgesic and antipyretic actions of R. sativus L. var caudatus. The study was carried out first time on this variety of radish so it will add new information regarding pharmacological potential of plant.
MATERIALS AND METHODS
The study was conducted at Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences in the months of September to November 2016.
Collection and extraction of plant material
The pods of Raphanus sativus L. were purchased from local market of Karachi identified from Herbarium, Department of Botany, University of Karachi. The plant material was air dried and subjected to grinding to make powder. The powdered plant material of R. sativus L. var. caudatus was subjected to extraction with ethanol using Soxhlet apparatus. Then the extract was subjected to rotary evaporator (Buchi) to obtain semi solid mass and then air dried.
Animal selection
Before the experiments the animals were placed in separate cages for 7 days. For evaluation of antipyretic and analgesic activity, total of 60 albino mice (25±2g) of either sex were used. Standard sized cages made up of iron were employed for animals housing at Animal House, Department of Pharmacology, University of Karachi. The animals were housed at at 25±1oC on 12/12 h light and dark cycle. All animals were allowed food and water ad libitum. University Board of Advanced Studies and Research approved this study with reference # 02419/Pharm.
Protocol of the experimental study
The protocol of the current study was designed on the basis of pilot study. Mice were divided into five groups (I, II, III, IV and V) of six animals in each group. Group I and II were kept as control and standard groups, administered with normal saline and aspirin (100 mg/kg), respectively. Group III, IV and V were set as treated groups, administered orally with ethanolic extract of R. caudatus in doses of 50 mg/kg, 100 mg/kg and 200 mg/kg, respectively (Ahmed et al., 2015).
The animals were subjected to following different tests to evaluate analgesic and antipyretic activities.
Analgesic activity
The anti-nociceptive activities of ethanolic extract of R. caudatus were carried out in albino mice by using acetic acid-induced writhing and tail flick methods, respectively.
For acetic acid-induced writhing test the anti-nociceptive activity of the ethanolic extract of R. caudatus was evaluated in five groups of adult albino mice (Koster, 1959). Each group contained six animals. Group I and II were kept on normal saline and aspirin (100 mg/kg), respectively. The mice of III, IV, and V groups (six animals each) were orally given the tested extract in three doses; 50, 100 and 200 mg/kg, respectively. Writhing was induced 30 min later by intraperitoneal injection of 0.6% v/v acetic acid solutionat a dose of 10 ml/kg. After the injection of acetic acid, numbers of writhes (abdominal contractions) in all animals were calculated immediately for 10 min. Analgesic activity interm of percent inhibition of writhing was calculated as follows (Ahmad et al., 2015).
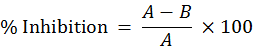
Where A is average number of writhing of control per group, and B is average number of writhing of test per group.
For tail flick test in mice Luiz et al. (1988) method was used to determine analgesic activity as the reaction time or latency period (flick the tail out of the hot water). The reaction time was measured by immersing the distal part of tail (3.5cm) into hot water bath maintained temperature at 51+0.5ºC (Luiz et al., 1988). This reaction time considered as zero reading. The reaction time in seconds also determined at a time interval of 30, 60, 90, 120, 150 min. All the experiments were carried out in triplicates. The data obtained were analyzed statistically to determine the level of significance.
Antipyretic activity
On the testing day food was withdrawn and animals were habituated to test condition for 2 h. Temperature was noted by utilizing calibrated thermometers in to the rectum of animals. The baseline temperature was set as 39.2 ºC to 39.8 ºC for any animal to be employed in to the test. After taking the rectal temperature at 0 h, the animals were injected subcutaneously with 20% w/v aqueous suspension of brewer’s yeast (1 ml / kg) (Vaz et al., 1996) and the animals with elevated temperatures up to 0.5ºC preferred for the study. The standard group was given Asprin and test groups were given plant extracts of different stated doses at 0 h time. The rectal temperatures of all mice were recorded at 1 h interval up to 05 hs after yeast injection.
Statistical analysis
All the results were represented as mean ± SEM and analyzed by applying SPSS- 20 using one-way analysis of variance (ANOVA) followed by post hoc Tukey HSD. The significant cut –off value was considered at p < 0.05.
RESULTS
Writhing induced test
Ethanolic extract of R. caudatus showed notable peripheral anti-nociceptive activity in acetic acid-induced writhing test in mice when compared with standard drug (aspirin). The results of analgesic activity of R. caudatus are tabulated in Table I. By increasing the dose (200 mg/kg), the antinociceptive efficacy was observed to be enhanced. It is evident that the means of increasing treatments were apparently lowered.
Tail flick test
In case of heat induced pain model, ethanolic extract of R. caudatus exhibited significant increase (p < 0.05) in reaction time when compared with control in mice showing central analgesic effect. The highest anti-nociceptive activity was observed at 150 minute after the treatment. The extract showed significant anti-nociceptive activity at the dose of 200 mg/kg and remarkable (p < 0.05) analgesic effect was observed as compared to other groups (Fig. 1).
Yeast-induced pyrexia
In the present investigation, ethanolic extract of R. caudaus produced significant (p < 0.05) reduction in body temperature of yeast induced mice at different tested doses. it is noted that the means of increasing treatments were apparently lowered. To test each treatment to others, post hoc Tukey HSD was used. It was observed by post hoc analysis that by increasing the dose, anti-pyretic effects were potentiated (Table I).
DISCUSSION
Non-steroidal anti-inflammatory drugs (NSAIDs) are among very popular medication especially for analgesic and anti-inflammatory properties. In spite of these benefits, NSAIDs are also responsible for serious adverse effectsincluding gastrointestinal and cardiovascular problems (Sostres et al., 2010).Thus current studies were focused to identify plant derived pharmacologically active compounds to overcome such type of problems.
Table I. Analgesic effect and antipyretic activity of ethanolic extracts of Raphanus caudatus (RC) and aspirin on acetic acid induced writhing test and yeast-induced pyrexia, respectively in mice.
|
Control (n=6) |
RC 50 mg/kg (n=6) |
RC 100 mg/kg (n=6) |
RC 200 mg/kg (n=6) |
Aspirin 100 mg/kg (n=6) |
|
|
No. of writhing (Mean±SEM) |
20.50±1.22 |
16.16±0.47 |
11.22±0.27* |
7.83±0.29* |
8.35±0.44* |
|
% inhibition |
0.00 |
21.17 |
45.26* |
61.80* |
59.26* |
|
Rectal temperature (ºC) (Mean±SEM) |
|||||
|
After yeast injection at 0h |
39.43±0.04 |
39.76±0.09 |
39.22±0.14 |
39.06±0.03 |
39.17±0.09 |
|
After drug administration |
|||||
|
1h |
39.22±0.23 |
39.59±0.14 |
39.06±0.29 |
38.73±0.19 |
38.77±0.14 |
|
2h |
39.15±0.23 |
37.61±0.05 |
38.75±0.15 |
38.56±0.22* |
38.59±0.05* |
|
3h |
39.30±0.21 |
37.33±0.12 |
38.30±0.04* |
37.82±0.08* |
38.26±0.12* |
|
4h |
39.23±0.10 |
37.06±0.20 |
38.02±0.28* |
37.66±0.33* |
38.07±0.20* |
|
5h |
39.07±0.14 |
39.90±0.28 |
37.92±0.24* |
37.42±0.27* |
37.77±0.28* |
RC, Raphanus caudatus; n=6; One-Way ANOVA followed by post hoc Tukey HSD; *p < 0.05 significant when compared to control.
The present study indicated R. caudatus as analgesic and antipyretic agent for the first time so it has added new information regarding this pharmacological potential of plant.
Brassicaceae is a plant family of great significance. The plants of this family have been reported for variety of activities. R. sativus L. originally belongs to Asia and Europe (Gutiérrez and Perez, 2004). R. sativus L. var. caudatusare actually pods of R. sativus. The vernacular name in Pakistan is “Mungra” and rat-tailed radish in English. This edible part of radish is available in Pakistan between the months of November and March and is cooked in the form of delicious dishes (Khare, 2007).
Already different plants have been reported to possess analgesic and antipyretic activities (Almeida et al., 2001; Khattak et al., 1985).The results of present study showed significant antipyretic and analgesic potential of R. caudatus.
To evaluate central and peripheral analgesic activity, two tests were utilized i.e. acetic acid induced writhing test and tail flick test, respectively. The ethanolic extract of R. caudatus exhibited notable activities. The current study is supported by an earlier study that indicated radish seeds in prevention of pain after hemorrhoid surgery (WU et al., 2010). Similarly methanolic extract of aerial parts of Raphanus sativus var. hortensis indicated analgesic potential (Sharmin and Mohammed, 2014).
The acetic acid and tail flick latency time are responsible to release prostaglandins and other endogenous substances from the nerve endings and abdominal constrictions (writhes) are produced due to interaction of prostagalndin to pain receptors (Bentley et al., 1983). Thus analgesic activity in the present writhing test might be attributed to R. caudatus involvement in prostaglandin pathway.
Regarding antipyretic activity evaluation, ethanol extract of R. caudatus showed noteworthy activity in Yeast induced pyrexia model and it is comparable with standard antipyretic drug (Asprin). Nevertheless the extract showed antpyretic activity in dose dependant manner. Prostaglandins have been known since long for their role in the pyrexia (Milton, 1982; Sugita et al., 2016; Engström et al., 2012). So it is obvious that inhibition in prostaglandin pathway could lead to antipyretic potential. Flavonoids are plant derived compounds (also present in R. caudatus) reported to inhibit prostaglandins (Ferrandiz and Alcaraz, 1991; Baumann et al., 1980). Thus the occurrence of flavonoids in R. caudatus could be linked to its antipyretic activity in yeast induced pyrexia method. With regard to antipyretic potential of radish limited research data is available but previously different plants showed antipyretic effects (Ikram et al., 1987; Awaad et al., 2011; Muhammad et al., 2012; Ajaz et al., 2019; Vinod and Malgi, 2019)
Isothiocyanates, sulphur containing compounds, have been reported in the plants of the Brassicaceae family (Bednarek, 2012). These compounds are present in these plants as glucose inolatesprecursors. GC–MS analysis of R. caudatus in aprevious study had revealed the presence of two isothiocyanates compounds named sulforaphane and sulforaphene (Pocasap et al., 2013). On the other hand, phenolic compounds have also been reported in R. caudatus (Beevi et al., 2012; Takaya et al., 2003). Thus the presence of these constituents may be responsible for observed analgesic and anti-pyretic activity of R. caudatus in the present study.
Thus the present study identified R. caudatus as analgesic and antipyretic agent. The results recommend use of this plant in pain and pyrexia. Moreover, the study is limited because of animals thus it should be replicated on large number of animals and also in humans to develop this plant as useful medicines in such ailments.
CONCLUSION
Conclusively, the present study reportedin vivo analgesic and anti-pyretic effects of ethanolic extract of Raphanus sativus L. var. caudatus. In future, there is dire need to carry out the fractional extraction ofethanolicextract with different solvents based on polarity to identify the active constituents with exact analgesic and antipyretic mechanistic action of R. caudatus.
This study discovered the analgesic and antipyretic pharmacological potential of R. caudatus that can be beneficial in pains and pyrexia conditions. This study will help the researcher to uncover the pharmacological potential and possible mechanism of action. Thus, a new theory of pharmacological actions of this radish variety may be arrived at.
ACKNOWLEDGEMENT
The authors are grateful to Department of Pharmacology, Faculty of Pharmacy, University of Karachi for providing excellent support during the research project.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Ahmed, S., Naved, A., Khan, R.A. and Siddiqui, S., 2015. Analgesic activities of methanol extract of Terminalia chebula fruit. Pharmacol. Pharm., 6: 547. https://doi.org/10.4236/pp.2015.612056
Ajaz, M., Akram, M., Asif, H.M., Hussain, A., Shah, S.M.A., Tahir, I.M., Daniyal, M. and Khan, F.S., 2019. Phytochemical screening and antipyretic effects of hydroalcoholic extracts of selected medicinal plants of Rawalakot, Azad Jammu and Kashmir in albino rats. Pak. J. pharmaceut. Sci., 32: 581-592.
Almeida, R., Navarro, D. and Barbosa-Filho, J., 2001. Plants with central analgesic activity. Phytomedicine, 8: 310-322. https://doi.org/10.1078/0944-7113-00050
Awaad, A.S., El-Meligy, R., Qenawy, S., Atta, A. and Soliman, G.A., 2011. Anti-inflammatory, antinociceptive and antipyretic effects of some desert plants. J. Saudi chem. Soc., 15: 367-373. https://doi.org/10.1016/j.jscs.2011.02.004
Baumann, J., Bruchhausen, F.V. and Wurm, G., 1980. Flavonoids and related compounds as inhibitors of arachidonic acid peroxidation. Prostaglandins, 20: 627-639. https://doi.org/10.1016/0090-6980(80)90103-3
Bednarek, P., 2012. Sulfur-containing secondary metabolites from arabidopsis thaliana and other brassicaceae with function in plant immunity. Chembiochem, 13: 1846-1859. https://doi.org/10.1002/cbic.201200086
Beevi, S.S., Mangamoori, L.N. and Gowda, B.B., 2012. Polyphenolics profile and antioxidant properties of Raphanus sativus L. Natl. Prod. Res., 26: 557-563.https://doi.org/10.1080/14786419.2010.521884
Bentley, G., Newton, S. and Starr, J., 1983. Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br. J. Pharmacol., 79: 125-134. https://doi.org/10.1111/j.1476-5381.1983.tb10504.x
Engström, L., Ruud, J., Eskilsson, A., Larsson, A., Mackerlova, L., Kugelberg, U., Qian, H., Vasilache, A.M., Larsson, P. and Engblom, D., 2012. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology, 153: 4849-4861. https://doi.org/10.1210/en.2012-1375
Ferrandiz, M. and Alcaraz, M., 1991. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions, 32: 283-288. https://doi.org/10.1007/BF01980887
Gilani, A.H. and Ghayur, M.N., 2004. Pharmacological basis for the gut stimulatory activity of Raphanus sativus leaves. J. Ethnopharmacol., 95: 169-172. https://doi.org/10.1016/j.jep.2004.06.038
Gutiérrez, R.M.P. and Perez, R.L., 2004. Raphanus sativus (Radish): Their chemistry and biology. Sci. World J., 4: 811-837. https://doi.org/10.1100/tsw.2004.131
Ikram, M., Khattak, S.G. and Gilani, S.N., 1987. Antipyretic studies on some indigenous Pakistani medicinal plants: II. J. Ethnopharmacol., 19: 185-192. https://doi.org/10.1016/0378-8741(87)90040-7
Khare, C., 2007. Indian medicinal plants: An illustrated dictionary library of congress control number: 2007922446 ISBN: 978-0-387-70637-5 Springer-Verlag Berlin. Heidelberg, Springer science+ Business Media, LLC.
Khattak, S.G., Gilani, S.N. and Ikram, M., 1985. Antipyretic studies on some indigenous Pakistani medicinal plants. J. Ethnopharmacol., 14: 45-51. https://doi.org/10.1016/0378-8741(85)90027-3
Koster, R., 1959. Acetic acid for analgesic screening. Fed. Proc., 1959: 412-last page.
Luiz, C., Mirtes, C., Sigrid, L., Mizuekirizawa, M., Cecilia, G. and Jrotin, G., 1988. Studies on medicinal plants of Sri Lanka, toxicity of some traditional medicinal herbs. J. Ethnopharmacol., 24: 205-211.
Magesh, S., Rajappa, R. and Jayathi, G., 2016. Protective effect of Raphanus sativus on D-galactosamine induced nephrotoxicity in rats. Nutr. Clin. Métab., 30: 22-28.
Mandal, S., Yadav, S., Singh, R., Begum, G., Suneja, P. and Singh, M., 2002. Correlation studies on oil content and fatty acid profile of some Cruciferous species. Genet. Resour. Crop Evol., 49: 551-556. https://doi.org/10.1023/A:1021210800414
Mikaili, P., Nezhady, M.A., Shayegh, J. and Asghari, M.H., 2010. Study of antinociceptive effect of Nepeta meyeri, Raphanus sativus and Origanum vulgare extracts in mouse by acute pain assessment method. Int. J. acad. Res., 2: 1-3.
Milton, A., 1982. Prostaglandins and fever. Trends Pharmacol. Sci., 3: 490-492. https://doi.org/10.1016/0165-6147(82)91250-0
Muhammad, N., Saeed, M. and Khan, H., 2012. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement. Altern. Med., 12: 59. https://doi.org/10.1186/1472-6882-12-59
Pinky, B.R.T., Banna, H., Al-Labib, B., Al-Mahamud, R., Rahman, S., Rahman, M.M. and Rahmatullah, M., 2015. Analgesic activity of boiled Brassica oleracea L. var. capitata (Cabbage) leaves. World J. Pharm. pharmaceut. Res., 4: 113-119.
Plaisance, K.I. and Mackowiak, P.A., 2000. Antipyretic therapy: Physiologic rationale, diagnostic implications, and clinical consequences. JAMA Int. Med., 160: 449-456. https://doi.org/10.1001/archinte.160.4.449
Pocasap, P., Weerapreeyakul, N. and Barusrux, S., 2013. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef). J. Funct. Fds., 5: 1372-1381. https://doi.org/10.1016/j.jff.2013.05.005
Sangthong, S., Weerapreeyakul, N. and Barusrux, S., 2014. 795: Plausible extraction technique for cancer chemopreventive isothiocyanate compounds from the Raphanus sativus L. var. caudatus Alef. Eur. J. Cancer, 50: S192. https://doi.org/10.1016/S0959-8049(14)50700-9
Sharmin, J. and Mohammed, R., 2014. Methanolic extract of aerial parts of Raphanus sativus var. hortensis shows antihyperglycemic and antinociceptive potential. World J. Pharm. pharmaceut. Sci., 3: 193-202.
Shimada, S.G., Otterness, I.G. and Stitt, J.T., 1994. A study of the mechanism of action of the mild analgesic dipyrone. Agents Actions, 41: 188-192. https://doi.org/10.1007/BF02001915
Sostres, C., Gargallo, C.J., Arroyo, M.T. and Lanas, A., 2010. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol., 24: 121-132. https://doi.org/10.1016/j.bpg.2009.11.005
Sugita, R., Kubota, K., Sugimoto, K., Tachida, Y., Shibayama, T., Kiho, T., Kawakami, K. and Shimada, K., 2016. A novel selective prostaglandin E 2 synthesis inhibitor relieves pyrexia and arthritis in Guinea pigs inflammatory models. J. Pharmacol. Sci., 130: 128-135. https://doi.org/10.1016/j.jphs.2016.01.009
Sultana, S., Nandi, J.K., Rahman, S., Jahan, R. and Rahmatullah, M., 2014. Preliminary antihyperglycemic and analgesic activity studies with Angiopteris evecta leaves in Swiss albino mice. World J. Pharm. Pharmaceut. Res., 3: 1-12.
Takaya, Y., Kondo, Y., Furukawa, T. and Niwa, M., 2003. Antioxidant constituents of radish sprout (kaiware-daikon), Raphanus sativus L. J. Agric. Fd. Chem., 51: 8061-8066. https://doi.org/10.1021/jf0346206
Vaz, Z.R., Yunes, R. and Calixto, J., 1996. Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4, 6-dimethoxy benzofuran, a novel xanthoxyline derivative on chemical and thermal models of nociception in mice. J. Pharmacol. exp. Ther., 278: 304-312.
Vinod, M. and Malgi, R., 2019. Antipyretic activity of Abutilon indicum (L.) sweet leaf extract. J. Pharmacogn. Phytochem., 8: 440-442.
Wu, S., Xia, J., Bi, Y., Lin, J. and Zhang, Q., 2010. Nursing application of umbilicus therapy with radish seed for preventing pain after hemorrhoid surgery. Clin. Med. Engin., 5: 52-58.
Wu, S.B., Wang, F., Jin, X.B., Liu, T., Wu, L.R., Wang, W., Mei, D., Zheng, Z. and Zhu, J.Y., 2014. Two new natural products from the roots of Raphanus sativus L. Adv. Mater. Res., 884: 548-552. https://doi.org/10.4028/www.scientific.net/AMR.884-885.548
To share on other social networks, click on any share button. What are these?










