Production and Quality of M2 Generations of Indigofera Zollingeriana Result from Gamma-Ray Irradiation as Animal Feed and its Effect on Soil Characteristics on Saline Soil
Research Article
Production and Quality of M2 Generations of Indigofera Zollingeriana Result from Gamma-Ray Irradiation as Animal Feed and its Effect on Soil Characteristics on Saline Soil
Rijanto Hutasoit1,3, Edison Purba2*, Simon Petrus Ginting3, Nevy Diana Hanafi2
1Graduate school, Faculty of Agriculture, Universitas Sumatera Utara, Jl. Dr. A. Sofian No.3, Padang Bulan, Medan 20155, Indonesia; 2Faculty of Agriculture, Universitas Sumatera Utara, Jl. Dr. A. Sofian No.3, Padang Bulan, Medan 20155, Indonesia; 3National Research and Innovation Agency, Jl. Raya Jakarta-Bogor No.32, Cibinong, Bogor, Jawa Barat, Indonesia
Abstract | The study was aimed to investgate utilization M2 generation of Indigofera zollingeriana results from gamma-ray irradiation as a roughage animal feed and its effect in soil characteristics on saline soil. The study used a randomized block design (RBD) with four treatments of gamma-irradiated M2 generation I. zollingeriana (0, 100, 200, and 300 gray) and six replications. The data were analyzed by ANOVA and then evaluated using an orthogonal polynomial contrast test. In this study, the soil characteristics were analyzed descriptively. The findings showed that there was no significant difference (P > 0.05) between the M2 I. zollingeriana generations in terms of quantity and quality. Numerically, 300 Gy treatment produced the most dry matter production (11.08 t ha-1 y-1). When compared with those that are not irradiated, the impact of radiation can result in an increase in dry matter yield of 1.2 t ha-1 y-1. The highest crude protein content (26%) was seen in the 300 Gy treatment. The digestibility of dry and organic matter peaked at 200 Gy (73.65 and 72.26%, respectively). The 200 Gy treatment had the highest proline concentration (65450 ppm). The population of phosphate-solubilizing bacteria was highest at 100 Gy (2.06 x 109 CFU/g). It was concluded that M2 I. zollingeriana irradiation treatment could produce more production and quality compared to non-iradiated treatments and could increase the population of phosphate-solubilizing microbes in saline soil.
Keywords | Indigofera zollingeriana, Mutan 2 generation, Irradiation, Salinity, Production
Received | April 04, 2024; Accepted | June 15, 2024; Published | August 05, 2024
*Correspondence | Edison Purba, Faculty of Agriculture, Universitas Sumatera Utara, Jl. Dr. A. Sofian No.3, Padang Bulan, Medan 20155, Indonesia; Email: edisonpurba@usu.ac.id
Citation | Hutasoit R, Purba E, Ginting SP, Hanafi ND (2024). Production and quality of m2 generations of indigofera zollingeriana result from gamma-ray irradiation as animal feed and its effect on soil characteristics on saline soil. Adv. Anim. Vet. Sci. 12(9): 1689-1699.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.9.1689.1699
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
The expansion of livestock, particularly ruminants, should be accompanied by the development of forage production systems for feed supply. Indigofera zollingeriana is a type of legume good for consumption to stimulate the growth performance of livestock, particularly ruminant livestock, which impact on productivity, and increases the number of livestock kept (Smýkal et al., 2014; Ginting et al., 2020).
(Abdullah and Suharlina 2010) found that the dry mater production of I. zollingerianara was 40.96 t/ha/year, with a protein content of 27.60%, and in vitro dry matter digestibility of 67-81%. (Tarigan et al., 2010) observed a 60-day production rate of 31.23 t/ha/year, with CP content of 25.50%-25.81%, in vitro dry and organic matter digestibility (77.13 and 68.10% respectively). (Ali et al., 2021) studied I. zollingeriana on peatlands with a two-month cutting interval produced CP content of 27.45%, crude fiber of 16.50%, crude fat of 2.11%, ash of 7.55%, and BETN of 46.39%.
As a legume plant, it has rhizobium in its root nodules, which can increase the N concentration in the soil (Kumari et al., 2010). Soil biophysical improvements were also reported by (Agisti et al., 2014) using legume cover crops in former coal mining areas and had a real effect on increasing organic C and total N, soil pH, and increasing the available P from 24–25 ppm to 55–64 ppm.
Farmers’ efforts to develop I. zollingeriana are constrained by land availability due to land use competition (Budiari and Suyasa, 2019). The very rapid increase in human population requires a place to live, so agricultural land is increasingly reduced and much fertile agricultural land is being converted (Jalaludin et al., 2010; Harahap et al., 2017), forcing the conversion of grazing land for residential or industrial purposes (Mapiye et al., 2007). In connection with this problem, one alternative is to expand livestock areas toward marginal land. Marginal land is problematic land and has high limiting factors for plants. One of the marginal lands that has high potential in Indonesia is the coastal saline land, with a potential size of 108,000 km (BPS, 2022).
Several studies have been carried out to improve the quality of saline land, including the use of organic materials (Arifiani et al., 2018; Parnianto et al., 2022), the use of mulch to save soil moisture in the dry season (Purwaningrahayu and Taufiq, 2018), and the application of rhizosphere bacteria to increase the weight of cucumber fruit (Alfya et al., 2017). However, the efforts mentioned above have not shown significant results in the development of crops and require very high costs, so saline land has not been widely used. Based on the problems above, an alternative that can be done is to create new salinity-tolerant genetics. According to (Zeng et al., 2002), obtaining salt tolerance in rice genotypes base on physiological characteristics will be more profitable in the long term than dealing with salinity problems in agricultural production.
Plant breeding involves selecting from a diversity of genotypes with high yields. It is necessary to form a diverse population first. In feed crop commodities, research on new superior varieties that are tolerant to saline soil is limited. The information obtained is still limited to increasing genetic diversity through gamma-ray irradiation in I. zollingeriana plants (Hutasoit et al., 2022). The results of radiosensitivity analysis through the Lethal Dose 50 (LD 50) test obtained the best growth in four dose treatments, including 0, 100, 200, and 300 Gray, which is called mutant 1 (M1). The four M1 mutants can be used as genetic material to form new superior varieties that are tolerant to salinity, and then the research was continued with adaptation tests on saline soil to obtain M2. This is an absolute requirement in the formation of new superior varieties of plants. A promising genotype of I. zollingeriana will be obtained that grows well in conditions of environmental stress by taking into account the parameters of agronomic and physiological changes in plants (Mustikarini et al., 2022; Niedbała et al., 2022).
MATERIALS ND METHODS
This activity was carried out in saline soil with a salt content of 12 dS/m. The soil structure was 91% sandy, 4% silt, and 5% clay. pH 5.6, total N 0.10%, organic C 1.0%, total P2O5 7 mg/100 g, P available 26.2 ppm.
The genetic material used was four M2 generations of I. zollingeriana resulting from gamma-ray irradiation. Namely: 0 Gy, 100 Gy, 200 Gy, and 300 Gy. The seeds were planted in polybags measuring 10 × 15 cm and containing soil media. The growing plants were watered and cleaned of weeds for one month until they were approximately 10 cm tall and moved to the research location.
Dry Matter Yield
Plant biomass production, including leaf weight and twig weight, started to be harvested at the beginning of flowering (10%) and occurred 180 days after planting with a 60-day cutting interval. The plants were cut one meter above ground level. Data from each plot and the number of harvests were used to calculate production (t ha-1 y-1). Plant samples were taken (300 g/plot) and then dried at 65  for 72 hours in an oven to obtain dry matter (DM) production.
for 72 hours in an oven to obtain dry matter (DM) production.
Leaf/Stem Ratio
The proportions of leaves and stems were measured at each harvest. A 500 gr/plot sample was taken to determine the ratio of leaves and stems. After separation, each fraction was weighed to calculate the leaf-to-stem ratio. All the data were averaged at the end of the experiment.
Plant mortality
The mortality of the plant was characterized by wilted leaves and dry, brittle stems and was calculated based on the following formula:
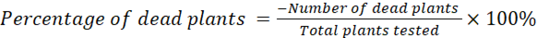
Nutritional Compositions
The dried samples were ground using a hammer mill with a sieve with a diameter of 1.0 mm and then analyzed for the content of crude protein (Kjeldahl), crude fiber, and crude fat. Part of the samples were put in an electric furnace at 600 °C for 2 hours, then weighed to determine the ash content (AOAC, 2005). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were analyzed according to the method of (Van Soest et al., 1991).
In vitro DM and OM digestibilities were carried out according to the method of (Tilley and Terry 1963). Cow rumen fluid obtained from the slaughterhouse was used. Rumen fluid was collected into an empty flask containing hot water to maintain the temperature in the flask around 39-40°C. Samples weighing 1.5 g from each treatment were put into a fermenter tube, then 18.0 ml of artificial saliva solution (McDougall solution) was added, then inoculated with 12.0 ml of rumen fluid. Each in vitro medium was given CO2 gas for ± 30 seconds to keep it in anaerobic conditions. The tube was placed in a water bath shaker at 39ºC shaker and incubated. After 48 hours, drip 0.2 ml of saturated HgCl2 (2-3 drops). Next, the tube was centrifuged at a speed of 5000 rpm for 20 minutes. After the supernatant was discarded, the precipitate resulting from centrifugation in a fermenter tube was then dissolved with 30 ml of HCl-pepsin solution (0.2%) in an acidic atmosphere and incubated for 48 hours in an aerobic atmosphere at a temperature of 39-40°C . Next, the solution is filtered. The filtrates were then dried in an oven at 105°C for 24 hours and then weighed to determine the dry matter digestibility (IVDMD). The samples were then dried in an electric furnace at 600°C for 24 hours and then weighed to determine the organic matter digestibility (IVDOM).
A total of 0.5 g of leaf samples (fully developed leaves) were used to analyze the proline content using the method of (Bates et al., 1973). Proline concentration was determined by a pure proline standard curve and calculated based on fresh weight. Measurements were modified using a spectrophotometer.
Tannin Content
Incubation was carried out using the method of (Abdulrazak and Tsutomu 1999). Samples of 200 mg were put into a 10 ml tube filled with 70% acetone and shaken for 90 minutes at 30°C , at 130 cycles per minute. The solution was centrifuged at 3000 gratification at 4°C for 20 minutes, then stored in the refrigerator at 4°C . To determine the total phenol, put 0.05 ml of the original extract into a test tube. Prepare a blank containing 0.1 ml of distilled water and a standard solution (tannic acid) (0.5 mg/ml of solvent; 0.02, 0.04, 0.06, 0.08, and 0.1 ml). Put 100 mg of PVP into a test tube, add 5 ml of original extract to the tube, and put it on ice for 5 minutes. Vortex put it back on ice for 7 minutes. The tube was centrifuged at 3000 g for 10 minutes. Take the supernatant. Take 0.1 ml of extract into a test tube, add 0.9 ml of distilled water, 0.5 ml of folin solution, and 2.5 ml of Na2CO3 solution, leave for 35 minutes, and read at a wavelength of 725 nm. Total tannin = total phenol – total phenol PVP.
Soil characteristics
Soil samples were taken before planting and after harvesting I. zollingeriana plants. Soil extraction was carried out compositely using a soil drill with a depth of 20 cm. On each plot, taken from five different points and weighing as much as 500 g. The next chemical analysis of the soil is carried out, including soil texture (pipette method), pH (pH meter), organic C (Walkey and Black), nitrogen (Kjedhal), potential phosphorus and potassium (HCl 25%), available P (Bray I), available K (Morgan), Ca and Mg (AAS), K and Na (flamephotometry), and cation exchange capacity (CEC) (distillation, ammonium acetate at pH 7).
Soil microorganisms analysis was carried out by counting colonies of nitrogen-fixing bacteria and phosphate-solubilizing bacteria. N-fixing bacteria were counted using the plate count method, which was inoculated before the nutrient agar medium. Meanwhile, P-solubilizing bacteria use Pikovskaya media (Saraswati, 2007). Colonies of N-binding bacteria were characterized by a pink, round, and convex color, while P-solubilizing bacteria were characterized by a clear color using the following formula:

df = dilution factor in the petri dish whose colonies are counted
dm = dry matter of soil sample (g) = wet weight x (1- water content)
Statistical Analysis
The experimental design was a randomized block design (RBD), with four generations of M2 I. zollingeriana resulting from gamma ray irradiation. Namely: 0 Gy, 100 Gy, 200 Gy, and 300 Gy with six replications.
The statistical analysis production and quality were analyzed by ANOVA, and when there was a significant difference, the data was further analyzed with the orthogonal polynomial contrast test in SPSS v.25. The soil characteristics were analyzed descriptively.
RESULTS AND DISCUSSION
Dry Matter Production
The characteristics of dry matter (DM) production and growth of the four M2 generations of I. zollingeriana resulting from gamma-ray irradiation on saline soil are presented in Table 1. The results showed that DM production was not significantly different (P > 0.05) across M2 generations. Numerically, the highest DM production was found in the 300 Gy treatment (11.08 t ha-1 y-1), with treatments of 0, 100, and 200 Gy, respectively, obtaining 9.8, 8.31, and 8.88 t ha-1 y-1. Potential yield increased by 1.2 t ha-1 y-1 (12.14%) compared to non-irradiation treatment.
Table 1: DM production and growth characteristics of I. zollingeriana on saline soil.
|
Parameter |
Radiation Dosage (Gray) |
|||
|
0 |
100 |
200 |
300 |
|
|
DM Production (t ha-1 y-1) |
9.88a |
8.31a |
8.88a |
11.08a |
|
Leaf/stem ratio |
0.70a |
0.72a |
0.64a |
0.69a |
|
Single leaf area (cm2) |
5a |
6a |
5a |
5a |
|
Compound leaf area (cm2) |
80a |
98a |
73a |
89a |
|
Mortality (%) |
3a |
4.33a |
4.33a |
3a |
Different superscripts on the same line indicate significant differences (P<0.05)
DM: dry matter
Leaf/stem Ratio
Statistically, there was no significant difference in the leaf/stem ratio (P > 0.05) between the four genotypes tested on saline soil. The results obtained ranged from 0.64 to 0.70 (average 0.68). Numerically, the leaf/stem ratio increased at the 100 Gy level by 0.2 from the control. However, the ratio decreased at the 200 and 300 Gy levels.
Nutritional Content
The effect of gamma-ray irradiation on the nutrient contents of I. zollingeriana is presented in Table 2. There were no significant effects of radiation levels (P > 0.05) on nutrient contents. The data shows that the dry matter content obtained was comparable between the 0 and 100 Gy treatments, 23.82 and 23.51%, respectively, then numerically decreased in the 200 and 300 Gy treatments to 20.08 and 22.63%.
In Vitro Digestibility
In vitro digestibility of I. zollingeriana was not significantly different (P > 0.05) among irradiation doses. Dry and organic matter digestibility were numerically higher in the 200 Gy treatment (73.65 and 72.26%, respectively). There was a positive effect by increasing the radiation dose to a limit of 200 Gy, then decreasing at a dose of 300 Gy to 69.63 and 68.05%.
Table 2: Nutritional content and the digestibility of M2 I. zollingeriana in saline soil.
|
Parameter |
Radiation Dosage (Gray) |
|||
|
0 |
100 |
200 |
300 |
|
|
Dry matter (%) |
23.82a |
23.51a |
20.08a |
22.63a |
|
Ash content (%) |
7.97a |
7.83a |
7.7a |
7.17a |
|
Crude protein (%) |
25.39 a |
25.16a |
25.86a |
26.81a |
|
Crude fiber (%) |
22.48a |
23.6a |
23.02a |
24.57a |
|
Crude fat (%) |
2.89a |
2.63a |
2.77a |
2.85a |
|
ADF (%) |
32.7a |
34.6a |
33.04a |
35.48a |
|
NDF (%) |
43.88a |
43.76a |
44.72a |
45.41a |
|
IVDMD (%) |
71.02a |
70.73a |
73.65a |
69.63a |
|
IVOMD (%) |
70.94a |
70.15a |
72.26a |
68.05a |
Different superscripts on the same line indicate significant differences (P<0.05).
ADF: acid detergent fibre; NDF: neutral detergent fibre; IVDMD: in vitro dry matter digestibility; IVOMD: in vitro organic matter digestibility
Physiological Characteristics
Proline:The physiological characteristics of I. zollingeriana plants resulting from gamma-ray irradiation in Table 3 showed that there were no significant differences between the treatments. The research shows that I. zollingeriana plants in saline soil make physiological adaptations by producing proline to maintain cell turgor.
Table 3: Physiological characteristics of I. zollingeriana on saline land.
|
Radiation Dosage (Gray) |
Proline content (ppm) |
Tannin Content (%) |
|
0 |
60937a |
0.15a |
|
100 |
61959a |
0.15a |
|
200 |
65450a |
0.13a |
|
300 |
55788a |
0.13a |
Different superscripts in the same column indicate significant differences (P<0.05)
Based on Table 3, the proline content continues to increase with increasing radiation dose. Numerically the treatments of 0, 100, and 200 Gy, respectively, obtained 60937, 61959, and 65450 ppm, but proline accumulation decreased drastically at the highest radiation dose (300 Gy) even under control treatment (55788 ppm).
Tannin:The tannin content in this study was classified as very low, ranging from 0.13-0.15% compared to that reported by (Antari et al., 2022), ranging from 2.4-7.7% for several types of Indigofera in Indonesia. Although statistically, it was not significantly different, increasing the radiation dose was able to reduce tannin levels in I. zollingeriana plants by 0.2% from the control at levels of 200 and 300 Gy.
Soil Characteristics
Soil Microorganisms: Soil microorganisms before and after planting I. zollingeriana as a result of gamma irradiation on saline soil are presented in Table 4.
Table 4: Soil microorganisms analysis before and after planting M2 I. zollingeriana in saline soil.
|
Radiation Dosage (Gray) |
Nitrogen-fixing bacteria (CFU/g) |
Phosphate solubilizing bacteria (CFU/g) |
||
|
Start |
End |
Start |
End |
|
|
0 |
1.74 x 108 |
3.63 x 109 |
3.40 x 104 |
9.98 x 108 |
|
100 |
1.45 x 108 |
6.02 x 108 |
2.72 x 106 |
2.06 x 109 |
|
200 |
1.83 x 108 |
1.75 x 108 |
3.72 x 106 |
7.41 x 107 |
|
300 |
1.69 x 108 |
3.72 x 106 |
6.55 x 108 |
|
The variables are presented descriptively.
CPU/g: Colony forming unit/gram
Soil microorganisms analysis was carried out by counting colonies of nitrogen-fixing bacteria and phosphate-solubilizing bacteria. This variable is presented descriptively.
Chemical analysis of soil: Chemical analysis of soil before and after planting M2 generations of I. zollingeriana resulted from gamma-ray irradiation on saline soil is presented in Table 5.
In general, observations were made to determine changes in soil structure and chemistry before and after planting I. zollingeriana in saline soil. Without involving the influence of irradiation on plants. The discussion of the changes obtained is explained descriptively.
Dry matter production
The high yields obtained in the 300 Gy treatment are most likely due to new traits in plants through genetic changes after receiving gamma irradiation, there is an increase in mutant lines, genes whose role is being able to provide higher dry matter production in salt stress conditions which is a measure of tolerance plants against salinity stress. Mutation breeding contributes to a significant increase in plant production (Yasmin and Arulbalachandran, 2022), the physical mutagen gamma ray irradiation acts as a more effective tool to induce new traits in Indigofera plants to determine the potential impact on plant growth and production.
Compared with the results of previous research, biomass production in this study was relatively low. In the M1 generation, around 38.54 t ha-1 y-1 was obtained (Hutasoit et al., 2022). This is most likely due to differences in the type of soil used. M1 was planted in optimal soil conditions that had sufficient nutrient content for crop production, while M2 planting was carried out on marginal soil (saline soil) resulting in a decrease in crop production of 24.74% from M1. This low production is because saline soil contains excessive amounts of soluble salts which interfere with plant growth (Hailu and Mehari, 2021).Increasing the concentration of dissolved salts results in low plant osmotic pressure, low N, P, K and Ca contents and chlorophyll degradation. This inhibits the absorption of water and nutrients which takes place through the process of osmosis. The amount of water entering the roots decreases, resulting in reduced water supplies in plants (Kamsurya, 2020; Balasubramaniam et al., 2023).In general, there are two basic mechanisms for plant responses to the effects of salinity, namely osmotic and ionic mechanisms. The osmotic mechanism is a rapid reaction of plants by limiting water absorption in the root area due to salinity. Meanwhile, the ionic mechanism is the ability of plants to overcome intercellular poisoning due to an excess of certain ions (Ouhaddou et al., 2023).
Leaf/steam ratio
These results are relatively low compared to research that has been carried out on optimal land. Among other, (Banurea et al., 2017) reported 0.86 with different plant spacing treatments (Banurea et al., 2017). (Wagiu et al., 2020) obtained a ratio of 1.15 with different cutting intensities, while (Sambuaga et al., 2020) obtained even higher results
with an average value of 2. The low leaf/stem ratio in this study was probably due to the influence of the saline soil
Table 5: Chemical analysis of soil before and after planting M2 I. zollingeriana in saline soil.
|
Time |
Texture (%) |
pH |
Organic matter (%) |
C/ N |
P2O5 |
K2O |
Bray 1 P2O5 |
Mor-gan K2O |
Ca |
Mg |
K |
Na |
am-ount |
CEC |
BS |
||||
|
Sand |
Dust |
Clay |
H2O |
KCL |
C |
N |
mg/100g |
ppm |
ppm |
Cmol/kg |
% |
||||||||
|
Start |
91 |
4 |
5 |
5.6 |
4.5 |
1.00 |
0.10 |
10 |
7 |
26 |
26.2 |
27 |
1.88 |
0.25 |
0.4 |
0.12 |
2.29 |
3.69 |
62 |
|
End |
92 |
4 |
4 |
5.3 |
4.3 |
1.30 |
0.12 |
11 |
8 |
21 |
39.8 |
35 |
1.73 |
0.22 |
0.5 |
0.15 |
2.15 |
4.31 |
50 |
The variables are presented descriptively.
pH: Potential of Hydrogen; KCL: Kalium clorida; C: Carbon; N: Nitrogen; P2O5: difosfor pentoksida; K2O: Kalium Oksida; Ca: Calsium; Mg: Magnesium; K: Kalium; Na: Natrium; CEC: Cation exchange capacity; BS: Base saturation.
used which had a high NaCl concentration (dS/m >12). Water absorption through roots in saline soil often experiences problems because it is bound by other minerals, resulting in dryness in plants (Ievinsh, 2023). Drought causes structural changes in plants to maintain turgor. Smaller stomata per unit leaf area and increased succulence result in a lower leaf ratio, which causes a decrease in the proportion of leaves in plants (Dos Santos et al., 2022; Singh et al., 2021). The leaf/stem ratio related to the nutritional quality of Indigofera plants. The higher the proportion of leaf fraction indicates the higher the nutritional quality of the plants because some nutrients (protein, fat) are higher in the leaves than in the stems. The lower crude fiber content indicates a softer leaf texture and tends to be preferred by livestock. According to (Shehu et al., 2001; Gustavsson and Martinsson, 2004) a high proportion of leaves is an indication that the feed is of good quality and can increase feed consumption and nutrient intake in livestock (Gustavsson and Martinsson, 2004; Shehu et al., 2001).
In the 200 Gy treatment, a morphological adaptation mechanism occurred in saline soil by reducing leaf size. The size of the leaf area in this study is relatively narrow, ranging from 73-98 m2. This trait closely related to the plant’s mechanism for minimizing the risk of its life. Plants will reduce the size of their leaves, then narrow and shed them, which is one of the tolerance mechanisms for salinity stress to reduce high water requirements or to optimize water reception by plants. The highest mortality rate (4.3%) of the population was observed in the 100 and 200 Gy treatments. Salt stress affects plant growth with stressful conditions resulting in leaves and stems drying out and then dying (Negrão et al., 2017; Zhao et al., 2021).
Nutritional content
Even though I. zollingeriana is grown on saline soil, the crude protein (CP) content is relatively high, ranging from 25.39–26.81%. This yield is higher than that planted on peat land (24.34%), reported by (Ali et al., 2021). In this study, the CP content numerically increased with an increasing radiation dose. The same results were obtained in previous research on the M1 generation (Hutasoit et al., 2022). The highest CP was found in the 300 Gy treatment (29.70%). The effect of irradiation was able to mutate plant genes, causing changes in their genetic traits in a positive direction that can be passed on to the next generation (Riviello-Flores et al., 2022). The CP in the M2 generation is relatively lower than the CP in the M1, most likely due to the low ratio of leaves obtained in the M2 generation, so the nutritional content is low (Shaaban et al., 2023).
The numerically lowest ADF content was found in the 0 Gy treatment (32.7%), while NDF was at 100 Gy (43.76%). Numerically, the higher the radiation dose up to 300 Gy, the higher the ADF and NDF content (35.48 and 45.41%, respectively). There was a correlation with the level of digestibility obtained in this study; the higher the radiation dose, the lower the feed digestibility.
In Vitro Digestibility
The level of digestibility obtained in this study was higher compared to that reported by (Tarigan et al., 2018), who reported in vitro digestibility dry matter (IVDMD) of 73.62% and in vitro digestibility organic matter (IVDOM) of 71.84% in goats fed green concentrate containing I. zollingeriana. (Gunun et al., 2022) reported that the addition of 20% Indigofera in concentrate feed could increase IVDMD from 54.9% to 60.2% and IVDOM from 58.8% to 64.1%. The high digestibility obtained in this study shows that Indigofera plants have good quality as animal feed. The low leaf/stem ratio obtained did not affect the digestibility value of Indigofera plants. This is most likely due to the low content of ADF and NDF obtained with an average yield of 33.95 and 44.44% recpectively, so they are easily degraded in the rument. ADF and NDF in forages are the insoluble portions containing cellulose, lignin, and silica, which are understood as the cell wall fraction.
Research shows the high content of ADF and NDF at an irradiation dose of 300 Gy obtained 35.48 and 45.41%, respectively (Table 2), negatively correlated with the digestibility of DM and OM (69.63 and 68.05%, respectively). According to (Turangan et al., 2018; Christyanto and Utama 2021), high levels of ADF and NDF can reduce feed digestibility because they are insoluble in acid detergents and neutral detergents and cannot be digested by digestive enzymes.
Physiological characteristics
Proline:Even though the average proline content at 300 Gy was lower, the biomass dry matter production produced was higher (Table 1). This probably occurs because among the population there are mutant plants that are tolerant to salinity. The plants do not experience high stress, so they do not need to produce the secondary metabolite proline in this environment. Thus, metabolic processes continue to run normally and do not affect plant production. The high tolerance of Indigofera plants to saline soil is closely related to high radiation doses. At the level of 300 Gy, were able to change the DNA structure in I. zollingeriana plants, causing changes in the codon amino acids. Then the amino acid sequence in the protein strand changes, and the protein experiences both structural and physiological changes. If mutations occur in the cell membrane protein, then the membrane will be more tolerant in regulating the flow of ions and water entering the cells, so that cell metabolism is not disturbed and will be more resistant to salinity stress.
This is what causes cells to continue to grow and develop, so that plants continue to produce optimally. Meanwhile, increasing proline levels up to 200 Gy causes a decrease in biomass dry matter production. High levels of stress force plants to increase levels of proline to maintain plants in stressful conditions, one of which is osmoregulatory (Chun et al., 2018; Spormann et al., 2023). The presence of proline can be used as an indicator of resistance to drought stress caused by saline soil; this happened in the 200 Gy treatment so that plants could survive.
Tannin:The low tannin content in this study shows that the I. zollingeriana plant is very good for use as animal feed because the tannin levels obtained are still below the normal threshold. Tannins are secondary plant metabolite compounds that are anti-nutritive and can cause poisoning if consumed excessively by livestock. (Bustanussalam et al., 2018) reported that high tannin content, if given to livestock, can have a negative impact, and according to (FAO, 2005), tannin levels above 4% can inhibit the growth of ruminants and even cause death. Tannins are polyphenols that can directly or indirectly affect intake and digestion. Its ability to bind molecules and form complexes depends on the structure of the polyphenols and macromolecules involved; thus, this will have a negative impact on livestock growth (Besharati et al., 2022; Espinoza-Velasco and Ramírez-Mella, 2022).
Soil characteristics
Soil microorganisms: Observations are presented descriptively. The population of nitrogen-fixing bacteria before planting I. zollingeriana was almost the same in each area, ranging from 1.45 x 108 to 1.83 x 08 CFU/g. Based on Indonesian Government Regulations (Permentan, 2019), the standard amount of N-binding bacteria in soil is ≥107 CFU/g, thus the soil used in this research meets the standard quality set by the government. At the end of the study, by planting I. zollingeriana in saline soil, the number of N-fixing bacteria increased, except for the 200 Gy treatment, where there was a slight decrease. The population of N-fixing bacteria in this study was classified as high; the highest numbers were sequentially found in the 0, 300, 100, and 200 Gy treatments, respectively, which obtained 3.63 x 109, 1.26 x 109, 6.02 x 108, and 1.75 x 108 CFU/g, indicating the potential for plant I. zollingeriana to produce good soil quality, most likely because this plant is a type of legume with many root nodules resulting from the symbiosis of rhizobium bacteria, which can absorb free nitrogen from the environment and convert it into a form that can be utilized by plants, which is called nitrogen fixation (Concha et al., 2020).
There was a decrease in soil nitrogen-fixing bacteria at 200 gy from 1.83 x 108 to 1.75 x 108. The impact of gamma-ray irradiation on this is closely related to This is due to the narrower leaf area and low leaf/stem ratio (Table 1). so that a few leaves are dropped. This incident is closely related to the growth of soil microbes, which really need organic material from plants for their survival. On the other hand, there is a morphological adaptation mechanism in saline soil to maintain its survival, the occurrence of mutations that provide results according to the desired goals, supported by physiological characterization, and an increase in proline concentration at 200 Gy radiation (65450 ppm) as a marker of tolerance to salinity stress. However, low leaf fraction also has an impact on reducing production (Table 1). At an irradiation dose of 300 Gy, high production was obtained, the nutrient content was moderate, although the digestibility was slightly lower, but microbial development was relatively high (1.26 x 109). This treatment is superior to other treatments in supporting feed availability on saline soil area.
The number of phosphate solubilizing bacteria (PSB) in this study showed an increase in population both before and after the research was carried out and after the research was carried out. Previously, the control treatment plot had less PSB (3.40 x 104), while the irradiation treatment plot was higher and relatively the same: 100, 200, and 300 Gy, respectively, had 2.72 x 106, 3.72 x 106, and 3.72 x 106 CFU/g. At the end of the study, the PSB population increased in all treatments, both control and irradiation, and was more variable. The highest population was found in the 100 Gy treatment (2.06 x 109 CPU/g), which was higher than the control (9.98 x 108 CFU/g). Irradiation treatment were quite effective in increasing the amount of PSB up to a dose limit of 100 Gy, followed by treatments of 300 Gy (6.55 x 108 CFU/g) and 200 Gy (7.41 x 107 CFU/g). These results show that irradiation of 100 Gy is the right dose for I. zollingeriana to have a positive effect on increasing PSB in saline soil. This is supported by the leaf morphology (Table 1), which shows that the leaf/stem ratio, the area of single and compound leaves, has a higher size. As animal feed, this fraction has very high palatability because its texture is softer than twigs or stems. However, in terms of biomass production, it is relatively low; irradiation at a low dose of 100 Gy has not been able to increase the biomass production of I. zollingeriana plants on saline soil.
The high PSB population in the 100 Gy treatment is most likely due to the creation of an environment conducive to PSB growth. The high leaf fraction it has can affect light, temperature, CO2, pH, water, and soil organic matter originating from the organic tissue of I. zollingeriana in the form of leaves, twigs, and branches that fall to the soil surface and inactive roots that decompose into compost, as well as soil organisms such as bacteria, fungi, earthworms, and insects, which play an important role in increasing the need for soil nutrients in the soil. PSB growth metabolic process (Yani and Botanri, 2022). The effect of increasing PSB will greatly influence the increase in available P in the soil (Lovitna et al., 2021). P uptake activity through the release of organic acids such as fumarate, lactate, and citrate can break down P ions bound to soil cations such as Al, Ca, Fe, and Mg and then convert them into forms that are available for plant uptake (Tian et al., 2021; Timofeeva et al., 2022).
Chemical analysis of soil: There were changes in soil texture and chemistry before and after planting the M2 I. zollingeriana generation. Sandy texture changes from 91% to 92%, and clay content changes from 5% to 4%. There was an increase in organic C levels from 1% to 1.30%, most likely influenced by vegetation in the form of leaves and twigs that fell to the ground. Even though it is still in the low category, organic C is very necessary as an energy source for soil organisms and triggers nutrient availability for plants (Subowo, 2010; Sagiarti et al., 2020). Nitrogen (N) levels increased by 0.1%, this is closely related to the I. zollingeriana plant, as a legume plant has root nodules and rhizobium, which can fix N from the air so that it is available in the soil (Darma and Dhonanto, 2021).
The P2O5 content increased from 7 to 8 mg/100 g in the low category, but K2O decreased to the medium category (21 mg/100 g). The available P content in the soil increased from 26.2 to 39.8 ppm. This is closely related to the increase in phosphate-solubilizing bacteria (Table 4). According to (Dewi et al., 2023), phosphate-solubilizing bacteria have the potential to increase P availability in soil. The activity of the group of phosphate-solubilizing bacteria secretes organic acids that can extract phosphate from its insoluble form to make it available so that plants can absorb elemental P to meet their needs (Campos et al., 2018). The cation exchange capacity (CEC) obtained in this study was classified as very low, even though there was an increase from 3.69 to 4.31. The low CEC is due to the saline soil used, which containing high levels of sand with a coarse texture and low amounts of organic matter. (Suryani, 2014; Puja and Atmaja, 2018) stated that organic materials have greater cation absorption capacity (Puja and Atmaja, 2018; Suryani, 2014). This means that the higher the organic matter content of the soil, the higher the CEC.
The percentage of base saturation (BS) has decreased from the medium category (62%) to low (50%). The decrease in the percentage of BS is likely due to the uptake of bases by plants. The absorbed base cations (K, Ca, Mg, or Na) are immediately replaced by H+ cations, so that the pH drops. Low base saturation means that many acid cations are strongly adsorbed in soil colloids (Felix et al., 2020).
CONCLUSION AND RECOMMENDATIONS
Research on M2 generation I. zollingeriana resulting from gamma ray irradiation on saline soil was able to increase the highest dry matter production at a dose of 300 Gy. Plant quality has the highest digestibility at a dose of 200 Gy, while soil biophysics shows that a dose of 100 Gy obtained the highest population of phosphate-solubilizing bacteria after planting I. zollingeriana.
ACKNOWLEDGMENTs
The authors thanks and highly appreciate to the National Research and Innovation Agency for its support in funding this research through the in house research program in the year of 2022.
NOVELTY STATEMENT
Gamma irradiation can be used to increase the dry matter production of I. zollingeriana in saline soil and raise the amount of phosphate-solubilizing bacteria in the soil.
AUTHOR’s CONTRIBUTION
Rijanto Hutasoit: Carried out the experiment, carried out the laboratory analysis, analyzed the data and drafted the manuscript. Edison Purba: Supervised the experiment and revised the manuscript. Simon Petrus Ginting: supervised the experiment. Nevy Diana Hanafi: Designed and supervised the experiment. All authors were responsible for the reading and approval of the final manuscript.
Conflict of Interests
All authors declare that they have no competing interests.
REFERENCES
Abdullah L, Suharlina (2010). Herbage yield and quality of two vegetative parts of indigofera at different times of first regrowth defoliation. Media Peternak. 33: 44-49. https://doi.org/10.5398/medpet.2010.33.3.169
Abdulrazak SA. Tsutomu F (1999). Animal Nutrition: A Laboratory Manual Laboratory of Animal science, Fac. Life Environ. Sci., Shimane Univ. Matsue-shi, 690 - 8504 Shimane, Japan First edition 1999.
Agisti A, Alami NH, Hidayati TN (2014). Isolasi dan Dentifiasi Bakteri Penambat Nitrogen Non Simbiotik pada Lahan Restorasi dengan Metode Legume Cover Crop (LCC) di Daerah Pasirian Lumajang Jawa Timur. J. Sains. Dan. Seni. Pomits., 3: 2301–9271.
Alfya cahyaty R, Aini N, Sumarni T (2017). Pengaruh Salinitas dan Aplikasi Bakteri Rhizosfer Toleran Salin Terhadap Komponen Hasil Tanaman Mentimun. Biotropika - J. Trop. Biol., 5: 133–137. https://doi.org/10.21776/ub.biotropika.2017.005.03.12
Ali A, Artika R, Misrianti R, Elviriadi EM, Poniran M (2021). Produksi Bahan Kering dan Kadar Nutrien Indigofera zollingeriana di Lahan Gambut Berdasarkan Umur Panen Berbeda Setelah Pemangkasan. J. Ilmu Nutr. Dan. Teknol. Pakan., 19: 30–35. https://doi.org/10.29244/jintp.19.2.30-35
Antari R, Anggraeny YN, Putri AS, Sukmasari PK, Krishna NH, Mariyono M, Aprilliza MN, Ginting S, (2022). Nutritive and antinutritive contents of Indigofera zollingeriana: Its potency for cattle feed in Indonesia. Livest. Res. Rural, Dev 34.
AOAC (2005). Official Methods of Analysis of AOAC international18th Edition, 2005.
Arifiani FN, Kurniasih B, Rogomulyo R (2018). Pengaruh Bahan Organik terhadap Pertumbuhan dan Hasil Padi (Oryza sativa L.) Tercekam Salinitas Vegetalika 7: 30. https://doi.org/10.22146/veg.38133
Balasubramaniam T, Shen G, Esmaeili N, Zhang H (2023). Plants’ Response Mechanisms to Salinity Stress. Plants 12. https://doi.org/10.3390/plants12122253
Banurea DP, Abdullah L (2017). Evaluasi produksi biomassa dan karakteristik tajuk Indigofera zollingeriana pada jarak tanam yang berbeda (Evaluation of biomass production and canopy characteristics of Indigofera zollingeriana on different plant spacing). Bul. Ilmu Makanan,.104: 1–11.
Bates LS, Waldren RP a, Teare ID (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207. https://doi.org/10.1007/BF00018060
Besharati M, Maggiolino A, Palangi V, Kaya A, Jabbar M, Eseceli H, De Palo P, Lorenzo JM (2022). Tannin in Ruminant Nutrition: Rev. Mol. 27: 1–26. https://doi.org/10.3390/molecules27238273
BPS (2022). Statistik Sumber Daya Laut Dan Pesisir 2022. Badan Pus. Stat. Katalog BPS / BPS Catalogue : 3312002.
Budiari NLG, Suyasa IN (2019). Optimalisasi Pemanfaatan Hijauan Pakan Ternak (Hpt) Lokal Mendukung Pengembangan Usaha Ternak Sapi. Pastura, 8: 118. https://doi.org/10.24843/Pastura.2019.v08.i02.p12
Bustanussalam YH, Rachman F, Septiana E (2018). Penentuan Kadar Antinutrisi Pada Tanaman Legume, in: Seminar Nasional Bioresources Untuk Pembangunan Ekonomi Hijau. p. 27.
Campos P, Borie F, Cornejo P, López-Ráez JA, López-García Á, Seguel A (2018). Phosphorus acquisition efficiency related to root traits: Is mycorrhizal symbiosis a key factor to wheat and barley cropping? Front. Plant Sci., 9: 1–21. https://doi.org/10.3389/fpls.2018.00752
Christyanto, Utama CS (2021). Kecernaan ADF, NDF dan Hemiselulosa Secara In Vitro pada Litter Fermentasi dengan Lama Peram yang Berbeda. J. Ilmu Ternak Univ. Padjadjaran, 21: 1. https://doi.org/10.30997/jpn.v7i2.4224
Chun SC, Paramasivan M, Chandrasekaran M (2018). Proline Accumulation Influenced by Osmotic Stress in Arbuscular Mycorrhizal Symbiotic Plants. Front. Microbiol., 9: 1–13. https://doi.org/10.3389/fmicb.2018.02525
Concha C, Doerner P, Gutiérrez R (2020). The impact of the rhizobia-legume symbiosis on host root system architecture. J. Exp. Bot., 71: 3902–3921. https://doi.org/10.1093/jxb/eraa198
Darma S, Dhonanto D (2021). Analisis kandungan N-Total dan pH tanah yang ditanami leguminosae cover crops (LCC) pada umur tanam serta dosis pengapuran berbeda. J. Trop. Agric. Food, 75–80. https://doi.org/10.35941/jatl.4.2.2022.5148.75-80
Dewi Hartati R, Suryaman M, Saepudin A (2023). The effect of phosfat solublizing bacteria at various soil pH on plant growth and yield of soybean (Glycine max (L.) Merr). JA-Crops J. Agrotechnology Crop Sci., 1: 26–34.
Dos Santos TB, Ribas AF, de Souza SGH, Budzinski IGF, Domingues DS (2022). Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2: 113–135. https://doi.org/10.3390/stresses2010009
Espinoza-Velasco B, Ramírez-Mella M (2022). Vegetable Tannins, Ruminal Microbiota and Ruminant Metabolism Interaction †. Trop. Subtrop. Agroecosystems 25. https://doi.org/10.56369/tsaes.3969
FAO (2005). State of the World’s Forests 2005. Food and Agriculture Organization of the United Nations, Rome.
Felix I, Rismaneswati, Lias SA (2020). Karkteristik Lahan Sawah Bukaan Baru Hasil Konversi Lahan Huta di Desa Kalosi Kecamatan Towuti Kabupaten Luwu Timur. J. Ecosolum, 9: 69–89.
Ginting SP, Tarigan A, Simanihuruk K, Antonius Solehuddin (2020). Effects of Two Different Energy Sources in Total Mixed Diets on the Performances and Blood Metabolites of Lactating Boerka Goats. J. Ilmu Ternak dan. Vet., 25: 32–39. https://doi.org/10.14334/jitv.v25i1.2196
Gunun N, Kaewpila C, Khota W, Polyorach S, Kimprasit T, Wasana P, Anusorn C, Metha W, Pongsatorn G (2022). The Effect of Indigo (Indigofera tinctoria L.) Waste on Growth Performance, Digestibility, Rumen Fermentation, Hematology and Immune Response in Growing Beef Cattle. Anim., 13. https://doi.org/10.3390/ani13010084
Gustavsson AM, Martinsson K (2004). Seasonal variation in biochemical composition of cell walls, digestibility, morphology, growth and phenology in timothy. Eur. J. Agron., 20: 293–312. https://doi.org/10.1016/S1161-0301(03)00041-8
Hailu B, Mehari H (2021). Impacts of Soil Salinity/Sodicity on Soil-Water Relations and Plant Growth in Dry Land Areas: A Review. J. Nat. Sci. Res., 12: 1–10.
Harahap F, Silveira S, Khatiwada D (2017). Land allocation to meet sectoral goals in Indonesia-An analysis of policy coherence. Land use policy 61: 451–465. https://doi.org/10.1016/j.landusepol.2016.11.033
Hutasoit R, Romjali E, Tarigan A, Sirait J, Ginting SP, Harahap MK (2022). The effect of gamma ray iradiation on the growth, production and quality of Indigofera zollingeriana to support the development of forage crops. IOP Conf. Ser. Earth Environ. Sci., 977. https://doi.org/10.1088/1755-1315/977/1/012139
Ievinsh G (2023). Water Content of Plant Tissues: So Simple That Almost Forgotten? Plants 12. https://doi.org/10.3390/plants12061238
Jalaludin A, Ngim J, Bakar BHJ, Alias Z (2010). Preliminary findings of potentially resistant goosegrass (Eleusine indica) to glufosinate-ammonium in Malaysia. Weed Biol. Manag., 10: 256–260. https://doi.org/10.1111/j.1445-6664.2010.00392.x
Kamsurya MY (2020). Perbaikan Produktivitas Lahan Salin yang Berkelanjutan : Review (Sustainable Improvement of Saline Productivity : Review). J. Agrohut., 11: 43–5.
Kumari BS, Ram MR, Mallaiah KV (2010). Studies on nodulation, biochemical analysis and protein profile ofRhizobium isolated from Indigofera species. Malays. J. Microbiol., 6: 133–139. https://doi.org/10.21161/mjm.20109
Lovitna G, Nuraini Y, Istiqomah N (2021). Pengaruh Aplikasi Bakteri Pelarut Fosfat Dan Pupuk Anorganik Fosfat Terhadap Populasi Bakteri Pelarut Fosfat, P-Tersedia, Dan Hasil Tanaman Jagung Pada Alfisol. J. Tanah dan. Sumberd. Lahan., 8: 437–449. https://doi.org/10.21776/ub.jtsl.2021.008.2.15
Mapiye C, Mwale M, Mupangwa JF, Mugabe PH, Poshiwa X, Chikumba N (2007). Utilisation of ley legumes as livestock feed in Zimbabwe. Trop. Grasslands, 41: 84–91.
Mustikarini ED, Prayoga GI, Santi R, Wardani K (2022). Adaptation test for upland rice genotypes in Balunijuk village rice fields with ultisol type. IOP Conf. Ser. Earth Environ. Sci., 1108. https://doi.org/10.1088/1755-1315/1108/1/012026
Negrão S, Schmöckel SM, Tester M (2017). Evaluating physiological responses of plants to salinity stress. Ann. Bot., 119: 1–11. https://doi.org/10.1093/aob/mcw191
Niedbała G, Tratwal A, Piekutowska M, Wojciechowski T, Uglis J (2022). A Framework for Financing Post-Registration Variety Testing System: A Case Study from Poland. Agron., 12. https://doi.org/10.3390/agronomy12020325
Ouhaddou R, Abdelilah M, Chayma I, Rachid L, Essaid AB, Mohammad-Reza H, Robin D, Marouane B (2023). Enhancing Maize Productivity and Soil Health under Salt Stress through Physiological Adaptation and Metabolic Regulation Using Indigenous Biostimulants. Plants, 12(21): 3703. https://doi.org/10.3390/plants12213703
Parnianto H, Hasanah U, Widjajanto D (2022). Reklamasi tanah salin menggunakan bahan organik dan pencucian di Desa Sidondo, Kecamatan Sigi Biromaru, Kabupaten Sigi. e-J. Agrotekbis 10: 82–90.
Permentan (2019). Permentan RI Nomor 01. Nomor 01. :1-47. Peraturan Menteri Pertanian - tentang pendaftaran pupuk organik pupuk hayati dan pembenah tanah.
Puja IN, Atmaja IDA (2018). Kajian Status Kesuburan Tanah untuk Menentukan Pemupukan Spesifik Lokasi Tanaman Padi. Agrotrop 8: 1–10.
Purwaningrahayu RD, Taufiq A (2018). Pemulsaan dan Ameliorasi Tanah Salin untuk Pertumbuhan dan Hasil Kedelai [Mulching and Amelioration Saline Soil for Growth and Yield of Soybean]. J Agron. Indones., 46: 182–188. https://doi.org/10.24831/jai.v46i2.16517
Riviello-Flores M, de la L, Cadena-Iñiguez J, Ruiz-Posadas LDM, Arévalo-Galarza M, de L, Castillo-Juárez I, Hernández MS, Castillo-Martínez CR (2022). Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties. Plants 11: 1–19. https://doi.org/10.3390/plants11091161
Sagiarti T, Okali, D, Markina G (2020). Analisis C-Organik, Nitrogen Dan C/N Tanah Pada Lahan Agrowisata Beken Jaya Di Kabupaten Kuantan Singingi. J. Agrosains dan. Teknol., 5: 11. https://doi.org/10.24853/jat.5.1.11-18
Sambuaga DMA, Telleng MM, Anis SD, Sumolang CIJ (2020). Produktivitas Indigofera zollingeriana pada berbagai interval pemotongan. Zootec., 40: 646. https://doi.org/10.35792/zot.40.2.2020.29769
Saraswati R, Husen RDM, Simanungkalit (2007). Metode analisis biologi tanah. Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian.
Shaaban A, Mahfouz H, Megawer EA, Saudy HS (2023). Physiological Changes and Nutritional Value of Forage Clitoria Grown in Arid Agro-Ecosystem as Influenced by Plant Density and Water Deficit. J. Soil Sci. Plant Nutr., 23: 3735–3750. https://doi.org/10.1007/s42729-023-01294-4
Shehu Y, Alhassan WS Pal UR, Phillips CJC (2001). Yield and chemical composition responses of Lablab purpureus to nitrogen, phosphorus and potassium fertilisers. Trop. Grasslands 35; 180–185.
Singh M, Nara U, Kumar A, Choudhary A, Singh H, Thapa S (2021). Salinity tolerance mechanisms and their breeding implications. J. Genet. Eng. Biotechnol., 19: 1–18. https://doi.org/10.1186/s43141-021-00274-4
Smýkal P, Vernoud V, Blair MW, Soukup A, Thompson RD (2014). The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci., 5: 1–19. https://doi.org/10.3389/fpls.2014.00351
Spormann S, Nadais P, Sousa F, Pinto M, Martins M, Sousa B, Fidalgo F, Soares C (2023). Accumulation of Proline in Plants under Contaminated Soils—Are We on the Same Page? Antioxidants 12: 1–26. https://doi.org/10.3390/antiox12030666
Subowo G (2010). Strategi Efisiensi Penggunaan Bahan Organik Untuk Kesuburan Dan Produktivitas Tanah Melalui Pemberdayaan Sumberdaya Hayati Tanah. Biota., 8: 117–128.
Suryani I (2014). Kapasitas Tukar Kation (KTK) Berbagai Kedalaman Tanah Pada Areal Konversi Lahan Hutan. J. Agrisistem, 10: 99–106.
Tarigan A, Abdullah L, Ginting SP, Permana IG (2010). Produksi dan komposisi kimia serta kecernaan invitro Indigofera sp pada interval dan tinggi pemotongan berbeda. Jitv 15: 188–195.
Tarigan A, Ginting SP, Arief, Astuti DA, Abdullah L (2018). Body weight gain, nutrients degradability and fermentation rumen characteristics of boerka goat supplemented green concentrate pellets (GCP) based on indigofera zollingeriana. Pakistan J. Biol. Sci., 21: 87–94. https://doi.org/10.3923/pjbs.2018.87.94
Tian J, Ge F, Zhang D, Deng S, Liu X (2021). Roles of Phosphate Solubilizing Microorganisms from. Biology (Basel), 10; 158. https://doi.org/10.3390/biology10020158
Tilley JMA, Terry RA (1963). a Two-Stage Technique for the in Vitro Digestion of Forage Crops. Grass Forage Sci., 18: 104–111. https://doi.org/10.1111/j.1365-2494.1963.tb00335.x
Timofeeva A, Galyamova M, Sedykh S (2022). Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants, 11: 1–23. https://doi.org/10.3390/plants11162119
Turangan GG, Tulung B, Tulung YRL, Waani MR (2018). Kecernaan Ndf Dan Adf Yang Mendapat Suplementasi Urea Molasses Multinutrient Block (Ummb) Dari Beberapa Jenis Limbah Pertanian Dan Rumput Lapang Pada Sapi Peranakan Ongole (Po). Zootec, 38: 320. https://doi.org/10.35792/zot.38.2.2018.19916
Van Soest PJ, Robertson JB, Lewis BA (1991). Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci., 74: 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Wagiu IHGM, Kaunang CL, Telleng MM, Kaunang W (2020). Pengaruh intensitas pemotongan terhadap produktivitas Indigofera zollingeriana. Zootec, 40: 665. https://doi.org/10.35792/zot.40.2.2020.29881
Yasmin K1, Arulbalachandran D (2022). Gamma irradiation effects on crop plants. Res. J. Biotech. 17 (8): 126-135. https://doi.org/10.25303/1708rjbt1260135
Yani Kamsurya M, Botanri S (2022). Peran Bahan Organik dalam Mempertahankan dan Perbaikan Kesuburan Tanah Pertanian; Review. J. Agrohut, 13: 25–34. https://doi.org/10.51135/agh.v13i1.121
Zeng L, Poss JA, Wilson C, Draz ASE, Gregorio GB, Grieve CM (2002). Evaluation of salt tolerance in rice genotypes by physiological characters. Euphytica, 129: 281–292. https://doi.org/10.1023/A:1022248522536
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021). Regulation of plant responses to salt stress. Int. J. Mol. Sci., 22: 1–16. https://doi.org/10.3390/ijms22094609
To share on other social networks, click on any share button. What are these?






