Polyphenol Content and Antioxidant Activity of Native Cultivar of Cannabis sativa L. of Pakistan
Research Article
Polyphenol Content and Antioxidant Activity of Native Cultivar of Cannabis sativa L. of Pakistan
Hafiz Husnain Nawaz1*, Ayesha Ahsan2, Amir Afzal1, Muhammad Ashraf Sumrah1, Muhammad Jan1, Kashif Ali1, Muhammad Arsalan1 and Rizwan Latif3
1Center of Excellence of Olive Research and Training, Barani Agricultural Research Institute, Chakwal, Punjab Province, 48800, Pakistan; 2Department of Chemistry, Allama Iqbal Open University Sector H-8, Islamabad- 44000, Pakistan; 3Soil and Water Testing Laboratory, Talagang Road, Chakwal, Pakistan.
Abstract | Cannabis sativa is a high-value crop that grows naturally in Pakistan’s highlands. This is commonly known “Bhang” in Pakistan. This study is an attempt to recognize the C. sativa plant’s wonderful physiochemical qualities and socioeconomic benefits. Antioxidants abound in C. sativa extract, thanks to its high phenolic content. It’s a high-polyunsaturated acid crop that’s strong in antioxidants. Extraction from C. sativa seeds yielded a 33% yield of C. sativa oil, which was then analyzed for TPC and antioxidants in comparison to commercially available C. sativa oil. The Folin-Ciocalteu reagent and the α, α-diphenyl-β-picrylhydrazyl (DPPH) test were used to measure the total phenolic content of oil and leaf extracts. Bioactive components included in plant extracts, such as tocopherol, chlorophyll, carotenoids, phenolics, flavonoids, and other scavenging components, make them more potent. Thus, the 50µg/ml concentration plant extract solution has the lowest radical scavenging percentage, whereas the 1000µg/ml concentration plant extract solution has the highest DPPH percentage 80%. The results of the test reveal that the extract concentration had an influence on the assay’s absorbance, or, to put it another way, an increase in extract concentration produces a drop in absorbance, demonstrating an inverse relationship. The extract’s antioxidants inhibit DPPH, causing the absorbance to gradually diminish. Our study described that C. sativa have a wide range of volatile compounds with multiple range of physiochemical qualities and socioeconomic benefits so our study emphasizes on the future work with belief that C. sativa is a valuable crop that should be further investigated for commercial and culinary purposes.
Received | June 28, 2022; Accepted | August 13, 2022; Published | September 07, 2022
*Correspondence | Nawaz Hafiz Husnain, Center of Excellence of Olive Research and Training, Barani Agricultural Research Institute, Chakwal, Punjab Province, 48800, Pakistan; Email: h_husnain_012@yahoo.com
Citation | Nawaz, H.H., A. Ahsan, A. Afzal, M.A. Sumrah, M. Jan, K. Ali, M. Arsalan and R. Latif. 2022. Polyphenol content and antioxidant activity of native cultivar of Cannabis sativa L. of Pakistan. Pakistan Journal of Agricultural Research, 35(3): 514-522.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.3.514.522
Keywords | Cannabis sativa, Pakistan indigenous varieties, DPHH, Anti-oxidants ability, Cannabis sativa, Seed oil
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Pakistan has a lot of different plants and animals because it has a lot of good land for farming. A lot of crops and species aren’t used for business or money. Cannabis sativa is one of those crops in Pakistan that isn’t very well-known with the name of Bhang, even though it has a lot of good things about it. There is a goal for this study to look into and recognize the possible benefits of C. sativa. It will also look into what Cannabis sativa is, and how it might be different from other crops. The focus of this study is on C. sativa seed oil and C. sativa leaf extract. Cannabis sativa is an economically useful crop that may be grown and sold for profit (Jabeen et al., 2009).
Cannabis sativa, Cannabis indica, and Cannabis ruderalis are three species of the Cannabaceae family, which are also known as useful Cannabis sativa in Latin. These plants have prickly leaves, and their blooms are generally narrow and lengthy (Jabeen et al., 2009). Central Asia, comprising Russia, China, India, Pakistan, and Iran, is home to this species. It is known as bhang in the subcontinent and is a wild highland plant, although it is now widely farmed for commercial uses, mainly in northern parts and KPK (Bagci and Altan, 2003).
Cannabis sativa, Cannabis sativa L., is the oldest therapeutic plant (Hanif et al., 2017a), it’s also referred to as a ceremonial drug (Fischedick et al., 2010). It is an ancient plant, as shown by its existence in 4500-year-old Egyptian tombs (Bonini et al., 2018). Cannabis sativa milk is also used as a traditional beverage in south China (Hong et al., 2015). It has been used in fiber, woven net, and other applications for over 6000 years (Edison, 2009) It was said to have been utilized around 2700 BC by the Chinese (Hong et al., 2015).
Cannabis sativa is a plant with palmate compound leaves and greenish yellow blooms (Petrović et al., 2015). The fruit hardens as it ripens. Cannabis sativa seed has a distinct pleasant scent and flavor, and it takes about a week for it to germinate (Aladić et al., 2016). Cannabis sativa is farmed for the economic advantages of the oil derived from the seeds and the fibre it produces (Sastri, 1962).
Due of the multiple applications, industrial C. sativa with a lower level of d-9-tetrahydrocannabinol (THC) is mostly farmed and promoted. This plant has a variety of physiochemical characteristics, including narcotic and sedative effects in its branches and leaves (Latif and Anwar, 2009). It’s also used to make bhang (crushed C. sativa or hashish), ganja, and charas, which are all types of drugs (Jabeen et al., 2009). To get the important essential oils, C. sativa extract is used. These oils are made up of volatile chemicals like sesquiterpenes, monoterpenes, and many more. Cannabis sativa essential oil is also used in the cosmetics sector to create valued goods. Cannabis sativa is also utilized in aromatherapy and as a food ingredient, among other things (Turner et al., 1980). Cannabis sativa is also used to make a variety of insecticides (Moran et al., 1997).
Minerals (P, K, Mg, S, Ca, Zn, and Fe) are abundant in C. sativa seed, as are 10–15 percent fiber, 25–35 percent oil, 20–25 percent protein, and 20–30 percent carbs (Turner et al., 1980). This is why C. sativa oil is a better source of polyunsaturated fats (PUFAs) than olive oil. It has the right amount of both types of fats (Deferne and Pate, 1996). Cannabis sativa seed oil has a pleasant taste and a perfect and balanced ratio of omega 3 and omega 6 fatty acids, which are called polyunsaturated fatty acids (PUFAs) (Fischedick et al., 2010). This makes it different from other seed oils, which don’t have this ratio. The proper ratio of polyunsaturated fatty acids is critical for human health. Because C. sativa seed oil is high in linolenic acid, it’s an excellent ingredient for lipid-rich lotions and skin-penetrating light body oils (Oomah et al., 2002). These two PUFAs provide a wide range of health advantages, including hypolipidemic, antithrombotic, anti-inflammatory, and antiarrhythmic effects. Tocopherols, which are antioxidant molecules, are also found in substantial amounts in C. sativa seed oil (Simopoulos, 2002). Cannabidiol (CBD) is responsible for C. sativa’ s antibacterial, anti-epileptic, anticonvulsive, and anti-epileptic effects (Latif and Anwar, 2009).
Phenolics are the biggest class of secondary metabolites, created through the workings of secondary metabolism in plants. They have a wide range of structure and function. They come from a variety of metabolic routes (Reis Giada, 2013). These phenolics are frequently attached to the walls of proteins and carbohydrates and are soluble in nature. They’re created via the shikimic and acetic acid pathways (Anastasiadi et al., 2012). Polyphenolics, which are found in the walls of plant vacuoles, have a vital role in combating and destroying reactive oxygen species (ROS) (Edison, 2009). Phenolic substances are now recognised to be micronutrients that are better ingested raw rather than processed. There is now a new trend for people to use medicinal plants to make possible functional meals (Mwamatope et al., 2020).
In the human body, when food is digested, a lot of free radicals are made that are bad for other organisms (Ahmed et al., 2013). Constant illness, such as coronary illness and disease, occurs as a result of oxidative damage to the cell premises (Siger and Lampart-szczapa, 2008). Producers are the food chain’s only source of energy, which is supplemented by the delivery of nutraceuticals and therapeutically relevant compounds that the body demands on a regular basis (Ahmed et al., 2013).
Because plant cell reinforcements are known to have genotoxic effects on the body, they are chosen over manufactured ones based on normal proliferation (Chen et al., 2012). As a result, there has been a substantial movement toward developing natural cancer prevention medicines to replace the artificial ones. When compared to Vitamin E and C alone, several studies have demonstrated that flavonoids, anthocyanins, and the cathechin have a strong antioxidation movement (Yu et al., 2005). Plants counter-oxidants include flavonoids, tannins, coumarins, phenolic corrosives, and tannins.
Materials and Methods
Sampling
In September, wild Cannabis sativa plants were collected in Gujranwala and Narowal, Pakistan. Before being stored in an airtight container, the plants were completely sun-dried and pulverised. Cannabis sativa seeds that had been ripened and had a shining straw tint were obtained. An importer was used to get the C. sativa oil. To see whether commercial processing affected the quality of the oil, we compared commercial and extracted oil. All the oils were tested for their anti-inflammatory properties and total phenolic content.
Cannabis sativa seed oil extraction
Cannabis sativa seeds were thoroughly rinsed and dried in the sun. A total of 30g of seeds were weighed and crushed in a grinder. For oil extraction, the Soxhlet technique was used since it is a well-known method that provides for thorough extraction while maximizing solvent recovery (Weller, 2006). For this experiment, we employed a hexane solvent (280 mL). The heat was set to a comfortable range of 60 to 70 degrees Fahrenheit throughout the space. For a total of three hours, the reflux was hooked up. In order to get rid of the solvent, it was heated to 50 degrees Celsius on a hot plate and then slowly evaporated. To reduce harm from heat and light, residual oil was stored in a cold, dry area of a dark cabinet. The color and aroma of the oil were similar to those of commercial olive oil. Most people don’t comprehend this at all.
Total phenolic content
The total phenolic content of extracts may be determined using the Folin-Ciocalteu reagent, a colorimetric reagent that generates a colored complex (Singleton et al., 1999). Electron transfers from phenolics to phosphomolybdic or phosphotungstic form a blue-colored mixture at a wavelength of 765nm. Gallic acid (3, 4, 5-Trihydroxybenzoic acid) is a commonly used reagent for comparing plant TPC (Singleton et al., 1999).
F-C reagent + Phenolics + Alkali → Blue Colored Complex
Reagents preparation
The leaves and oil were dissolved in 6ml of hexane and 6:4 Methanol, and then 3ml of water was added to the mixture. It was centrifuged for five minutes after vortex for five minutes. After vortex for 5 minutes and centrifuging for 5 minutes, 3 mL of the water-methanol combination was added to the supernatant, which was then collected. The sample solution is 100ml of this extract diluted. 1000ml of water was added after distilled water was dissolved in 200gm of Na2CO3 and heated. From a stock solution made by dissolving 0.500g of gallic acid in 10ml of methanol, diluted concentrations of 50g, 100g, 150g, 250g, and 100g were created. For the experiment, 100 litres of extract, 2 litres of distilled water, and 200 litres of Folin-Ciocalteu reagent were all incubated for 30 minutes at room temperature with the other ingredients. For one hour at room temperature, 1 mL of Na2CO3 solution was added, vortexed, and the mixture was incubated (Nawaz et al., 2018).
Spectrophotometric analysis
A UV-Spectrophotometer was used to measure total phenolic content at a wavelength of 765nm (T90-UV Spectrophotometer, PG Instruments Ltd). The goal is to use the intensity of light observed to measure the amount (concentration) of a given chemical compound. It turns from blue to yellow over time because of the effect that phenolics have on the F-C reagent, which makes the colour change over time (Yu et al., 2005).
Formula for determination of GAE/100g
Dilution Factor= 0.1, Final Extract Volume= 5ml, Aliquot =0.1ml, Plant Mass= 2.5, Dilution Factor =0.1, Aliquot= 0.1ml
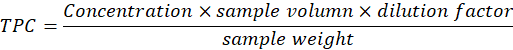
Antioxidant capacity of Cannabis sativa
Solution preparations: 0.0238g of DPPH dissolved in methanol and 100ml of water were used to make the solution 610 (-6) M DPPH. It may be kept in a dark, cool setting for up to 15 days, preferably in the refrigerator. A fresh DPPH working solution is made by diluting 10ml of DPPH stock solution to 100ml for the assay. Methanol and leaf powder were mixed for 15 minutes on a hot plate after centrifuging, discarding the supernatant, and adding more solvent. Supernatant mixtures were formed after three rounds of extractions. To make a 5000g/ml solution, they were diluted to 100ml using methanol. The dilutions for the 1000g, 500g, 250g, 100g, and 50g/ml tests were made from this stock solution. The oil was extracted using a novel process that employed 2 mL of oil in 6 mL of hexane. Methanol and water (6:4) were poured to 3 millilitres and vortexed for 5 minutes. The supernatant was recovered after centrifugation. To maximise the oil’s antioxidant action, the procedure was done three more times. The DPPH experiment was carried out using a mixture of the three extracts. The activity of commercial oil and extracted oil in scavenging DPPH radicals was examined.
Table 1: Calculation of total oil content in Cannabis sativa seed originated in Pakistan.
|
1 |
Weight of beaker |
13.6g |
|
2 |
Weight of beaker + oil |
23.525g |
|
3 |
The weight of oil |
23.525 – 13.6 = 9.925g |
|
4 |
Percentage yield |
9.925/30 = 0.33 x 100 |
|
5 |
Percentage yield of hemp seed oil |
33% |
Results and Discussion
This oil had a greenish yellow tint and an oily scent, with a fine C. sativa consistency (Table 1)
Total phenolic content
Calibration curve: Excel was used to calibrate the absorbance and determine the unknown extract volumes using gallic acid concentrations Figure 1.
Regression equation: The calibration curve revealed that concentration and absorbance had a direct connection. The unknown concentrations’ x was then determined using the regression equation.
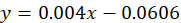
x= unknown concentration of samples, y= absorbance of sample.
The data in Sui-paper Siang’s Physiochemical and quality characteristics of cold pressed C. sativa, flax, and canola seed oils are quite comparable to those shown in Figure 2. Phytochemicals, tocopherols, chlorophyll, and other components provide plant extracts a greater overall phenolic concentration. Because of gamma-tocopherol and other essential phenolic acids, C. sativa seed oil has a high total phenolic content. As a consequence of this study, the extraction process yields C. sativa oil that is higher in total phenolic content than commercial oil.
Antioxidant capacity of Cannabis sativa
Percentage inhibition of DPPH: The test findings show that the extract concentration influenced the absorbance of the assay, or, to put it another way, an increase in extract concentration causes a decrease in absorbance, exhibiting an inverse connection. The extract’s antioxidants inhibit DPPH, causing the absorbance to gradually diminish. Observations were collected after 15 and 30 minutes of the experiment, indicating a gradual decrease in absorbance.
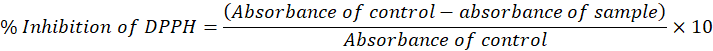
The working solution absorbance was found to be 0.511, which is lower than the recommended range of 0.5 to 0.6, according to the literature. The working solution should be maintained in the dark and oxygen contact should be avoided to prevent light-assisted oxidation. A fresh purple colour is good for working solutions; those that have become orange owing to oxidation cannot be used for the test. Before making a functional solution, the stock solution must be kept for three hours. Each test involves the development of a novel working solution. However, the plant stock solution could be kept refrigerated for a few days, but the dilutions had to be produced from scratch. DPPH is fully soluble in methanol, which makes it an ideal extraction solvent (Table 2).
The DPPH test reliably identifies inhibition because it is scavenged when it comes into touch with antioxidants, and its colour is released, becoming orange or yellow. More colour is emitted and the absorbance lowers because concentrated plant samples have a significant scavenging action. The plots revealed that as plant concentration rose, so did the amount of DPPH scavenging. There is a clear link between concentration and radical scavenging activity, as seen by a steep curve. Because of the presence of tocopherol, chlorophyll, carotenoids, phenolics, flavonoids, and other bioactive components in plant extracts, they have increased scavenging action. This means that when testing plant extract solutions, the lowest radical scavenging percent is found in the 50g/ml concentration plant extract solution, whereas the highest DPPH percent is found in the 1000g/ml concentration plant extract solution (Table 3).
Oil’s DPPH scavenging activity is weaker than that of plant extracts because it lacks chlorophyll and other green pigments. When the absorbance of extracted and commercial oil was compared, the commercial oil’s DPPH scavenging activity was found to be much lower than that of the extracted oil. Scavenging is permitted up to 37 percent for extracted oil, but only 30 percent for commercial oil, which is a substantial difference in terms of efficiency. Commercial things are thought to be physically and chemically less competent than biologically or chemically generated materials, according to common thinking. Cannabis sativa oil’s beneficial components are more likely to be lost during commercial processing.
Table 2: Percentage inhibition of DPPH of Cannabis sativa plant extracts.
|
DPPH Assay |
Abs (P1) |
Abs (P2) |
Abs (P3) |
% DPPH scavenging |
||
|
2ml DPPH working soln. |
0.511 |
0.511 |
0.511 |
P1 |
P2 |
P3 |
|
2ml, 50µg/ml+2ml working soln. |
0.191 |
0.189 |
0.218 |
62% |
63% |
57% |
|
2ml, 100µg/ml+2ml working soln. |
0.179 |
0.172 |
0.191 |
64% |
66.2% |
62% |
|
2ml, 250µg/ml +2ml working soln. |
0.140 |
0.152 |
0.165 |
72% |
70% |
67% |
|
2ml, 500µg/ml+2ml working soln. |
0.109 |
0.141 |
0.152 |
78% |
72% |
70% |
|
2ml, 1000µg/ml+2ml working soln. |
0.101 |
0.132 |
0.141 |
80% |
74% |
72% |
Table 3: Percentage inhibition of DPPH of Cannabis sativa extracted oil.
|
DPPH assay Extract + working soln. |
Extracted O1 (abs) |
Commercial O2 (abs) |
Inhibition percentage O1 |
Inhibition percentage O2 |
|
20µL +2ml working soln. |
0.426 |
0.506 |
17% |
2% |
|
60µL+2ml working soln. |
0.388 |
0.449 |
24% |
12% |
|
80µL+2ml working soln. |
0.378 |
0.439 |
26% |
14% |
|
100µL+2ml working soln. |
0.324 |
0.361 |
36% |
29% |
|
120µL+2ml working soln. |
0.321 |
0.354 |
37% |
30% |
Cannabis sativa, according to publicly available statements, is a plant of significant conservative and organic value. In Punjab, it is exclusively grown for dairy cow feed, which is the only manner it is permitted to be grown (Bagci and Altan, 2003). Cannabis sativa might be declared a valuable plant that should be researched and exploited as a result of the inquiry (Hendriks et al., 1978). For birds and dairy animals, C. sativa seeds are a great source of protein (250-350 g/kg) and fiber. Cannabis sativa seed includes PUFA, such as 3 and 6, which are necessary unsaturated fats that should be incorporated in one’s diet (78 to 85 percent) (Hong et al., 2015). Fish flesh is the sole source of 3 and 6 for a big percentage of the Pakistani population. In the diet, C. sativa seed oil provides a superb 3:1 ratio of unsaturated fats. Cannabis sativa seed is utilized as an edible oil in many regions of the world, including our region, since it lacks synergistic effects (Hanif et al., 2017). Cannabis sativa seed oil, according to the published notification, may be utilized to substitute any of our privately produced oil seed crops. Cannabis sativa seed oil has a higher proportion of unsaturated fats than other seed oils, making it advantageous for cardio-vascular illnesses (Nawaz et al., 2018). Pakistan is a horticulture nation with a superb soil profile that produces a very nutritious rural product. Unfortunately, C. sativa is not a natural crop in Pakistan, therefore C. sativa seed oil must be imported from outside. It is not a costly oil, and Pakistan may be able to export it to worldwide markets, therefore establishing new trade (Hanif et al., 2017). It also has a high yield rate of about 31%, showing that it is a productive crop. Cannabis sativa oil was separated in this research utilizing a Soxhlet apparatus with a three-hour reflux period and a water shower temperature of no more than 80 degrees celsius (Akhtar et al., 2021). For oil extraction, hexane was employed as the dissolvable, and it came in a 250ml container to guarantee safe refluxing (Hong et al., 2015). After separation, the oil was heated and mixed in order to get easy dissolvable ejection. Hexane is the most widely used dissolvable for oil extraction because of its instability and nontoxicity, as well as its ability to dissolve oil (Nawaz et al., 2022). Hexane is the most commonly used dissolvable for oil extraction because of its instability and nontoxicity, as well as its ability to dissolve oil. It was not possible, however, to give a purity grade of 100 percent to the oil generated during the soxhlet extraction technique owing to inadequate solvent removal. In this study, the extraction yield was 33%, which was somewhat greater than what had been reported in the previous literature (Oomah et al., 2002). Gamma-tocopherol is found in high concentration in Cannabis sativa seed oil, which helps to keep lipids stable and extend the shelf life of C. sativa products. Cannabis sativa oil contains gamma linolenic acid, which is a natural moisturizer and anti-aging component that should be investigated by the cosmetic industry (Jin et al., 2020).
Cannabis sativa plants have a high cell reinforcement content, which helps to alleviate oxidative pressure by functioning as free radical scavengers and disease protection. The antioxidant potential of C. sativa plants and C. sativa seed oil was examined using DPPH (Chen et al., 2012). When it comes to antioxidants, DPPH is a free radical in and of itself, which makes the method helpful. The experiment was conducted on harvested C. sativa plants that had been diluted to different concentrations. To measure the extract’s total antioxidant capacity, the solvent was not eliminated throughout the liquid/ liquid extraction method (Fischedick et al., 2010). The researchers compared plant samples from Gujranwala, Narowal, and Lahore. Gujranwala-grown C. sativa had the highest radical scavenging capacity, whereas Lahore-grown C. sativa had the lowest (Bautista et al., 2021). The total phenolic content of C. sativa oil and C. sativa plants is far greater than that of other oils and plants, making it a natural free radical scavenger (Serdar, 1998). Eliana Vonapartis and colleagues conducted a study on C. sativa seed oil in 2015 and reported that it contained 2224 mg of phenol per 100 grammes of GAE. It was claimed in Physicochemical and quality characteristics of cold-pressed C. sativa, flax, and canola seed oils, published by Sue-Siang and John Birch, that the total phenolic content of C. sativa oil extract was comparable to that reported in Physicochemical and quality characteristics of cold-pressed C. sativa, flax, and canola seed oils (Hanif et al., 2017). The total phenolic content of C. sativa plant extract was identical to what Eliana Vonaparti reported in her study Seed composition of eleven industrial Cannabis sativa cultivars allowed for production in Canada. The total phenolic content extracted is somewhat lower than previously reported. Vitamin E, an important antioxidant and biomolecule, is abundant in C. sativa seed oil and C. sativa essential oils. Pakistani agricultural products have a higher content of vitamin E than the rest of the globe (Kovalchuk et al., 2021). According to research conducted in Pakistan, the tocopherol content of C. sativa seed oil is 60.40 1.40 mg/kg for alpha-tocopherol and 650.00 4.50 mg/kg for gamma-tocopherol, with alpha-tocopherol containing the highest concentration at 60.40 1.40 mg/kg (Hanif et al., 2017). In accordance with the literature, the C. sativa plant has a significant amount of alpha-tocopherol, while C. sativa seed oil contains a significant amount of gamma-tocopherol. Finally, in this location, there are little extensive research on the characterization of C. sativa plants, which may be used.
Conclusions and Recommendations
The experiment was conducted on hemp plants harvested and diluted to various concentrations. The solvent was not removed throughout the liquid/liquid extraction process to determine the extract’s total antioxidant capacity. Plant samples from the areas of Gujranwala, Narowal, and Lahore were compared. The maximum radical scavenging potential was found in Gujranwala-grown hemp, whereas the least was found in the Lahore area. Hemp oil and hemp plants have much more great total phenolic content, a natural, free radical scavenger. According to a research experiment, the tocopherol content of hemp seed oil is 60.40 1.40 mg/kg for alpha-tocopherol and 650.00 4.50 mg/kg for gamma-tocopherol. This content has broad-spectrum benefits, but due to unknown aspects of hemp oil, we cannot get the maximum benefits, so that further studies could reveal more befits and proper usage of hemp seed oil. Accumulated results described that hemp plant contains a high amount of -tocopherol, and hemp seed oil is also high in -tocopherol. Finally, the area lacks comprehensive research on the characterization of hemp plants, which should be capitalized on.
Acknowledgement
We are thankful to the department of chemistry, Allama Iqbal open University, Islamabad for providing facility to conduct this research trial and the financial support.
Novelty Statement
Our results highlighted the possibility of also using this part of the plant, which represents a valuable source of natural antioxidants and a rich fount of polyphenols, which represent bioactive compounds not common in other typical plants. It is, therefore, desirable to continue to expand the understanding of this actual topic to estimate their efficacy for future applications for nutraceutical purposes.
Author’s Contribution
Hafiz Husnain Nawaz: Conceived the ideas of research and conducted experiment.
Ayesha Ahsan: Helped in management of article.
Amir Afzal: Worked for language improvement of manuscript.
Muhammad Ashraf Sumrah: Prepared first draft.
Muhammad Jan: Thoroughly review the article and improved the article time to time.
Kashif Ali: Provided technical input.
Muhammad Arsalan: Helped for manuscript improvement.
Rizwan Latif: Reviewed and corrected the final draft.
Conflict of interest
The authors have declared no conflict of interest.
References
Ahmed, D., S. Zara and H. Baig. 2013. In vitro analysis of antioxidant activities of oxalis corniculata linn. fractions in various solvents. Afr. J. Tradit. Complement. Altern. Med., 10): 158–165. https://doi.org/10.4314/ajtcam.v10i1.21
Akhtar, S., M.A. Sumrah, M. Faisal, M. Jan, M.R. Anser, M.A. Iqbal, H. Nawaz and M. Rafique. 2021. Economics and marketing of olive in Punjab, Pakistan. J. Econ. Impact, 3(3): 202-208. https://doi.org/10.52223/jei3032110
Aladić, K., S. Vidović, J. Vladić, D. Balić, H. Jukić and S. Jokić. 2016. Effect of supercritical CO2 extraction process parameters on oil yield and pigment content from by-product cannabis sativa cake. Int. J. Food Sci. Technol., 51: 885–893. https://doi.org/10.1111/ijfs.13041
Anastasiadi, M., H. Pratsinis, D. Kletsas, A. Skaltsounis and S. Haroutounian. 2012. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT-Food Sci. Technol., pp. 316–322. https://doi.org/10.1016/j.lwt.2012.04.006
Bagci, E. and Y. Altan. 2003. Chemotaxonomic approach to the fatty acid and tocochromanol content of Cannabis sativa L. (Cannabaceae). Turk. J. Bot., 27: 141–147.
Bautista, J.L., S. Yu and L. Tian. 2021. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega, 6(8): 5119-5123. https://doi.org/10.1021/acsomega.1c00318
Bonini, S.A., Premoli, M., Tambaro, S., Kumar, A., Maccarinelli, G., Memo, M. and Mastinu, A., 2018. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol., 227: 300-315. https://doi.org/10.1016/j.jep.2018.09.004
Chen, T., J. He, J. Zhang, X. Li, H. Zhang, J. Hao and L. Li. 2012. The isolation and identification of two compounds with predominant radical scavenging activity in cannabis sativaseed (seed of Cannabis sativa L). Food Chem., pp. 1030–1037. https://doi.org/10.1016/j.foodchem.2012.03.009
Deferne, J.L. and D. Pate. 1996. Cannabis sativa seed oil: A source of valuable essential fatty acids. J. Int. Cannabis sativa Assoc. 3: 4–7.
Edison, B., 2009. Analysis of tocopherols by high performance liquid chromatography. J. Chem., 6: 395–398. https://doi.org/10.1155/2009/415864
Fischedick, J.T., A. Hazekamp, T. Erkelens, Y.H. Choi and R. Verpoorte. 2010. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry, pp. 2058–2073. https://doi.org/10.1016/j.phytochem.2010.10.001
Hanif, M.A., H. Nawaz, S. Naz, R. Mukhtar, N. Rashid, I.A. Bhatti and M. Saleem. 2017a. Raman spectroscopy for the characterization of different fractions of Cannabis sativa essential oil extracted at 130 °C using steam distillation method. Spectrochimica. https://doi.org/10.1016/j.saa.2017.03.072
Hendriks, H., T. Malingre, S. Batterman and R. Bos. 1978. The essential oil of Cannabis sativa L. Pharm. Weekbl., pp. 413–424.
Hong, S., K. Sowndhararajan, T. Joo, C. Lim, H. Cho, S. Kim, G.Y. Kim and J.W. Jhoo. 2015. Ethanol and supercritical fluid extracts of Cannabis sativa seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac. J. Reprod., 4: 147–152. https://doi.org/10.1016/S2305-0500(15)30012-9
Jabeen, A., M.A. Khan, M. Ahmad, M. Zafar and F. Ahmad. 2009. Indigenous uses of economically important flora of Margallah hills national park, Islamabad, Pakistan. Afr. J. Biotechnol., 8(5): 763-784.
Jin, D., Dai, K. and Xie, Z. 2020. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep., 10: 309. https://doi.org/10.1038/s41598-020-60172-6
Kovalchuk, A., B. Wang, D. Li, R. Rodriguez-Juarez, S. Ilnytskyy, I. Kovalchuk and O. Kovalchuk. 2021. Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? Aging (Albany NY), 13(2): 1571. https://doi.org/10.18632/aging.202500
Latif, S. and F. Anwar. 2009. Physicochemical studies of Cannabis sativa (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur. J. Lipid Sci. Technol., 111: 1042–1048. https://doi.org/10.1002/ejlt.200900008
Moran, J., R. Klucas, R.J. Grayer, J. Abian and M. Becana. 1997. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med., 22: 861–870. https://doi.org/10.1016/S0891-5849(96)00426-1
Mwamatope, B., D. Tembo, I. Chikowe, E. Kampira and C. Nyirenda. 2020. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr., https://doi.org/10.1016/j.sciaf.2020.e00481
Nawaz, H., A. Nawaz and A. Ahsan. 2021. Total phenolic content and antioxidant potential of local varieties of Cannabis sativa in Pakistan (Cannabis sativa).
Nawaz, H.H., U. Anam, M.N. Rajaofera, Q. He, W. Liu and W. Miao. 2018. Development of SNP-Based markers to identify Colletotrichum gossypii in upland cotton. Plant Dis., 102(7): 1426-1433. https://doi.org/10.1094/PDIS-10-17-1672-RE
Nawaz, H.H., R. Dogar, M. Ijaz, A. Sumrah, R. Ansar, S.R. Ali, M. Wajid and W. Miao. 2022. First report of crown gall disease caused by Agrobacterium radiobacter on olive plants in Layyah, Punjab, Pakistan. Plant Disease, (Ja). https://doi.org/10.1094/PDIS-12-21-2805-PDN
Nawaz, H.H., M.N. Rajaofera, Q. He, U. Anam, C. Lin and W. Miao. 2018. Evaluation of antifungal metabolites activity from Bacillus licheniformis OE-04 against Colletotrichum gossypii. Pestic. Biochem. Physiol., 146: 33-42. https://doi.org/10.1016/j.pestbp.2018.02.007
Oomah, B.D., M. Busson, D. Godfrey and J. Drover. 2002. Characteristics of Cannabis sativa (Cannabis sativa L.) seed oil. Food Chem., pp. 33–43. https://doi.org/10.1016/S0308-8146(01)00245-X
Petrović, M., Z. Debeljak, N. Kezić and P. Džidara. 2015. Relationship between cannabinoids content and composition of fatty acids in Cannabis sativa seed oils. Food Chem., pp. 218–225. https://doi.org/10.1016/j.foodchem.2014.08.039
Reis Giada, M.L., 2013. Food phenolic compounds: Main classes, sources and their antioxidant power. Intechopen. https://doi.org/10.5772/51687
Sastri, B.N., 1962. The wealth of India. A dictionary of Indian raw materials and industrial products. Raw Mater. pp. 6.
Serdar, Ü., 1998. Selection of chestnuts (C. sativa Mill.) in sinop vicinity. In: II International Symposium on Chestnut 494. pp. 327-332. https://doi.org/10.17660/ActaHortic.1999.494.50
Siger, A. and M.N.K. Lampart-szczapa. 2008. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids, pp. 137–149. https://doi.org/10.1111/j.1745-4522.2007.00107.x
Simopoulos, A.P., 2002. The importance of the ratio of omega-6/ omega-3 essential fatty acids. Biomed. Pharmacother., pp. 365–379. https://doi.org/10.1016/S0753-3322(02)00253-6
Singleton, V.L., R. Orthofer and R.M. Lamuela-Raventós. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol., 299: 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Turner, C.E., M. Elsohly and E. Boeren. 1980. Constituents of Cannabis sativa L. XVII: 169–243. https://doi.org/10.1021/np50008a001
Weller, L.W., 2006. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol., pp. 300–312. https://doi.org/10.1016/j.tifs.2005.12.004
Yu, L., K. Zhou and J. Parry. 2005. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and Cannabis sativa seed oils. Food Chem., pp. 723–729. https://doi.org/10.1016/j.foodchem.2004.06.044
To share on other social networks, click on any share button. What are these?







