Polyethylene Glycol (Peg) Mediated In Vitro Characterization of Sugarcane (CP-77/400) Calli and Regenerated Plantlets
Research Article
Polyethylene Glycol (Peg) Mediated In Vitro Characterization of Sugarcane (CP-77/400) Calli and Regenerated Plantlets
Ayesha Gul1, Mohammad Sayyar Khan1*, Mazhar Ullah1 and Iqbal Munir3
1Genomics and Bioinformatics Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan; 2Department of Agronomy, The University of Agriculture Peshawar, Pakistan; 3Biochemistry Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan.
Abstract | Drought stress negatively affects sugarcane growth and productivity. Polyethylene glycol (PEG) serves as an indicator and inducer of osmotic stress in plants. In the present research, PEG stress was applied to sugarcane calli of CP-77/400 and the physiological and biochemical responses of the stressed and the control calli were measured. The calli were grown on MS media and were then transferred to different PEG concentrations (0%, 2.5%, 5% and 7.5%). Data was taken after 30 and 60 days of treatment. Relative growth of call showed significant decrease after 30 and 60 days. Control had maximum relative growth (3.33), while calli on 7.5% PEG showed the least growth rate i.e 1.33. Similarly, non-stressed control calli had higher water content i.e. (20%) while 7.5% stressed calli showed lowest water content i.e. (5%). Catalase activity was not significantly different between control and the stressed calli after 30 days at all PEG %. However, the catalase activity was significantly increased (0.14 µMole ml-1 min-1) as compared to that of the control (0.06 µMole ml-1 min-1) at 7.5% PEG media after 60 days of stress application. PEG stress showed a significant increase in proline content. Control calli showed less amount of proline content i.e. 1.34 µMg-¹ and 1.4 µMg-¹ while 7.5% PEG had high amount of proline i.e. 4.2 µMg-¹ and 7 µMg-¹ after 30 and 60 days, respectively. In case of sugar, a significant increase was observed when calli were exposed to PEG stress. Control calli had less sugar content (1.3 µMg-¹ and 1.5 µMg¹) while 7.5% PEG had significantly high sugar content (20 µMg-1and 27 µMg-¹, respectively). Moreover, the plantlets regenerated from the selected calli showed improved root and shoot growth on media containing 7.5% PEG. Our results revealed that invitro analysis of sugarcane calli against PEG may be a useful strategy for initial screening and selection of stress tolerant sugarcane lines to be used in the future breeding programs.
Received | May 29, 2018; Accepted | October 06, 2021; Published | October 31, 2021
*Correspondence | Mohammad Sayyar Khan, Genomics and Bioinformatics Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan; Email: sayyarkhankazi@aup.edu.pk
Citation | Gul, A., M.S. Khan, M. Ullah and I. Munir. 2022. Polyethylene glycol (Peg) mediated In Vitro characterization of sugarcane (Cp-77/400) calli and regenerated plantlets. Sarhad Journal of Agriculture, 38(1): 01-10.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.01.10
Keywords | Sugarcane, Drought, Polyethylene glycol, Osmotic stress, Tissue culture
Introduction
Sugarcane (Saccharum officinarum) belongs to family Poacae and has a great importance throughout the world for nutritional and economical purposes (Ather et al., 2009). Sugarcane is also important for bioenergy production in many tropical and subtropical areas of the world (Waclawovsky et al., 2010). Despite one of the largest sugarcane growing countries, Pakistan lacks behind many countries in average cane yield (Ullah et al., 2016). Pakistan produces about 47.33 tons per hectare which is far below the existing potential due to improper techniques and unsuitable genotypes (Ali et al., 2008). Several biotic and abiotic stresses might be responsible for annual losses in sugarcane growth and production throughout the world (Hussain et al., 2003; Ghazanfar et al., 2017).
Among other factors, abiotic stresses adversely affect plant growth and productivity (Kumar, 2013). In particular, drought stress is the major constraint in production of crops including sugarcane. Conventional breeding has been used for development of drought tolerant sugarcane varieties. However, limited success in conventional breeding is due to prolonged juvenile period, heterozygosity and unsuitable flowering conditions. Therefore, different methodologies have been utilized to develop competent sugarcane varieties of strong agronomic characteristics (Khan et al., 2008). Over the past few decades, tissue culture techniques have been effectively used to develop stress tolerance in crop plants. Drought tolerant cell lines were developed in several plants species through in vitrosomaclonal variation such as sunflower (Hassan et al., 2004), soybean (Sunaryo et al., 2016), mung bean (Gulati and Jaiwal, 1993), tomato (Kulkarni and Deshpande, 2007), maize (Matheka et al., 2008), rice (Wani et al., 2010) and sugarcane (Begum et al., 2011).
Polyethylene glycol (PEG), a high molecular weight compound, is used to induce drought stress in sweet potato under in vitro conditions (Sunaryo et al., 2021). PEG is non-penetrable and non-toxic substance that lowers water potential and thus enhances and thus induces drought stress (Nadir et al., 2018). Therefore, PEG can be efficiently used for in vitro selection of drought tolerant plants. PEG could be used to develop drought tolerant sugarcane lines by tissue culture techniques (Parade et al., 2005). PEG has been extensively used to establish drought tolerant callus lines of pearl millet and wheat (Ashraf et al., 2003; Wahid et al., 2007). Previously, the sugarcane cultured cells were induced to produce osmolytes and antioxidants under PEG stress (Patade et al., 2011; 2012). In addition, PEG was used as a selection agent for screening sugarcane varieties for drought tolerance (Musa, 2011). Moreover, Begum et al. (2011) used PEG for screening of sugarcane soma clones generated through tissue culture for drought tolerance.
In the present study, we report selection and characterization of PEG induced sugarcane calli lines with respect to physiological and biochemical responses compared with non-stressed Calli lines. In vitro plantlets were then regenerated from the stressed and non-stressed calli lines and their root and shoot growth were analysed on media containing PEG as a selection agent.
Materials and Methods
The present research was performed at Genomics and Bioinformatics Laboratory, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan during 2017- 2018.
Plant materials and explants preparation
Sugarcane variety, CP-77/400 was used for callus induction and subsequent analysis of PEG mediated physiological and biochemical responses between stressed and non-stressed calli lines. Lower portions of the inner leaf whorls 12-16 inches below the upper expanded leavesof mature sugarcane plants grown in the field were collected and used as explant for callus induction. The explants were cut into small pieces and then washed 2-3 times with tap water followed by washing with sterile distilled water for a few minutes. After that explants were sterilized with 70% alcohol for 30 seconds. The sterilized explants were washed 3-5 times with distilled water and then dried on filter paper before inoculation on media for callus induction in laminar airflow cabinet.
Preparation of callus induction media
Calli were induced on Murashige and Skoog media (1962) supplemented with 5 mg L-1 2,4-D as auxin and 10% coconut water as previously described (Ullah et al. 2016). The pH of MS media was adjusted to 5.8 and then autoclaved at 120oC and 15 lb pressure for 15 minutes. The media was solidified by 6-8% agar.
Inoculation of explants and callus induction
The inner immature leaf whorls were cut into 3-4 mm portions and were then inoculated on sterilized solid MS medium. Calli were kept at 26±1°C under 30 µM−2 S−1 light with 16/8 hours light and dark periods. Callus induction was started on explants after 8-10 days of inoculation. The calli were then sub-cultured on fresh MS media after every two weeks. After four weeks, calli isolated from explants were cultured on the same media for another four weeks.
In vitro PEG stress
After eight weeks on culture media, calli were transferred to MS media containing different PEG (Molecular weight 6,000) concentrations such as 0% (control), 2.5%, 5%, and 7.5%. At least six plates with five calli per each PEG concentration were maintained. Changes in morphology and color were regularly observed and recorded on the stressed and non-stressed calli.
Physiological and biochemical analysis
Relative growth rate (RGR): RGR was estimated after 30 and 60 days of experiment according to the method of Amin et al. (2013). MS media along with petri plates were weighed before and after inoculation of calli in order to estimate the initial weight of calli. The RGR was calculated according to the following formula.
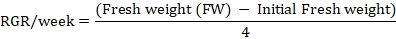
Water content: Fresh callus sample (500 mg) was taken and placed in oven for 50 hrs at 75oC. The dry callus was again weighed. Water content of calli was determined based on the following formula described by Patade et al. (2012).
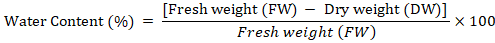
Catalase activity: Catalase activity was determined based on the method of Chandlee and Scandalios (1984). A sample of 1 g frozen callus was crushed using an ice-chilled pestle and mortar with 1ml 50mM extraction buffer (Potassium Phosphate buffer (pH 7.2) + PVP 5%). The slurry obtained was centrifuged at 12,000 rpm for 15 minutes at 4°C. After centrifugation, 100 ul supernatant was added to 900µl of 10m MReaction Mixture (Potassium Phosphate buffer (pH 7.0) + 10mM H2O2), which was then analyzed for catalase activity. The samples were vortexed and after 5 minutes, absorbance was measured at 290nm.Catalase activity was expressed in unit mg-1 protein. One unit showed the quantity of enzyme catalyzing the decomposition of 1 nmol H2O2 per m1-1mg-1 protein. Catalase activity was determined according to the following formula.
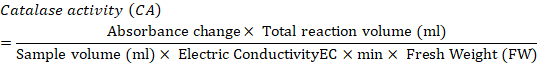
Proline and sugar analysis: Callus (500) mg was crushed using mortar and pistil. Samples were added to the test tubes along with 5 ml Methanol: Chloroform: Distilled water in a ratio of 12:5:1. The samples were centrifuged at 5000 rpm for 5 min. The supernatant was transferred to new test tubes. Then, 2 ml chloroform and 3 ml distilled water was poured and shake well. The samples were used for proline and total soluble sugar analysis.
Proline was calculated according to the procedure of Bates et al. (1973). Sample (0.5 ml) was mixed with 0.5 ml methanol: distilled water along with addition of 1 ml acetic acid. The sample was heated at 100oC for 45 min after addition of 1 ml Ninhydrin solution. After cooling, 5 ml toluene was added in the mixture. Upper layer of the sample was separated and used for proline measurement at 520 nm absorbance. Toluene was used as blank in spectrophotometer.
Total soluble sugar content was determined as previously described (DuBois et al., 1956). Sample (1 ml) was mixed with 1 ml distilled water in a test tube. Then, 5 ml sulphuric acid and 1 ml phenol were added to the sample in the fume hood. The mixture was cooled at room temperature and shaked for 12 min. Total soluble sugar content was measured in sample at 490 nm absorbance against D-glucose (blank) using spectrophotometer.
Plant regeneration and PEG stress application
After 60 days of stress application, calli from both control (0% PEG) and from the media containing 7.5% PEG were transferred to normal callus induction media and maintained for four weeks. The calli were then transferred to shoot induction media containing the appropriate cytokinins concentrations as previously reported (Ullah et al., 2016). After four weeks culturing on shoot induction media, the individual shoots of both control and stressed calli were transferred to root inducing media as previously reported (Ullah et al., 2016). The root inducing media was also supplemented with 7.5% PEG as a selection agent. Root and shoot growth of both selected and non-selected calli were recorded for a period of four weeks.
Statistical analysis
All data were examined in three replicates (n = 3). The data was statistically analysed through ANOVA, followed by Least Significance difference (LSD). P values ≤ 0.05 were considered as significant. Data in the form of means and standard errors were used to construct graphs by Sigma Plot for Windows ver. 10.0 (Systat Software, San Jose, CA, USA).
Table 1: Effect of PEG stress on morphology and survival of sugarcane calli after 30 and 60 days of the start of stress application. Means within the same column with different letters are significantly (P≤0.05) different as revealed by LSD test.
|
PEG (%) |
30 days |
60 days |
||
|
Callus morphology |
Survival (%) |
Callus morphology |
Survival (%) |
|
|
0 (control) |
Yellow |
99.2 ± 0.8a |
Yellow |
97.5 ± 1.3a |
|
2.5 |
Yellow |
96.2 ± 3.0a |
Yellow |
90.1 ± 2.8b |
|
5.0 |
Brown yellowish |
90.1 ± 4.2a |
Off white yellowish |
84.5 ± 5.1b |
|
7.5 |
Black/white |
85.4 ± 3.4b |
Brown yellowish |
76.5 ± 3.7c |
Results and Discussion
Effect of PEG concentrations on morphology and survival of calli
Calli on non-stressed media remained yellow in coloration during the experiment, while the stressed calli changed coloration from yellowish to brown and black (Figures 1 and 2). Survival of the calli was negatively affected by the increasing PEG concentrations (Table 1). After 30 days of experiment, calli on 7.5% PEG media showed survival of 85% as compared to 99% of the non-stressed control. Drastic changes in survival of calli were more pronounced after 60 days of stress application. Calli on media containing 2.5%, 5% and 7.5% PEG showed significantly reduced survival percentage as compared to that of control (Table 1).
Effect of PEG stress on callus relative growth rate and water content
Relative growth rate of sugarcane calli was significantly affected by application of PEG stress (Table 2 and 3). Calli of non-stressed media showed comparatively higher relative growth rate i.e. 3.33 unit after 30 days of experiment (Table 2). Relative growth rate of calli significantly (P ≤ 0.05) decreased at PEG concentrations of 5% and 7.5%. Lowest relative growth rate (1.33 unit) was observed at 7.5% PEG concentration. After 60 days, relative growth rate of calli increased in all treatments except at 7.5% PEG concentration (Table 3). High relative growth (5.7) of calli was found in the absence of PEG while lowest growth rate of calli was observed at 7.5% PEG concentration (1.23).
It was observed that PEG stress had a significant (P ≤ 0.05) impact on the water content of calli (Table 3). Water content also decreased with increase in PEG concentration. After 30 days at 5 and 7.5% PEG concentrations, the relative water content was significantly different from control and 2.5% PEG showing 13.43 and 11.53% respectively. Calli of control had accumulated 19.8% water content at the end of 60 days. However, calli water content decreased with
Table 2: Effect of PEG stress on physiological and biochemical parameters of sugarcane calli after 30 days of stress application. Data are averages of five replicates ± SD. Means within the same column with different letters are significantly (P≤0.05) different as revealed by LSD test.
|
Treatment |
Relative growth rate |
Relative Water Content (%) |
Catalase activity (U mg-1 ) |
Proline content ( µM g-1 FW) |
Sugar content ( µM g-1 FW) |
|
Control |
3.33 ± 0.11a |
29.73 ± 0.23a |
0.02 ± 0.01a |
1.27 ± 0.06c |
1.34±1.20c |
|
2.5% |
2.88 ± 1.12a |
21.33 ± 0.19b |
0.05 ± 0.02a |
1.33 ± 0.05c |
4.17± 0.36c |
|
5% |
1.83 ± 0.15b |
13.43 ± 0.31c |
0.06 ± 0.05a |
3.66 ± 0.05b |
12.16± 5.90b |
|
7.5% |
1.33 ± 0.05c |
11.53 ± 0.26c |
0.09 ± 0.04a |
4.26 ± 0.25a |
20.33± 5.78a |
Table 3: Effect of PEG stress on physiological and biochemical parameters of sugarcane calli after 60 days of stress application. Data are averages of five replicates ± SD. Means within the same column with different letters are significantly (P≤0.05) different as revealed by LSD test.
|
Treatment |
Relative growth rate |
Water content (%) |
Catalase activity (U mg-1 ) |
Proline content ( µM g-1 FW) |
Sugar content ( µM g-1 FW) |
|
Control |
5.70 ± 0.51a |
19.80 ± 0.01a |
0.06 ± 0.01b |
1.45 ± 0.05c |
1.51 ± 1.32c |
|
2.5% |
5.43 ± 0.49a |
12.80 ± 0.02b |
0.08 ± 0.01b |
1.66 ± 0.11c |
3.51 ± 3.51b |
|
5% |
2.43 ± 0.49b |
6.62 ± 0.05c |
0.09 ± 0.03b |
5.56 ± 0.15b |
15.53 ± 2.13b |
|
7.5% |
1.23 ± 0.11c |
5.26 ± 0.04c |
0.13 ± 0.05a |
6.83 ± 0.56a |
26.56 ± 4.61a |
increasing PEG concentration. Water content of calli was lowest (6.62% and 5.26%) at 5% and 7.5% PEG, respectively (Table 3).
Catalase activity
Catalase activity of calli was unaffected with 30 days of PEG application (Table 2). However, catalase activity increased in calli exposed to high concentration of PEG (7.5%) for 60 days while increase in catalase activity at 2.5% and 5% were not significantly different from that of the control (Table 3).
Accumulation of free proline
Proline accumulation was found to be positively correlated with PEG stress. Proline content of 1.27 µM g-1 FW was measured in control calli after 30 days experiment. PEG stress at 2.5% had no-effect on proline accumulation. However, 5% and 7.5% PEG concentrations significantly (P ≤ 0.05) increased proline content of calli (3.66-4.26 µM g-1 FW) as compared to that of the control calli (Table 2). Similar trend was observed for proline accumulation in calli after 60 days of PEG application (Table 3). PEG application (5 and 7.5% PEG) significantly increased the proline content.
Accumulation of total soluble sugars
Sugar content of calli increased with increasing PEG concentrations. After 30 days of PEG stress, control calli accumulated 1.34 µM g-1 FW total soluble sugars. However, 5% and 7.5% PEG concentrations significantly (P ≤ 0.05) increased sugar content of calli (12.16-20.33 µM g-1 FW) as compared to that of the control calli (Table 2). Similar trend was observed after 60 days of PEG application (Table 3). The stressed calli (5 and 7.5% PEG) showed significantly (P ≤ 0.05) high sugar contents.
Plant regeneration and PEG stress application
After two to three weeks, multiple shoots emerged from the calli on both stressed and control media (Figure 3A). Here, it was observed that organogenesis was comparatively slow in stressed calli than the non-stressed control calli. After four weeks culturing on shoot induction media, the individual shoots from both control and stressed calli were transferred to root inducing media as previously reported (Ullah et al., 2016). The root inducing media was also supplemented with 7.5% PEG as a selection agent. Root and shoot growth of both selected and non-selected calli were recorded for a period of four weeks. It was observed that the root and shoot growth in non-selected control calli were comparatively slower on media containing 7.5% PEG (Figure 3B). On the contrary, the selected calli showed better root and shoot growth on media containing 7.5% PEG (Figure 3C and D).
PEG mediated in vitro physiological and biochemical responses were analysed in sugarcane calli ofCP77/400 variety. The calli exposed to various
PEG concentration for 30 and 60 days showed severe loss in relative growth rate as compared to that of non-stressed control calli. In response to drought and salt stresses, plants undergo a series of molecular, biochemical and physiological changes to adjust osmotic and ionic homeostasis. These changes in turn, negatively affect the water uptake potential rendering growth reduction. PEG stress negatively affects plant growth by imposing osmotic stress and a decrease in water potential (Aazami et al., 2010; Kumar et al., 2011). PEG stress has a negative impact on the water uptake from soil and then its transport to leaves and other parts of the plant. Chazen et al. (1995) observed significant impact of PEG and NaClstress on the water transport potential in plants. Impaired water uptake and limited transport to leaves resulted in severe growth retardation. One of the defense strategies to cope with osmotic stress is that plants avoid stress by using less amount of water in their cells. This in turn, decreases the cells growth at the expense of survival under stress condition. This phenomenon of stress avoidance and reduced growth was described in sugarcane (Wahid and Ghazanfar, 2006). Studies on sunflower, sugarcane and tomato also reported growth losses when PEG was added to the medium (Hassan et al., 2004; Errabii et al., 2008; Aazami et al., 2010). In the present study, sugarcane calli showed severe loss in growth at increasing PEG concentrations. In addition, it was also observed that the water content was significantly reduced in calli subjected to PEG stress. This loss in water content and relative growth rate might be a strategy to spend more energy on the expression of stress-responsive mechanisms and finally stress avoidance and tolerance (Patade et al., 2012).
In the present study, the sugarcane calli showed increased catalase activity when exposed to increasing PEG stress in the medium. Under stress conditions, plants trigger production of antioxidant enzymes to get rid of the reactive oxygen species (ROS) and their related damages to membranes and plant organelles (Davies, 1987; Fridovich, 1986). Several studies reported induction of antioxidant enzymes under drought and salt stress conditions. Patade et al. (2011) and (2012), reported PEG induced production of antioxidents including catalase in sugarcane callus cultures. Similar induction of antioxidant enzymes was observed in tobacco callus cultures subjected to PEG stress (Bueno et al., 1998). Li and van Stadan (1998) observed increased antioxidant activities in callus cultures generated from drought tolerant maize varieties. Our results are in complete agreement with those mentioned in the above studies.
In the present study, calli subjected to various PEG concentrations accumulated high proline content. Proline is an important osmoprotectant that confers protection to cellular organells, membranes and proteins under osmotic and oxidative stresses (Handa et al., 1986). This means the stress induced proline accumulation improves growth and overall survival of callus under stress condition. Previously, Shah et al. (2012) observed rapid induction in proline content in callus cultures of rice selected under 20% PEG stress than the non-selected control callus cultures. In addition, other studies also reported PEG induced proline accumulation in tobacco, sunflower, sorghum, and rice (Gangaopadhyay, 1997; Hassan et al., 2004; Bhaskaran et al., 1985; Aqeel-Ahmad et al., 2007). Patade et al. (2012) reported increased proline accumulation in sugarcane callus cultures under PEG stress. Our results are fully supported by the findings of the above studies. Moreover, Errabii et al. (2007) reported fourfold higher proline accumulation in sugarcane cultures subjected to PEG stress in in vitro conditions. Overall, proline accumulation protects cellular organelles and proteins from degradation under PEG mediated osmotic and oxidative stresses (Handa et al., 1983).
In the present study, calli under PEG stress were found with increased sugar accumulation. Plants tend to accumulate osmolytes like sugars due to osmotic stress for their osmotic adjustment (Munns, 2005). Plants divert most of the stored energies to produce these compounds and as a result, the overall growth is decreased (Munns and Tester, 2008). In our study, the PEG stressed calli accumulated significantly higher sugars than that of the control calli. Increase in sugars content was found positively correlated with increasing PEG concentration. Also, its production was negatively correlated with overall growth of the calli that indicates cells spend their energies to produce sugars at the expense of growth retardation in order to avoid the damaging effects of stress.
Further, the selected and non-selected control calli were regenerated into plantlets which were then tested for shooting and rooting on media containing 7.5% PEG. It was observed that plantlets from the selected calli showed improved growth as compared to that of non-selected calli. Previously, plant regeneration was reported from PEG tolerant callus lines of rice and tomato plants (Siddeswar and KaviKishor, 1989; Singh and Sharma, 2008).
Conclusions and Recommendations
Sugarcane calli responded efficiently to PEG stress in terms of physiological and biochemical changes. In comparison to the non-stressed control calli, the stressed calli accumulated higher catalase, proline and soluble sugars. All these compounds are major components involved in plant adaptation to stress condition. It is concluded that comparatively high accumulation of these stress-responsive compounds might be responsible for the improved root and shoot growth of plantlets regenerated from the selected calli on PEG supplemented medium.
Acknowledgements
We are highly grateful to Pakistan Science Foundation for funding the present research [Grant No./Project No. PSF/NSLP/KP-AU (281)].
Novelty Statement
The study assess an important aspect (in vitro) of development of drought tolerant in sugarcane lines.
Author’s Contribution
Ayesha Gul: Conducted experiments.
Mohammad Sayyar Khan: Conceived and designed the experiment and assisted in experiments and manuscript write-up.
Mazhar Ullah: Assisted in data analysis and manuscript preparation.
Iqbal Munir: Critically checked the manuscript.
Conflict of Interest
The authors declare no conflict of interest
References
Aazami, M.A., M. Torabi and E. Jalili. 2010. In vitro response of promising tomato genotypes for tolerance to osmotic stress. Afr. J. Biotechnol., 9(26): 4014–4017.
Ali, A., S. Nazi, F.A. Siddique and J. Iqbal. 2008. An efficient protocol for large scale production of sugarcane through micro propagation. J. Bot., 40(1): 139-149.
Amin, N.U., G. Sanga, N. Ara, S.H. Shah and F. Ullah. 2013. Effect of various concentrations of calcium chloride on callus growth and potassium nutrition of calli cultures of potato (solanumtuberosum). Pak. J. Bot., 45(1): 209-214.
Aqeel-Ahmad, M.S., F. Javed and M. Ashraf. 2007. Iso-osmotic effect of NaCl and PEG on growth, cations and free proline accumulation in callus tissue of two indica rice (Oryza sativa L.) genotypes. Plant. Growth. Regul., 53:53–63. https://doi.org/10.1007/s10725-007-9204-0
Ashraf, M., A.Kausar and M.Y. Ashraf. 2003. Alleviation of salt stress in pearl millet (Pennisetumglaucum (L.) R. Br.) through seed treatments. Agronomie., 23:227-234. https://doi.org/10.1051/agro:2002086
Ather, A., S. Khan, A. Rahman and M. Nazir. 2009. Optimization of the protocols for callus induction, regeneration and acclimatization Of Sugarcane CV. THATTA-10. Pak. J. Bot., 41(2): 815-820.
Bates, L.S., R.P. Waldren and I.D. Teare.1973. Rapid determination of free proline for water stress studies. Plant. Soil., 39:205–207. https://doi.org/10.1007/BF00018060
Begum, M.K., M.O. Islam, M.A.S. Miah, M.A. Hossain and N. Islam. 2011. Production of somaclone in vitro for drought stress tolerant plantlet selection in sugarcane (Saccharumofficinarum L.). Agriculturists, 9:18–28. https://doi.org/10.3329/agric.v9i1-2.9475
Bhaskaran, S., R.H. Smith and R.J. Newton.1985. Physiological changes in cultured Sorgham cells in response to induced water stress 1. Free proline. Plant. Physiol., 79:239–248. https://doi.org/10.1104/pp.79.1.266
Bueno, P., A. Piqueras, J. Kurepa, A. Savoure, N. Veerbruggen, M.V. Montegu and D. Inze. 1998. Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in BY-2 cell cultures. Plant. Sci., 138(1):27–34. https://doi.org/10.1016/S0168-9452(98)00154-X
Chandlee, J.M. and J.G. Scandalios. 1984. Analysis of variants affecting the catalase development program in maizscutellum. Theor. Appl. Genet., 69: 71–77. https://doi.org/10.1007/BF00262543
Chazen, O., W. Hartung and P.M. Neumann. 1995. The different effects of PEG 6000 and NaCl on leaf development are associated with different inhibition of root water transport. Plant. Cell. Environ., 18:727-735. https://doi.org/10.1111/j.1365-3040.1995.tb00575.x
Davies, K.J.A. 1987. Protein damage and degradation by oxygen radicals. J. Biol. Chem., 262:9895–9901. https://doi.org/10.1016/S0021-9258(18)47553-9
DuBois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers and F. Smith. 1956. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem., 28:350–356. https://doi.org/10.1021/ac60111a017
Errabii, T., C.B. Gandonou, H. Essalmani, J. Abrini, M. Idaomar and N.S. Senhaji. 2008. Growth, proline and ion accumulation in sugarcane callus cultures under drought-induced osmotic stress and its subsequent relief. Afr. J. Biotechnol., 5:1488–1493.
Errabii, T., C. Gandonou, H. Essalmani, J. Abrini, M. Idaomar and N.S. Senhaji. 2007. Effects of NaCl and mannitol induced stress on sugarcane Saccharum sp. callus cultures. Acta. Physiol. Plant, 29:95-102. https://doi.org/10.1007/s11738-006-0006-1
Fridovich, I. 1986.Biological effects of superoxide radical. Arch. Biochem. Biophys., 247:1–11. https://doi.org/10.1016/0003-9861(86)90526-6
Ghazanfar, M.U., W. Raza, S.K. Gondal. 2017. Screening of sugarcane cultivars against colletotrichumfalcatum causing Red Rot disease and its control with different fungicides under laboratory conditions. Pak. J. Phytopath., 29(1): 103-110. https://doi.org/10.33866/phytopathol.029.01.0381
Gangopadhyay, G., S. Basu, B.B. Mukherjee and S. Gupta. 1997. Effect of salt and osmotic shocks on unadapted and adapted callus lines of tobacco. Plant. Cell. Tiss. Org. Cult., 49:45–52. https://doi.org/10.1023/A:1005860718585
Gulati, A. and P. Jaiwal. 1993. Selection and characterization of mannitol-tolerant callus lines of Vignaradiata (L.) Wilczek. Plant. Cell. Tissue. Organ. Cult., 35-41. https://doi.org/10.1007/BF00048461
Hussain, A., Z.I. Khan, M.H. Rashid, M. Ashraf and M.S. Akhtar. 2003. Soil Salinity Effects on Sugarcane Productivity, Biochemical Characteristics, and Invertase Activity. Pak. J. Life Soc. sci., 1(2): 114-121.
Handa, S., A.K. Handa, P.M. Hasegawa and R.A. Bressan. 1986. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant. Physiol., 80: 938–45. https://doi.org/10.1104/pp.80.4.938
Handa, S., R.A. Bressan, A.K. Handa, N.C. Carpita and P.M. Hasegawa. 1983. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant. Physiol., 73:834–43. https://doi.org/10.1104/pp.73.3.834
Hassan, N.S., L.D. Shaaban, A.H. El-Sayed and E.E. Seleem. 2004. In Vitro Selection for Water Stress Tolerant Callus Line of Helianthus annus L., CvMyak. Int. J. Agric. Biol., http://www.ijab.org.
Khan, S.A., H. Rashid, F.M. Chaudhary, Z. Chaudhary and A. Afroz. 2008. Rapid micro propagation of three elite Sugarcane (Saccharumofficinarum L.) Varieties by shoot tip culture. Afr. J. Biotechnol., 7:2174-2180.
Kulkarni, M. and U. Deshpande. 2007. In Vitro screening of tomato genotypes for drought resistance using polyethylene glycol. Afr. J. Biotechnol., 6:691-696.
Kumar, R.R., K. Karjol and G.R. Naik. 2011. Effect of polyethylene glycol induced water stress on physiological and biochemical responses in Pigeon pea (Cajanuscajan L. Millsp.). RRST-Plant Physiol., 3:148–152.
Kumar, M. 2013. Crop plants and abiotic stresses. J. Biomol. Res. Ther., 4172: 2167-7956. https://doi.org/10.4172/2167-7956.1000e125
Li, L. and J. van Stadan. 1998. Effects of plant growth regulators on the antioxidant systems in callus of two maiz cultivars subjected to water stress. Plant. Growth. Regul., 24:55–66. https://doi.org/10.1023/A:1005954532397
Mathica, J.M., E. Magiri, A.O. Rasha and J. Machuka 2008. In vitro Selection and Characterization of Drought Tolerant Somaclones of Tropical Maize (Zea mays L.). Biotechnology,7:641-650. https://doi.org/10.3923/biotech.2008.641.650
Munns, R. 2005. Genes and salt tolerance: bringing them together. New Phytol., 167:645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
Munns, R. and M. Tester. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol., 59:651-681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant., 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Musa, Y. 2011. The use of Polyethylene glycol (PEG) as selection agent of callus and plantlets of some sugarcane varieties for drought tolerance. J. Agrivigor., 10(2):130–140.
Nadir, M., R. Syahrir and Syamsia. 2018. In vitro selection of a drough tolerant callus of dwarf Napier grass (Pennisetumpurpureum Cv. Mott). IOP. Con. Ser. Earth Environ. Sci., 1:20–24. https://doi.org/10.1088/1755-1315/156/1/012024
Parade, V.Y., P. Suprasanna, V.A. Banat and U.G. Kulkarni. 2005. Selection for abiotic (salinity and drought) stress tolerance and molecular characterization of tolerant lines in sugarcane”. Poster presentation in National Conference on “Biotechnological Aspects towards Cultivation, Utilization and Disease Management of Plants, held at Lal Bahadur Shastra Mahavidyalaya, Dharmabad, Nanded. Plant. Biotechnol. J., 8: 263–27.
Patade, V.Y., S. Bhargava and P. Suprasanna. 2011. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: growth osmolyte accumulation and antioxidant defense. J. Plant. Interact., 6(4):275–282. https://doi.org/10.1080/17429145.2011.557513
Patade, V.Y., S. Bhargava and P. Suprasanna. 2012. Effects of NaCl and iso-osmotic PEG stress on growth, Osmolytes accumulation and antioxidant defense in cultured sugarcane cells. Plant. Cell. Tissue. Organ. Cult., 108:279–286. https://doi.org/10.1007/s11240-011-0041-5
Sunaryo, W., D. Darnaningsih and N. Nurhasanah. 2021. Selection and regeneration of purple sweet potato calli against drought stress simulated by polyethylene glycol [version 2; peer review: 1 approved, 1 approved with reservations]. F100 Research. 8:10. https://doi.org/10.12688/f1000research.16993.1
Shah, A.H., S.H. Shah, H. Ahmad and Z.A. Swati. 2012. Adaptation to polyethylene stress maintains totipotency of cell lines of Oryza sativa L. CV Swat-1 for a longer period. Pak. J. Bot., 44(1):313–316.
Siddeswar, G. and P.B. KaviKishor.1989. Plant regeneration from polyethylene glycol adapted callus of rice. Curr. Sci., 58:926–928.
Singh, A.K. and D.R. Sharma. 2008. In vitro screening and regeneration of water stress tolerant culture of tomato. Indian. J. Plant. Physiol., 13(1):33–38.
Sunaryo, W., W. Widoretno, Nurhasanah and Sudarsono. 2016. Drought tolerance selection of soybean lines generated from somatic embryogenesis using osmotic stress simulation of poly-ethylene glycol (PEG). Nusant. Biosci., 8:45-54. https://doi.org/10.13057/nusbiosci/n080109
Ullah, M., H. Khan, M. S. Khan, A. Jan, K. Ahmad and A.W. Khan. 2016. In vitro plant regeneration of sugarcane (Saccharumofficinarum L.); The influence of variety, explant, explant position and growth regulators. ARPN J. Agric. Biol. Sci., 11:267-273.
Waclawovsky, J., P. M. Sato, C.G. Lembke, P.H. Moore and G.M. Souza1. 2010. Sugarcane for bioenergy production: an assessment of yield and regulation of salt tolerance of sugarcane. J. Plant. Physiol., 163:723-730.
Wahid, A. and A. Ghazanfar. 2006. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant. Physiol., 163:723-30. https://doi.org/10.1016/j.jplph.2005.07.007
Wahid, A., M. Perveen, S. Gelani and S.M.A. Basra. 2007. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant. Physiol., 164:283-294. https://doi.org/10.1016/j.jplph.2006.01.005
Wani, S.H., P.A. Sofi, S.S. Gosal and N.B. Singh. 2010. In vitro screening of rice (Oryza sativa L.) callus for drought tolerance. Commun. Biomet. Crop. Sci., 5:108-115.
To share on other social networks, click on any share button. What are these?








