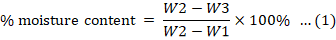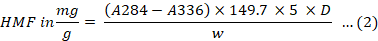Physicochemical Properties and Hydroxymethylfurfural (5-HMF) Content of Kelulut Honey Processed Using Ultrasound Method
Physicochemical Properties and Hydroxymethylfurfural (5-HMF) Content of Kelulut Honey Processed Using Ultrasound Method
Nor Adila Zulkifli1, Nurul Zaizuliana Rois Anwar1*, Zalilawati Mat Rashid1, Zarinah Zakaria1, Norshazila Shahidan2, Lee-Hoon Ho1 and Faridah Yahya3
1Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia; 2Faculty of Science and Technology, Programme of Food Biotechnology, Universiti Sains Islam Malaysia, 71800 Nilai, Negeri Sembilan, Malaysia; 3Faculty of Fisheries and Food Science, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia.
Abstract | Honey produced by stingless bee is commonly known as Kelulut honey. Honey is frequently heated to purify, filter, and permit packing in order to inhibit the growth of microbes. However, heating could increase the amount of the potentially cancer-causing substance hydroxymethylfurfural (HMF) in honey and will reduce the physicochemical and nutritional attributes due to its heat sensitivity. Hence, ultrasound which is a non- thermal processing was chosen as an alternative method to minimize the quality loss during the processing of Kelulut honey. The purpose of this study was to investigate the effect of ultrasound processing on the quality of Kelulut honey. The ultrasound processing was conducted at five different amplitudes (20, 40, 60, 80, and 100%) and three different treatment times (5, 10 and 15 minutes). Kelulut honey samples were analysed for total soluble solids, water activity, moisture content, pH, and the amount of HMF. Conventional heating was conducted at 60 °C for 30 minutes as a comparison. The results showed that the ultrasound processing increased the total soluble solids of Kelulut honey from 65.32 °Brix to 73.00 °Brix and the pH value from 2.97 to 3.62. Ultrasound processing also reduced the moisture content of Kelulut honey from 33.08% to 25.40% and water activity from 0.8188 to 0.7580. For the HMF value, ultrasound processing slowed down the formation of HMF in Kelulut honey compared to conventional heating as it only increased from 14.33 mg/kg to 34.44 mg/kg. According to these findings, the application of ultrasounds at 20% for 5 minutes was the most suitable combination of amplitude and treatment time as it could minimize the increase in HMF. Thus, it can be concluded that ultrasound processing can be used as an alternative method to the producers in providing minimally-processed Kelulut honey and maintaining the quality of honey.
Received | February 22, 2024; Accepted | August 30, 2024; Published | October 14, 2024
*Correspondence | Nurul Zaizuliana Rois Anwar, Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia; Email: zaizuliana@unisza.edu.my
Citation | Zulkifli, N.A., N.Z.R. Anwar, Z.M. Rashid, Z. Zakaria, N. Shahidan, L.H. Ho and F. Yahya. 2024. Physicochemical properties and hydroxymethylfurfural (5-HMF) content of kelulut honey processed using ultrasound method. Sarhad Journal of Agriculture, 40(Special issue 1): 142.151.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.142.151
Keywords | Kelulut honey, Ultrasound, Thermal processing, Physicochemical, 5- hydroxymethylfurfural (5-HMF)
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Honey, a nutrient-rich sweet liquid food, provides numerous health benefits (Bogdanov et al., 2008). Its chemical composition, flavor, and aroma are influenced by various factors, including botanical source, geographical location, environmental conditions, bee species, and storage conditions (Costa et al., 2015).
Kelulut honey, produced by Kelulut bees, stands out for its superior medicinal and nutritional properties compared to honey from Apis mellifera (Zhao et al., 2016). The quality of Kelulut honey can vary depending on the bee species and the location of the beehives (Ramly et al., 2021). Furthermore, Sujanto et al. (2022) highlighted that variations in plant diversity at harvesting sites contribute to differences in the physicochemical properties and mineral content of Kelulut honey.
Assessing the purity of honey involves analyzing its physicochemical parameters, including moisture content, total soluble solids, color, pH, free acidity, 5-hydroxymethylfurfural content, sugar content and intensity, and amino acid content (Razali et al., 2018). HMF, a cyclic aldehyde (C6H6O3), forms during sugar decomposition when heated or acidified (Bakhiya et al., 2009). Its formation occurs naturally during the Maillard reaction, facilitated by simple sugars and water in an acidic environment (Kowalski et al., 2013; Nozal et al., 2001). As HMF levels typically rise during processing and storage, it serves as a reliable indicator of honey freshness. Recent research has focused on understanding the physicochemical properties of honey. Notably, heat treatment negatively impacts honey’s nutritional value, texture, and flavor (Fauzi and Farid, 2015).
Honey is often heated before packaging to eliminate spoilage-causing bacteria and reduce moisture content, thereby minimizing fermentation (Subramaniam et al., 2007). Heating to 60°C or higher facilitates packaging and delays crystallization (Tosi et al., 2004). However, improper heat treatment can negatively impact honey quality and safety (Cozmuta et al., 2011).
Research indicates that thermal treatment can diminish enzymatic activity while increasing HMF concentration (Tosi et al., 2004). Given that heat processing can elevate HMF levels, regulatory bodies like Codex Alimentarius and the European Commission have established maximum HMF limits for honey (40 mg/kg, with a higher limit of 80 mg/kg for honey from tropical regions). This is crucial because HMF has been linked to cytotoxic, mutagenic, carcinogenic, and genotoxic effects in in vitro studies (Capuano and Fogliano, 2011). Analysing HMF concentration and enzymatic activity in honey is essential, as these factors serve as indicators of product quality (Subramaniam et al., 2007). Enzymatic activity significantly influences honey quality by affecting protein concentration, free amino acid profile, acidity, flavour, aroma, and pH (Chua and Adnan, 2014).
Consumer demand for minimally processed foods with enhanced nutritional and sensory qualities is on the rise. Consequently, traditional thermal pasteurization techniques are being supplanted by novel food processing technologies, particularly non-thermal methods (Jan et al., 2017). Non-thermal processing methods, utilizing low operating temperatures and short treatment durations, have demonstrated minimal to no adverse effects on the flavor and essential nutrients of food (Pina-Perez et al., 2016; Birmpa et al., 2013; Rawson et al., 2011). This has led to a surge in interest and research into non-thermal processing approaches (Frewer et al., 2011).
Ultrasound, a non-thermal processing method utilizing high-frequency sound waves, presents a compelling alternative to traditional heat treatment for Kelulut honey. This technique effectively eliminates spoilage-causing yeast, enhances the honey’s appearance, and inhibits granulation (Kabbani et al., 2011). Ultrasound processing offers several advantages over conventional methods, including improved sensory and nutritional value, reduced processing time, and lower operating temperatures.
Ultrasound encompasses a range of frequencies, categorized as power ultrasound (16-100 kHz), high-frequency ultrasound (0.1-1 MHz), and diagnostic ultrasound (1-10 MHz). High-power ultrasound, particularly in the 20-100 kHz range, can induce physical, mechanical, and chemical changes in materials by generating pressure, shear, and temperature gradients within the medium. Cavitation, a phenomenon involving the formation and implosion of bubbles and gas, can occur when using high-power ultrasound or power ultrasound at lower frequencies (20-100 kHz) with high sound intensities (10-1000 W/cm2) (Feng and Yang, 2005). This phenomenon has been successfully employed to modify the functional properties of proteins in various food sources, including dairy, meat, grains, legumes, tubers, and fruits (O’Sullivan et al., 2016). Ultrasonication has emerged as a potential alternative to thermal processing for pasteurization (Mohideena et al., 2015). In the context of honey, ultrasound can dissolve existing crystals, retard crystallization, and maintain the honey in a liquid state for extended periods compared to heat-treated honey (Salazar et al., 2010).
This study aims to investigate the impact of ultrasound processing on the physicochemical properties and 5-HMF levels of Kelulut honey. By understanding the effects of this non-thermal processing method, we can explore its potential for preserving the quality and extending the shelf life of this valuable honey variety.
Materials and Methods
Kelulut honey sample
Freshly collected unprocessed Kelulut honey was purchased from a local beekeeper in Besut, Terengganu, Malaysia. A transparent glass bottle was used to keep the liquid honey and it was stored at room temperature (25 °C) until used for analysis (not more than 3 days).
Thermal processing
Approximately 25 g of unprocessed Kelulut honey was weighed and placed in a 25 ml test tube. Thermal processing was applied by immersing the test tube for 30 minutes in a warm water bath (Memmert model) set at 60°C.
Ultrasound processing
An ultrasonic instrument (Q500 Sonicators by QSonica) was used to treat Kelulut honey during the ultrasound processing. A volume of 30 ml of the unprocessed Kelulut honey was used and the treatment was carried out at a nominal frequency of 20 kHz. The samples were processed for 5, 10, and 15 minutes at amplitudes of 20, 40, 60, 80, and 100%.
Physicochemical analysis
All unprocessed, thermally processed and ultrasonically processed Kelulut honey samples were subjected to physicochemical analysis. Moisture content, water activity, pH, and total soluble solids were among the analyses that were performed. To obtain the average results, all analyses were performed in triplicate.
Total soluble solid
A digital refractometer (ATAGO, Tokyo, Japan) was used to determine the total soluble solid of Kelulut honey. About 0.3 mL of sample was placed on the refractometer’s glass prism and the measurement at 25°C in Brix was obtained.
Water activity
The water activity (Aw) of Kelulut honey was measured using a water activity meter (METER Aqualab 4TE) calibrated with salt solutions. Sample of Kelulut honey weighing around 2 g was placed on the sample plate, and measurements were taken until the results were shown together with an LED flash (Chong et al., 2017).
Moisture content
The moisture content of Kelulut honey was evaluated using the Association of Official Analytical Chemists (AOAC) procedures. In an oven set to 105°C, the crucible was dried for 4 hours with a cover on. The crucible was then cooled in a desiccator and weighed once it reached room temperature (W1). The crucible (W2) was then filled with 3 g of Kelulut honey sample. Then, the honey sample was placed uncovered in an oven at 105°C for around 24 hours. Finally, the sample was desiccated and weighed after reaching room temperature (W3). The percentage of moisture was calculated using the formula in Equation 1.
Where; W1 = Weight of crucible (g); W2 = Weight of crucible + weight of wet sample (g); W3 = Weight of crucible + weight of dried sample (g).
pH
A pH meter basic 20 was used to determine the pH of honey. The buffer solutions of pH 4, pH 7, and pH 10 were used to calibrate the pH meter. 10 grams of honey were dissolved in 75 milliliters of distilled water to create the honey solution. The pH electrode was placed in the solution after the mixture had been homogenised, and the pH value was recorded.
5-hydroxymethylfurfural (HMF)
The HMF was determined using White’s spectrophotometric method (White, 1979). After being diluted in 25 ml of distilled water, 5 g of Kelulut honey was added to a 50 ml volumetric flask, along with 0.5 ml of each two Carrez solutions. The flask was then filled with distilled water to a capacity of 50 ml. The solution was filtered using a filter paper, and the first 10 ml of the filtrate was discarded. In two separate test tubes, 5 ml of distilled water and 5 ml of sodium bisulfite solution (0.2%) were used as the sample and reference solutions, respectively. The absorbance of the sample solution in contrast to the reference solution was measured using 284 and 336 nm within an hour. The formula in Equation 2 was used to calculate the value of HMF:
Where; A284, A336 = absorbance readings.
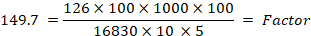
126= molecular weight of HMF; 16830 = molar absorptivity of HMF at 284 nm; 1000 = conversion g into mg; 10 = conversion 5 into 50 mL; 1000 = conversion g of honey into kg; 5 = theoretical nominal sample weight; D= dilution factor, in case dilution is necessary; W = weight in gram of the honey sample.
Stastical analysis
The results of the study were analysed using Analysis of Variance (ANOVA) with Tukey post hoc test. Two-way ANOVA was used to compare the means of the obtained data, and a significant difference was considered at p<0.05. Excel 2010 and SPSS 20 were used for all statistical analysis.
Results and Discussion
Effect of ultrasound processing on the physicochemical properties of Kelulut honey
Total soluble solids: Figure 1 shows the total soluble solids of ultrasonically processed Kelulut honey. It can be observed that the total soluble solids increased when Kelulut honey was processed using ultrasound processing. The lower amplitudes (20 and 40%) used did not significantly change the total soluble solids for all processing times applied. Further increment of amplitude to 60% significantly increased the total soluble solids to 71.52 °Brix and it did not change significantly at higher amplitudes (80 and 100%). For all the amplitudes applied, the total soluble solids increased significantly with the increasing of processing time. The highest total soluble solids were recorded at 100 % amplitude and 15 minutes of processing time. Total soluble solids may increase due to the improved extraction efficiency by the ultrasound applied. The observed increase in total soluble solid can be attributed to the enhanced extraction efficiency facilitated by ultrasound. Zou et al. (2010) suggest that ultrasound treatment can disrupt plant tissues and cell walls, improving the release of soluble compounds. This breakdown allows for greater passage of soluble solids and water across cell membranes, leading to higher TSS values in the treated honey.
Water activity
Figure 2 depicts the water activity of Kelulut honey after processing with ultrasound at various amplitudes and times. Overall, a reduction in water activity of ultrasonically processed Kelulut honey was observed. As the processing time and amplitude were increased, the water activity of the Kelulut honey significantly decreased (p<0.05). It can be deduced that increasing the ultrasound processing time and amplitude could reduce the water activity of Kelulut honey. Among all amplitudes and times of ultrasonic processing, the Kelulut honey processed at 100% amplitude for 15 minutes showed the lowest water activity value (0.7580). This observed reduction in water activity aligns with previous studies on various food products. For instance, Pingret et al. (2011) reported a decrease in water activity for chocolate mousse treated with ultrasound. Similarly, Stojkovic et al. (2020) and Shamaei et al. (2012) found that ultrasound application reduced water activity in honeydew honey and cranberries, respectively.
The mechanism behind this phenomenon is attributed to the sponge effect (De la Fuente-Blanco et al., 2006). Ultrasound-induced cavitation generates microchannels within and outside cells, facilitating water diffusion from the product’s core to its surface. Additionally, as Yao (2016) suggests, ultrasound creates air turbulence at the air-product interface, further promoting moisture removal.
Moisture content
Figure 3 demonstrates the impact of ultrasound processing on the moisture content of Kelulut honey. The results clearly indicate a reduction in moisture content with increasing ultrasound amplitude and processing time. The moisture content of ultrasonically processed Kelulut honey was reduced to a range of 30.33% to 25.40%. Initially, the increase of amplitude to 40% did not result in a significant change in the moisture content of Kelulut honey. A decrease in the moisture content was only observed when 60% amplitude was used and it remained decreased when higher amplitudes were applied. The lowest moisture content was recorded at 100% amplitude for 15 minutes. According to Bachirbey et al. (2017), when the crystallized honey was treated with ultrasound, the moisture content dropped from 30.32% (fresh honey) to 25.40%. Similarly, Stojkovic et al. (2020) reported a decrease in the moisture content of honeydew honey after being processed with ultrasound. The collapse of cavitation microbubbles generates shear forces within the honey, promoting mass transfer and facilitating the release of tightly bound (Scripcă and Amariei, 2021). This method would remove the tightly bound moisture in honey, resulting in evaporation (Shamaei et al., 2012). Furthermore, ultrasound treatment, particularly at higher amplitudes, increases thermal energy within the honey, leading to enhanced evaporation of water (Bachirbey et al., 2017).
pH
Figure 4 depicts the pH values of ultrasonically processed Kelulut honey. It shows that the pH values of Kelulut honey increased after the processing. The pH of Kelulut honey increased as the ultrasonic processing time and amplitude were increased. Furthermore, the amplitude was the most significant parameter (p<0.05) in raising the pH value. In particular, Kelulut honey processed at 100% amplitude had the highest pH in comparison to other amplitudes applied. Meanwhile, 15 minutes of processing time resulted in the highest pH increment among the processing times. This result was in contrast with Chaikham et al. (2016), who discovered that increase the amplitude process led to a decreasing in the pH value of lychee blossom and wildflower honey. This variation in how ultrasound affects pH could be due to differences in honey composition or specific processing parameters. Interestingly, a study on coconut milk (Sun et al., 2022) found that ultrasound significantly impacted how pH adjustments affected the milk’s properties. While honey and coconut milk are vastly different, this finding suggests that ultrasound might not directly increase Kelulut honey’s pH but rather interact with its components in a way that leads to a pH shift. For example, ultrasound could be influencing enzymes or amino acids present in the honey, indirectly impacting the overall pH.
Effect of ultrasound processing on the 5- hydroxymethylfurfural (HMF) content of Kelulut honey
Figure 5 shows the HMF content of Kelulut honey that has been subjected to a range of ultrasonic amplitudes and processing times. Based on the figure, the HMF concentration appears to be steadily increasing as the processing time and amplitude increased. The HMF value increased significantly (p<0.05) when the amplitude was increased indicating that high exposure to the ultrasonic waves could generate more heat, leading the HMF to rise substantially. The uneven oscillation of honey molecules during sonic cavitation causes the heat to rise. The bubbles in the honey started to develop at this point and they will continue to grow, oscillate, and finally asymmetrically implode with a lot of intensity (Peshkovsky and Peshkovsky, 2008). However, there is no significant difference between the processing time. This might be due to the processing time used in this study did not increase the temperature that was high enough to speed up the HMF formation. This also indicates that using the ultrasound processing time between 5 minutes to 15 minutes was safe. Furthermore, the results found that the Kelulut honey subjected to 100% amplitude for 15 minutes had the highest HMF content (34.44 mg/kg). This shows that the higher amplitude and time cause the temperature to increase and would increase the concentration of HMF. This conclusion was consistent with the findings of Mahmoud and Owayss (2008), who showed that HMF content increased when exposed to higher temperatures for extended periods of time.
Comparison of fresh, ultrasonically and conventionally processed of Kelulut honey
The physicochemical characteristics and HMF value of fresh, ultrasonically (20%, 5 minutes) and conventionally processed (60°C, 30 minutes) Kelulut honey were compared in Table 1. The process conditions of ultrasounds at 20% amplitude and 5 minutes were chosen as optimum conditions since it does not drastically increase the value of HMF. The HMF value was considered one of the quality indicators for honey. Kelulut honey had substantial changes in moisture content, water activity, pH, total soluble solids, and HMF value after being treated by thermal and non-thermal processing. Kelulut honey that was heated conventionally had a lower moisture content, water activity, and pH compared to ultrasonic processing. Meanwhile, ultrasonic processing of Kelulut honey recorded lower HMF and total soluble solids values than conventional heating.
The moisture content, water activity, pH, total soluble solids, and HMF value of Kelulut honey differed significantly between the two treatments applied. There was a significant difference in moisture content when Kelulut honey was treated with ultrasound and conventional heating. The results showed that
Table 1: Comparison of physicochemical characteristics and HMF value of fresh, ultrasonically and conventionally processed Kelulut honey.
|
Parameters |
Fresh Kelulut honey |
Ultrasonically processed |
Conventionally processed |
|
33.08 ±0.03 a |
30.33 ±1.23 b |
25.11±0.22 c |
|
|
Water activity (aw) |
0.8188±0.0001a |
0.7682±0.002 b |
0.5547±0.005 c |
|
pH |
2.97±0.005 a |
3.38±0.005 b |
3.08±0.010 c |
|
Total soluble solids (°Brix) |
65.32±0.03 a |
68.07±1.23 b |
73.29±0.21 c |
|
HMF (mg/kg) |
14.33±1.40 a |
15.37±5.60 a |
36.68±1.47 b |
Means with different letters within the same row represent significant difference.
moisture content decreased the least at conventional heating (25.11%) compared to ultrasound (30.33%). This showed that the heat supplied during conventional heating was more effective in reducing the moisture content of Kelulut honey. The surface of Kelulut honey went through an evaporation process as heat was applied during the conventional heating, which then decreased the moisture content. As the rate of evaporation increased, a longer conventional heating time was able to dramatically lower the moisture content. The time of the heating process also had a greater impact than the temperature, according to Subramaniam et al. (2007).
Water activity decreased significantly from 0.8188 (fresh honey) to 0.7682 when treated with ultrasound and 0.5547 when treated with conventional heating. This indicated that conventional heating reduces more water activity in Kelulut honey compared to ultrasound. The water activity in honey may be decreased by prolonged heating. According to these findings, conventional heating may reduce water activity in Kelulut honey by causing it to become supersaturated. This finding is consistent with the findings of Shafiq et al. (2014), who discovered that heating honey reduces its water activity.
In addition, both processing methods increased the pH value from the fresh sample (2.97). For the pH value, ultrasound recorded the highest pH value (3.38) compared to conventional heating (3.08). This finding was in contrast with Annapoorani et al. (2010) which stated that the pH of honey heated at 140 °C increased compared to the unheated and 60 °C heated samples. The conventional heating of Kelulut honey resulted in a lower pH value, which may be related to the production of organic acids from pollen during heating which also has an impact on the honey’s pH (Chaikham and Apichartsrangkoon, 2012).
The total soluble solids of conventionally heating kelulut honey were higher compared to ultrasound processing. This could be due to water evaporation, which increases honey concentration to some extent during heat processing.
The HMF value of Kelulut honey using conventional heating increased dramatically from 14.33 mg/kg to 36.68 mg/kg, which was greater than the HMF value of ultrasonically-processed Kelulut honey (15.37 mg/kg). Conventional heating had a major impact on the HMF value of Kelulut honey since it involved heat during processing. This shows that high temperatures and longer processing time increase the HMF content of Kelulut honey. These results are consistent with previous studies that discovered an increase in HMF concentration in honey, which signifies poor storage conditions and excessive heating (Fallico et al., 2004; Khalil et al., 2010). According to Martins and Van Boekel (2005) and da Silva et al. (2016), the HMF concentration is widely used as an indicator of overheating because it is known to increase quickly as the heating temperature rises. This is due to the sugar’s tendency to decompose when heat is applied to honey. Furthermore, prolonged heating time may result in a rise in HMF concentration. According to Ribeiro et al. (2012), the time variable is the most important element in increasing HMF concentration. This is consistent with the findings of this investigation, which showed an increase in HMF content as heating duration was increased. Additionally, a study by Mohd Baroyi et al. (2022) found that the absence of HMF in high-pressure processed Kelulut honey, which does not involve any heating mechanism, supports the findings of this study.
Conclusions and Recommendations
The use of ultrasound processing as a novel method for Kelulut honey affected its physicochemical quality. Furthermore, the changes in HMF caused by ultrasound processing were minimal compared to conventional heating. In conclusion, ultrasound can be a substitute method for conventional heating to fulfil consumers’ demand to have a minimally processed and fresh-like quality of Kelulut honey.
Acknowledgments
The authors like to thank the Ministry of Higher Education Malaysia (MOHE) for the research support (FRGS/1/2018/TK02/UNISZA/03/2) and the Faculty of Bioresources and Food Industry for the research facilities rendered.
Novelty Statement
The current study is novel as the application of ultrasound processing to Kelulut honey has not yet been investigated. Most of Kelulut honey producers apply conventional heating to liquid honey prior to packaging.
Author’s Contribution
Nor Adila Zulkifli: Conducted the experiment and drafted the manuscript.
Nurul Zaizuliana Rois Anwar: Supervised this study and principal investigator of the research grant.
Zalilawati Mat Rashid: Co-supervised this study.
Zarinah Zakaria: Helped in data analysis.
Norshazila Shahidan: Contributed the ideas to design and improve the experiments.
Lee-Hoon Ho: Contributed the ideas to design and improve the experiments.
Faridah Yahya: Proof-read the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Bachirbey, M., S. Dris, K. Bourihane, L. Chenni, S. Ouchemoukh, B. Yacine and L. Hayette. 2017. Deployment of response surface methodology to optimize liquefaction of crystallized honey by ultrasound. Acad. J. Sci. Res., 5(9): 403-411.
Annapoorani, A., K.R. Anilakumar, F. Khanum, N.A. Murthy and A.S. Bawa. 2010. Studies on the physicochemical characteristics of heated honey, honey mixed with ghee and their food consumption pattern by rats. Int. Quart. J. Res. Ayurveda, 31(2): 141. https://doi.org/10.4103/0974-8520.72363
Bakhiya, N., B. Monien, H. Frank, A. Seidel, G. Albrecht, and J. Hansruedi. 2009. Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the Maillard product 5-hydroxymethylfurfural. Biochem. Pharmacol., 78(4): 414-419. https://doi.org/10.1016/j.bcp.2009.04.017
Birmpa, A., V. Sfika and A. Vantarakis. 2013. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready to eat foods. Int. J. Food Microbiol., 167(1): 96–102. https://doi.org/10.1016/j.ijfoodmicro.2013.06.005
Bogdanov, S., T. Jurendic, R. Sieber and P. Gallmann. 2008. Honey for nutrition and health: A review. J. Am. Coll. Nutr., 27(6): 677-689. https://doi.org/10.1080/07315724.2008.10719745
Capuano, E. and V. Fogliano. 2011. Acrylamide and 5-hydroxymethylfurfural: A review on metabolism, toxicity, occurrence in food, and mitigation strategies. LWT Food Sci. Technol., 44(4): 793–810. https://doi.org/10.1016/j.lwt.2010.11.002
Chaikham, P., V. Kemsawasd and A. Apichartsrangkoon. 2016. Effects of conventional and ultrasound treatments on physicochemical properties and antioxidant capacity of floral honeys from Northern Thailand. Food Biosci., 15: 19–26. https://doi.org/10.1016/j.fbio.2016.04.002
Chaikham, P. and A. Apichartsrangkoon. 2012. Comparison of dynamic viscoelastic and physico-chemical properties of pressurised and pasteurised longan juices with xanthan addition. Food Chemistry., 134: 2194–2200. https://doi.org/10.1016/j.foodchem.2012.04.056
Chong, K.Y., N.L. Chin and Y.A. Yusof. 2017. Thermosonication and optimization of stingless bee honey processing. Food Science and Technology International, 23(7): 608–622. https://doi.org/10.1177/1082013217713331
Chua, L.S. and N.A. Adnan. 2014. Biochemical and nutritional components of selected honey samples. Acta Sci. Polon. Technol. Alimentaria, 13(2): 169–179. https://doi.org/10.17306/J.AFS.2014.2.6
Codex Alimentarius Commission, 2001. Revised codex standard for honey. Standards and standard methods. Rome, Italy: WHO and FAO.
Costa, L.C.V., E. Kaspchak, M.B. Queiroz, M.M. Almeida and L.B. Quast. 2015. Influence of temperature and homogenization on honey crystallization. Brazilian Journal of Food Technology, 18(2), 155-161. https://doi.org/10.1590/1981-6723.7314
Cozmuta, A.M., L.M. Cozmuta, C. Varga, M. Marian and A. Peter. 2011. Effect of thermal pro-cessing on quality of polyfloral honey. Romanian J. Food Sci., 1(1): 45-52. https://doi.org/10.1007/s13197-018-3341-5
Da Silva, P. M., C. Gauche, L. V. Gonzaga, A. C. O. Costa and R. Fett. 2016. Honey: Chemical composition, stability and authenticity. Food Chem., 196: 309–323. https://doi.org/10.1016/j.foodchem.2015.09.051
De la Fuente-Blanco, S., E. Riera-Franco de Sarabia, V.M. Acosta-Aparicio, A. Blanco-Blanco and J.A. Gallego-Juárez. 2006. Food drying process by power ultrasound. Ultrasonics, 44: e523–e527. https://doi.org/10.1016/j.ultras.2006.05.181
European Commission, 2001. Council directive 2001/110 relating to honey. Off. J. Eur. Commun.,
Fallico, B., M. Zappala, E. Arena and A. Verzea. 2004. Effect of conditioning on HMF content in Unifloral honeys. Food Chem., 85: 305-313. https://doi.org/10.1016/j.foodchem.2003.07.010
Fauzi, N.A. and M.M. Farid. 2015. High-pressure processing of Manuka honey: Brown pigment formation, improvement of antibacterial activity and hydroxymethylfurfural content. Int. J. Food Sci. Technol., 50(1): 178–185. https://doi.org/10.1111/ijfs.12630
Feng, H. and W. Yang. 2005. Power ultrasound. In: Hui Y.H. (ed.), Handbook Food Science, Technology and Engineering. New York: Marcel Dekker.
Frewer, L., K. Bergmann and M. Brennan. 2011. Consumer response to novel agrifood technologies: Implications for predicting consumer acceptance of emerging food technologies. Trends Food Sci. Technol., 22(8): 442–456. https://doi.org/10.1016/j.tifs.2011.05.005
Jan, A., M. Sood, S. Sofi and T. Norzom. 2017. Non-thermal processing in food applications: A review. Int. J. Food Sci. Nutr., 2(6): 171-180.
Kabbani, D., F. Sepulcre and J. Wedekind. 2011. Ultrasound-assisted liquefaction of rosemary honey: Influence on rheology and crystal content. J. Food Eng., 107(2): 173–178. https://doi.org/10.1016/j.jfoodeng.2011.06.027
Khalil, M.I., S.A. Sulaiman and S.H. Gan. 2010. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol., 48(8-9): 2388–2392. https://doi.org/10.1016/j.fct.2010.05.076
Kowalski, S., M. Lukasiewicz, A. Duda-Chodak and G. Zięć. 2013. 5-Hydroxymethyl-2-furfural (HMF) – Heat-induced formation, occurrence in food, and biotransformation: A review. Polish J. Food Nutr. Sci., 63(4): 207-225. https://doi.org/10.2478/v10222-012-0082-4
Mahmoud, A.A. and A.A. Owayss. 2008. Influence of heat treatment on formation of hydroxymethylfurfural and hydrogen peroxide as heating indicators of honey. Fayoum J. Agric. Res. Dev., 22(2): 155-164. https://doi.org/10.21608/fjard.2008.197494
Martins, S.I.F.S. and M.A.J.S. Van Boekel. 2005. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem., 90(1-2): 257–269. https://doi.org/10.1016/j.foodchem.2004.04.006
Mohd Baroyi, S.A.H., Y.A. Yusof, N.L. Chin, S.H. Othman and N.S.M. Ghazali. 2022. A comparative study of high-pressure processing and microwave pasteurization on the formation of hydroxymethylfurfural in stingless bee (Heterotrigona itama) honey. Longhua Chinese Med., 5: 22. https://doi.org/10.21037/lcm-22-13
Mohideena, F.W., K.M. Solvalb, J. Lia, J. Zhanga, A. Chouljenkoa, A. Chotikoa and S. Sathivela. 2015. Effect of continuous ultra-sonication on microbial counts and physico-chemical properties of blueberry (Vaccinium corymbosum) juice. LWT Food Sci. Technol., 60(1): 563-570. https://doi.org/10.1016/j.lwt.2014.07.047.
Nozal, M.J., J.L. Bernal, L. Toribio, J.J. Jimenez and M.T. Martin. 2001. High-performance liquid chromatographic determination of methyl anthranilate, hydroxymethylfurfural, and related compounds in honey. J. Chromatogr., 917(1-2): 95-103. https://doi.org/10.1016/S0021-9673(01)00702-6
O’Sullivan, J.J., M. Park, J. Beevers, R.W. Greenwood and I.T. Norton. 2016. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: A review. Food Hydrocoll., 57: 337-347.
Peshkovsky, S.L. and A.S. Peshkovsky. 2008. Shock-wave model of acoustic cavitation. Ultrason. Sonochem., 15(4): 618-628. https://doi.org/10.1016/j.ultsonch.2007.07.006
Pina-Pérez, M.C., D. Rodrigo and A. Martínez. 2016. Nonthermal inactivation of Cronobacter sakazakii in infant formula milk: A review. Crit. Rev. Food Sci. Nutr., 56(10): 1620-1629. https://doi.org/10.1080/10408398.2013.781991
Pingret, D., A.S. Fabiano-Tixier, E. Petitcolas, J.P. Canselier and F. Chemat. 2011. First investigation on ultrasound-assisted preparation of food products: Sensory and physicochemical characteristics. J. Food Sci., 76(2): C287–C292. https://doi.org/10.1111/j.1750-3841.2010.02019.x.
Ramly, N.S., I.S.R. Sujanto, A.A. Ghani, Y.H.J. Tang, N. Alias, M. Salmah, A.J. Zakaria and N. Ngah. 2021. Physicochemical properties and mineral elements in honey from various species of Malaysian stingless bee. Biosci. Res., 18(SI-2): 34–44.
Rawson, A., A. Patras, B. Tiwari, F. Noci, T. Koutchma and N. Brunton. 2011. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: A review of recent advances. Food Res. Int., 44(7): 1875–1887. https://doi.org/10.1016/j.foodres.2011.02.053
Razali, M., Z.A. Zainal, M. Maulidiani, K. Shaari, Z. Zamri, M.Z.M. Idrus, A. Khatib, F. Abas, Y.S. Ling, L.L. Rui and I.S. Ismail. 2018. Classification of raw stingless bee honeys by bee species origins using the NMR- and LC-MS-based metabolomics approach. Molecules, 23(9): 2160. https://doi.org/10.3390/molecules23092160
Ribeiro, R.O.R., S. Carneiro, C.E.T. Mársico, F.L. Cunha, C.A.C. Junior and S.B. Mano. 2012. Influence of the time/temperature binomial on the hydroxymethylfurfural content of floral honeys subjected to heat treatment. Ciência e Agrotecnol., 36(2): 204–209. https://doi.org/10.1590/S1413-70542012000200009
Salazar, J., J.A. Chavez, A. Turo and M.J. Garcia-Hernandez. 2010. Effect of ultrasound on food processing. In: J. Ahmed, H.S.S. Ramaswamy, Kasapis, and J.I. Boye (Eds.), Novel Food Processing: Effects on Rheological and Functional Properties. CRC Press. pp. 65-80
Scripcă, L.A. and S. Amariei. 2021. The use of ultrasound for preventing honey crystallization. Foods, 10(4): 773. https://doi.org/10.3390/foods10040773
Shafiq, H., F. Iftikhar, A. Ahmad, M. Kaleem and A.T. Sair. 2012. Effect of crystallization on the water activity of honey. Int. J. Food Nutr. Sci., 3(3): 1-6.
Shamaei, S., Z. Emam-Djomeh and S. Moini. 2012. Ultrasound-assisted osmotic dehydration of cranberries: Effect of finish drying methods and ultrasonic frequency on textural properties. J. Texture Stud., 43(2): 114-122. https://doi.org/10.1111/j.1745-4603.2011.00323.x
Stojković, M., D. Cvetković, A. Savić, L. Topalić-Trivunović, A. Velemir, S. Papuga and M. Žabić. 2021. Changes in the physicochemical, antioxidant, and antibacterial properties of honeydew honey subjected to heat and ultrasound pretreatments. J. Food Sci. Technol., 58(8): 2555–2566. https://doi.org/10.1007/s13197-020-04762-2
Subramaniam, R., H.U. Hebbar and N.K. Rastogi. 2007. Processing of honey: A review. Int. J. Food Prop., 10(1): 127-143. https://doi.org/10.1080/10942910600981708
Sujanto, I.S.R., N.S. Ramly, J.Y.H. Tang, A.A. Ghani, N. Alias, S. Mohamed and N. Ngah. 2022. Influence of spatial variation on the physicochemical properties and mineral content of stingless bee honey (Heterotrigona itama) in Terengganu, Malaysia. Asian J. Agric. Biol., 10(2): 1-11.
Sun, Y., H. Chen, W. Chen, Q. Zhong, Y. Shen and M. Zhang. 2022. Effect of ultrasound on pH-shift to improve thermal stability of coconut milk by modifying physicochemical properties of coconut milk protein. LWT Food Sci. Technol., 164: 113861. https://doi.org/10.1016/j.lwt.2022.113861
Tosi, E.A., E. Ré, H. Lucero and L. Bulacio. 2004. Effect of honey high temperature short time heating on parameters related to quality, crystallization phenomena, and fungal inhibition. Food Chem., 37(6): 669-678. https://doi.org/10.1016/j.lwt.2004.02.005
White, J.W., 1979. Spectrophotometric method for hydroxymethylfurfural in honey. J. Assoc. Off. Anal. Chem., 62(3): 509–514. https://doi.org/10.1093/jaoac/62.3.509
Yao, Y., 2016. Enhancement of mass transfer by ultrasound: Application to adsorbent regeneration and food drying/dehydration. Ultrason. Sonochem., 31: 512–531. https://doi.org/10.1016/j.ultsonch.2016.01.039
Zhao, J., X. Du, N. Cheng, L. Chen, X. Xue, J. Zhao, L. Wu and W. Cao. 2016. Identification of monofloral honeys using HPLC-ECD and chemometrics. Food Chem., 194: 167–174. https://doi.org/10.1016/j.foodchem.2015.08.010
Zou, Y., C. Xie, G. Fan, Z. Gu and Y. Han. 2010. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol., 11(4): 611-615. https://doi.org/10.1016/j.ifset.2010.07.002
To share on other social networks, click on any share button. What are these?