Physical Stimulations of Electrical Shock and High Light Intensity on the Growth Performance and Yield of Grey Oyster Mushroom (Pleurotus sajor-caju)
Physical Stimulations of Electrical Shock and High Light Intensity on the Growth Performance and Yield of Grey Oyster Mushroom (Pleurotus sajor-caju)
Roshita Ibrahim1*, Anis Nursyazwani Aminuddin1 and Mohd Nizam Lani2
1Faculty of Chemical Engineering and Technology, Universiti Malaysia Perlis, Uniciti Alam Campus, Sg. Chuchuh 02100 Padang Besar, Perlis, Malaysia; 2Faculty of Fisheries and Food Science, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia.
Abstract | High demand, low-cost production, and vigorous mycelium growth of grey oyster mushroom (Pleurotus sajor-caju) make it popular among small farmers to commercially cultivate it in Malaysia. This study explored the impact of different physical stimulations on the growth and yield of grey oyster mushrooms. The treatments included individual applications of electrical shock (12V; 13A) and high light intensity (1500 Lux) which were studied previously, as well as combined treatments of electrical shock with high light intensity (ES-HLI) and alternating treatments of these two stimuli (alternate ES-HLI). All the physically stimulated mushroom bags took a shorter spawning period (25-30 days) compared to the control (37 days). Electrical shock and combined ES-HLI treated bags had a faster mycelium growth rate of around 0.94 - 0.96 cm/day whereas control treatment had only 0.62 cm/day. The duration for the first mushroom harvesting was led by treatment of combined ES-HLI with 30.4 days. Electrical shock has also resulted in the highest yield and percentage biological efficiency of 378.5 g and 75.7% respectively. The average pileus diameter in control was only 6.16 cm, however, high light intensity treatment produced a larger size of mushroom with an average pileus diameter of 10.09 cm and had the lightest colour compared to other treatments. In conclusion, the application of electrical shock stimulation emerged as the most effective treatment among the various physical stimulants investigated in this study, attributed to its shorter spawning time and the highest yield of mushrooms produced.
Received | February 22, 2024; Accepted | September 04, 2024; Published | October 09, 2024
*Correspondence | Roshita Ibrahim, Faculty of Chemical Engineering and Technology, Universiti Malaysia Perlis, Uniciti Alam Campus, Sg. Chuchuh 02100 Padang Besar, Perlis, Malaysia; Email: [email protected]
Citation | Ibrahim, R., A.N. Aminuddin and M.N. Lani. 2024. Physical stimulations of electrical shock and high light intensity on the growth performance and yield of grey oyster mushroom (Pleurotus sajor-caju). Sarhad Journal of Agriculture, 40(Special issue 1): 81-88.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.81.88
Keywords | Grey oyster mushroom (Pleurotus sajor-caju), Physical stimulants, Electrical shock, High light intensity, Biological efficiency
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Mushrooms from the Pleurotus genus, like grey oyster mushrooms (Pleurotus sajor-caju), are getting attention in commercial cultivation due to their adaptability and diverse benefits. They are gastronomically, nutritionally, and medicinally valuable, making them suitable for large-scale cultivation on various substrates (Torres-Martínez et al., 2022; Zmitrovich and Wasser, 2016). Medicinally, these mushrooms are recommended for obese and diabetic individuals due to their low-calorie, low-sugar, and starch-free nature. Oyster mushrooms offer decent protein, high dietary fiber, amino acids, minerals, and vitamins. They contain 1.6 to 2.5% protein and niacin levels ten times higher than other vegetables, helping lower cholesterol and triglycerides in the blood (Ayimbila and Keawsompong, 2023; Naeem et al., 2020).
Oyster mushrooms grow well in favorable conditions and require strict hygiene practices. They thrive at temperatures between 20 to 30°C and with humidity levels of 80-90%, typically on agricultural waste substrates like kernel and sawdust (Tesfaw, 2015). In developing and underdeveloped countries, disposing of large amounts of agricultural waste poses environmental challenges. The resistance to degradation in these waste materials is mainly due to the presence of lignin. However, mushrooms, being efficient fungi, are effective in breaking down and metabolizing these residues (Kumar and Chandra, 2020).
The life cycle of a mushroom comprises two distinct phases: Vegetative and reproductive growth. During the vegetative growth phase, fungal mycelia extend linearly, breaking down complex substrate components into simpler molecules and absorbing them as nutrients. This phase typically lasts between 30 to 90 days, depending on the quality of the spawn and environmental conditions. When the environment provides low temperatures, high humidity, ample oxygen, and occasionally light, the mycelia shift from vegetative growth to reproductive growth. In this phase, they form a fruiting body, or mature mushroom, which takes several days to develop. Mushroom cultivation involves the artificial repetition of these two growth stages to produce the fruiting body (Riquelme et al, 2018).
Under typical conditions, it takes at least a month for the mycelium to fully grow in a standard 18-centimeter mushroom bag. The first mushroom harvest takes even longer, delaying income from sales. Mushroom cultivation is risky, requiring a clean house far from contaminants, as bags are easily affected. Contamination can spread, causing the entire mushroom house to be compromised. Addressing contamination is expensive and time-consuming, leading to temporary halts in production and financial losses.
Despite these challenges, the inconsistent mushroom supply has prompted research to find solutions for improved productivity. Hence, given this situation, there is a need for studies to address the current challenge. The application of suitable physical stimulants is believed to boost the mycelium growth rate. Growers could use these stimulants to expedite mycelium growth, reducing the production time and ultimately increasing mushroom yields. Some stimulations require multiple treatments applied in combination or alternating ways. The applications of these electrical shock and high light intensity stimulants were cost-effective as they could shorten the mushroom growth period, and the stimuli were very easy and simple to apply resulting in a higher yield of mushrooms (Ibrahim, 2023; Ibrahim et al., 2020).
Physical stimulants like sound, light, electrical shock, and cold shock have been linked to enhancing the growth and yield of mushrooms and other living organisms (Ibrahim et al., 2015). Studies have highlighted the positive effects of electrical shock and high light intensity on mushrooms, with instances of increased mushroom growth observed around lightning-hit areas (Ryall, 2010; Tsukamoto et al., 2005). The mechanism behind the mushroom outbreak is not entirely clear, but researchers propose two possibilities. One is the creation of cracks in mycelium hyphae caused by lightning, as mushroom fruit bodies develop from these cracks. The other involves the activation of enzymes; some studies show that applying a pulse electric field activates enzymes, leading to the active development of mushroom fruit bodies (Ibrahim et al., 2020; Atri et al., 2012; Tsukamoto et al., 2005).
The most effective physical method to boost vegetable production involves employing various physical factors or plant treatments, particularly focusing on dill seed enhancement through high light intensity and electro-culture treatment (Sharma et al., 2019). The term electro-culture has historical roots, where electrical treatments, such as applying an electric charge to the soil, were utilized in prehistoric times to increase crop growth and yield (Barman and Bhattacharya, 2016). It has been observed that plants’ roots respond to the electric pulse treatment by morphologically measured parameters, indicating an unknown signal generated by the electric pulse (Yi, 2012).
Light plays a crucial role in determining the yield and morphological characteristics of grey oyster mushrooms. It is involved in phototropic responses and the formation of reproductive structures, influencing the expansion of the pileus and playing a significant role in spore dissemination (Sharma et al., 2015). Studies by Miyazaki et al. (2011) and Ibrahim et al. (2015) have confirmed that the development of fruiting bodies, including stipe elongation and cap formation, is induced by light in standard mushroom cultivations. Light also plays a crucial role in the colour of mushrooms. Low light intensity or absence of light can result in pale, deformed mushrooms with elongated stalks and reduced cap color. Some mushrooms, like those of the Pleurotus species, require light for primordial formation (Abdullah et al., 2023).
The primary objective of this study is to explore the effects of particular physical stimulants, specifically high light intensity and electrical shock treatments, on the growth morphology, yield enhancement, and quality of grey oyster mushrooms (P. sajor-caju).
Materials and Methods
Mushroom bag preparation
The experimental phase of this study was carried out at the Food Microbiology Laboratory and Institute of Sustainable Agrotechnology, Universiti Malaysia Perlis. A Mushroom substrate comprising sawdust, rice bran, and calcium carbonate (CaCO3) in a proportion of 100:10:1 was mixed. This mixture was then filled into substrate bags, standardized at a length of 18 cm and diameter of 12 cm. The bags underwent sterilization using an autoclave at 121°C and 15 psi for 30 minutes. Following sterilization, the bags were left to cool overnight before the inoculation process. About 10 g of mushroom spawn was put/inoculated into each mushroom bag. After inoculation, all bags were placed in a dark spawning room with approximately 10% light exposure (successfully done by using 90% shade of black netting). To expedite mycelium growth, the mushroom bags were arranged vertically. The incubation temperature was maintained at around 25-30 ℃.
Physical stimulation treatments
Different physical stimulants were applied to the mushroom bags at five-day intervals. These actions were repeated until the fifth harvest. For electrical shock treatment, the mushroom bags were placed horizontally on a table. A wire was planted in the substrate from the mouth to the bottom of the mushroom bag. A voltage adapter that supplied 12V of electrical shock was used to run through the bag and held for 10 seconds. Before the wire was planted, 70% ethanol solution was sprayed on the wire and let dry as an aseptic technique to prevent contamination. After passing through the electrical current for 10 seconds, the wire was pulled out and adhesive tape was used to cover the hole caused by the wire planted earlier. The same procedures were carried out on each bag in the dark with at least 10% light exposure.
For high-light intensity treatment, the environment surrounding the mushroom bags was controlled to not exceed 3 lux. A 10-watt spotlight was set up and the distance between the spotlight and the light intensity of 1500 lux was measured using a Lux meter and marked. Mushroom bags were arranged vertically on the 1500 lux mark and were subjected to 10 pulses/blinkings of the bright light and each time the bright light was held for 2 seconds. The procedures were repeated on the other side of mushroom bags where the bags were turned to face the spotlight.
Combined treatments of ES-HLI were done by applying both electrical shock and high light intensity treatments on the same day at every 5-day interval. Whilst alternate treatment of ES-HLI was applied alternately every 5 days (i.e. on day 5 electrical shock treatment, on day 10 high light intensity treatment, on day 15 electrical shock treatment again, and so on).
After the mushroom bags were filled with mycelium, they were moved to a mushroom house, and the caps were opened to facilitate mushroom fruiting. The conditions inside the mushroom house were carefully managed, maintaining a temperature of approximately 25-30°C, humidity levels between 85-90%, and subdued lighting with less than 30% brightness.
Determination of growth performance
During the spawning period, the length of mycelium growth that traveled down the bag was measured and recorded. A ruler was used to measure the distance traveled by mycelium at five-day intervals. The number of days taken for the mycelium to fill up the bag (fully colonized) until the bottom of the bag was recorded for each treatment. Upon complete colonization of the bags by the mycelium, the bag caps were opened, and the duration for pinhead emergence was recorded, followed by the formation of fruiting bodies (indicating when the mushrooms fruiting bodies were fully developed and ready for harvest).
Determination of mushroom yield
The yield of mushrooms in terms of the total weight of fruiting bodies after five harvesting cycles and percentage biological efficiency were calculated. The freshly harvested fruit bodies were weighed and recorded. This method was repeated until the fifth harvesting. The formula below was used to calculate the percentage of biological efficiency of mushrooms (Ahmed et al., 2016).
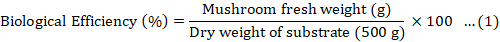
Determination of pileus diameter
The diameter of the pileus of freshly harvested mushrooms was measured using a ruler and recorded. The longest measurement of the mushroom was taken since the mushroom was not completely round.
Determination of pileus colour
The Minolta Chromameter was employed to analyze the pileus color of the harvested mushroom, utilizing the CIELAB color parameters L*, a*, and b* values. This measurement took place immediately after the harvesting process. Three distinct points were selected from the same surface of the harvested mushroom pileus, and the L*, a*, and b* values were determined. The L* value signifies a monochrome scale indicating the level of light reflected, where 100 represents pure white and 0 denotes matte black. The a* value ranges from greenness (-) to redness (+), while the b* value spans from blueness (-) to yellowness (+) (Ibrahim et al., 2017).
Experimental design and statistical analyses
This study utilized completely randomized design (CRD) to examine four different treatments: A control group (without any stimulant), electrical shock (ES), high light intensity (HLI), a combination of ES and HLI, and alternating ES and HLI. The results were analyzed using SAS version 16.0 statistical software. Differences among treatments were assessed with a One-Way Analysis of Variance (ANOVA), and significance was determined at (P < 0.05) using the Duncan Multiple Range Test (DMRT). Each treatment was represented by 5 mushroom bags.
Results and Discussion
Growth performance
Mycelium growth rate: Significant differences (P<0.05) in the mycelium growth rate were observed among different treatments, as indicated in Table 1. ES and combined ES-HLI treatments showed a faster growth rate of 0.962 cm/day and 0.94 cm/day respectively, followed by alternate ES-HLI (0.89 cm/day), HLI (0.78 cm/day) and the slowest was control (0.62 cm/day). Since in this study, the composition and condition of the substrate were standardized, therefore, it is evident that nutritional contents along with some other factors (physical stimulants) influence the differences in mycelium growth observed. Therefore, treatments like electrical shock and high light intensity most probably provoke and cause sufficient stress to the mycelium to shorten the period to fill up the bag as well as increase the growth rate (Ibrahim, 2023).
Table 1: The growth rate of mycelium to fill-up 18 cm length of mushroom bag subjected to different physical stimulant treatments.
|
Treatments |
Growth rate (cm/day) |
|
Control |
0.62±0.017ᵇ |
|
Electric shock (ES) |
0.96±0.240ᵃ |
|
High light intensity (HLI) |
0.78±0.060ᵃᵇ |
|
Combined ES-HLI |
0.94±0.160ᵃ |
|
Alternate ES-HLI |
0.89±0.150ᵃᵇ |
Note: Values represent the means of 5 replicates. Means (n=5) ± standard deviation. a-b: Values with different superscripts within the same column are deemed significantly different at a 5% level of significance (P<0.05).
Mycelium fill-up the bag, pinhead emergence and first harvesting
Referring to Table 2, it is evident that all bags subjected to physical stimulation significantly (P<0.05) demonstrated a shorter duration for mycelium colonization, ranging from 25 to 30 days, in contrast to the control group, which took 37 days. For pinhead emergence, treatment with HLI and combined ES-HLI took a shorter time (2.8-3.2 days), followed by alternate ES-HLI (3.4 days), ES (4.4 days) and the longest was control (10.8 days). Pinhead emergence was initiated by abrupt changes
Table 2: The duration for mycelium to fill up the bag, pinhead emergence, fruiting body formation and total time taken until the first mushroom harvesting subjected to different physical stimulant treatments.
|
Treatments |
Mycelium fill-up the bag (day) |
Pinhead emergence (day) |
Fruiting body formation (day) |
Total time taken until first harvesting (day) |
|
Control |
37.0±1.00ᵃ |
10.8±7.40ᵃ |
3.2±0.45ᵃ |
51.0a |
|
Electric shock (ES) |
25.0±5.39ᵇ |
4.4±2.51ᵃᵇ |
2.8±0.48ᵃ |
32.2b |
|
High light intensity (HLI) |
29.8±2.28ᵇ |
2.8±2.95ᵇ |
3.0±0.71ᵃ |
35.6ab |
|
Combine ES-HLI |
25.0±4.42ᵇ |
3.2±1.48ᵇ |
2.2±0.45ᵃ |
30.4c |
|
Alternate ES-HLI |
26.4±3.85ᵇ |
3.4±2.30ᵃᵇ |
3.0±1.00ᵃ |
32.8b |
Note: Values represent the means of 5 replicates. Means (n=5) ± standard deviation. a-c: Values with different superscripts within the same column are deemed significantly different at a 5% level of significance (P<0.05).
in the environment such as light intensity, exposure to high concentrations of carbon dioxide, increase in humidity and increase in temperature (Mandal and Sadhukhan, 2019). Khan et al. (2013) reported that small pinheads-like were formed 6-7 days after the spawn running and full colonization.
Fruiting body formation is another reproductive stage and final stage during mushroom cultivation (Khan et al., 2013). The time taken for fruiting body formation was recorded by counting from the day of pinhead emergence until the day developed fruiting bodies can be harvested. The results obtained from Table 2 show that there was no significant difference (P>0.05) in the number of days for fruiting body formation in all the treatments which ranged from 2.2 to 3.2 days.
Among all the treatments, the least total time taken for the first harvesting of mushrooms was led by treatment with combined ES-HLI (30.4 days), followed by HLI (35.6 days), ES (32.2 days) and Alternate ES-HLI (32.8 days) whereas control took the longest time of 51 days. Sakamoto (2018) reported that mushroom bags treated with high light intensity positively affect both the aggregation of hyphae and the maturation of the fruiting bodies. In addition, the normal expansion of pileus requires light and the formation of spores requires phototropism (Sakamoto, 2018; Khan and Chandra, 2017). This statement was complemented by the results obtained in this study where treatment involving high light intensity had a shorter period to complete both vegetative and reproductive stages.
Mushroom yield
The mushroom yield can be quantified directly by summing up the fresh weight of mushroom fruiting bodies across five harvesting cycles or indirectly by determining the biological efficiency percentage. Results presented in Table 3 highlighted the significant increase in mushroom yield from electrical shock treatment with a total weight of 375.5 g and 75.7% biological efficiency. The treatment with the second-highest yield was the alternate ES-HLI (319.5 g), succeeded by combined ES-HLI, HLI, and the control. Notably, there was no significant difference (P>0.05) in yields among these three treatments, ranging from 259.5 to 285.0 g, and the corresponding percentages were 51.9-57.0%. In general, treatments that involved electrical shock had relatively higher yields. Electrical shock-related research done by Ibrahim et al. (2020) on mushrooms also displayed a better yield of mushrooms. Similar results were also reported by Takaki et al. (2014) where the fruit body formation of mushrooms increased 1.3–1.9 times as measured by the total weight when using electrical stimulation. The migrating electrical charges through the substrate particles may induce a change in environments and activate specific enzymes in mycelium to produce a higher yield of mushroom fruiting bodies.
Table 3: Yield of grey oyster mushroom in term of total weight and percentage biological efficiency (of 5 harvesting cycles) subjected to different physical stimulant treatments.
|
Treatment |
Total weight (g) |
Biological efficiency (%) |
|
Control |
259.5±31.19ᵇ |
51.9±6.24ᵇ |
|
Electric shock (ES) |
378.5±39.03ᵃ |
75.7±7.81ᵃ |
|
High light intensity (HLI) |
275.5±29.28ᵇ |
55.1±5.86ᵇ |
|
Combine ES-HLI |
285.0±29.32ᵇ |
57.0±5.86ᵇ |
|
Alternate ES-HLI |
319.5±30.59ᵃᵇ |
63.9±612ᵃᵇ |
Note: Values represent the means of 5 replicates. Means (n=5) ± standard deviation. a-b: Values with different superscripts within the same column are deemed significantly different at a 5% level of significance (P<0.05).
According to Ibrahim (2023), The fundamental process of these physical stimulant treatments (in this case electrical shock) involves subjecting the mushroom hyphae to specific conditions (such as intensity, duration, and treatment frequency) to induce stress and create injuries or ruptures. This, in turn, triggers enzyme activity and boosts cell metabolisms for recovery. However, the stimulant treatment must be optimal, as inadequate or excessive treatment may lead to reduced or negative effects.
Pileus diameter
Table 4 shows the average diameter of mushroom pileus from different physical stimulant treatments. The results show that the high light intensity treatment has significantly (P<0.05) the longest diameter indicating the largest pileus of 10.09 cm compared to other treatments. This is followed by combined ES-HLI (9.81 cm), alternate ES-HLI (9.76 cm) and electrical shock (9.02 cm). However, mushrooms from control bags were relatively smaller in size with a mean of only 6.48 cm pileus diameter. Large pileus size may be one of the contributors to higher mushroom yield.
Table 4: The diameter of pileus of grey oyster mushroom subjected to different physical stimulant treatments.
|
Treatment |
Pileus diameter (cm) |
|
Control |
6.16±1.20c |
|
Electric Shock (ES) |
9.02±2.89b |
|
High Light Intensity (HLI) |
10.09±2.44ᵃ |
|
Combine ES-HLI |
9.81±1.95ab |
|
Alternate ES-HLI |
9.76±1.83ab |
Note: Values represent the means of 5 replicates. Means (n=5) ± standard deviation. a-b: Values with different superscripts within the same column are deemed significantly different at a 5% level of significance (P<0.05).
Table 5: The color of P. sajor-caju subjected to different physical stimulant treatments.
|
Treatments |
Colour |
||
|
L* value |
a* value |
b* value |
|
|
Control |
62.35±4.23ᵇ |
4.50±0.46ᵃ |
14.77±0.80ᵃ |
|
Electric shock (ES) |
64.43±4.30ᵇ |
4.21±0.63ᵃ |
15.69±1.86ᵃ |
|
High light intensity (HLI) |
72.63±7.74ᵃ |
2.65±1.28ᵇ |
14.08±1.11ᵃ |
|
Combine ES-HLI |
67.00±8.82ᵃᵇ |
3.49±1.25ᵃᵇ |
14.80±1.65ᵃ |
|
Alternate ES-HLI |
65.72±2.33ᵃᵇ |
3.97±0.28ᵃ |
15.00±0.66ᵃ |
Note: Values represent the means of 9 replicates. Means (n=9) ± standard deviation. a-b: Values with different superscripts within the same column are deemed significantly different at a 5% level of significance (P<0.05).
Pileus color
The color of mushroom pileus subjected to different physical stimulant treatments was compared by using a chromameter as shown in Table 5. There were 3 parameters for color where the color L* value represents the lightness of the pileus color which ranges from 0 (black) to 100 (white). According to the results presented, there are significant differences (P<0.05) in color L* values among different treatments. The lightest pileus color was from high light intensity treatment (72.63) followed by combined ES-HLI, alternate ES-HLI, electrical shock, and control with color L* values of 67.00, 65.72, 64.43 and 62.348 respectively. In this study, mushroom bags subjected to high light intensity were exposed to 1500 lux for a short time. Ballettini et al. (2019) claimed that light intensity influenced the colour of the pileus. Insufficient amounts of light resulted in the pale-colored pileus. However, the results obtained in this study showed the opposite outcome.
Significant distinctions (P<0.05) in color a* values (indicating redness) were noted across all the treatments. Mushrooms from control, ES and alternate ES-HLI treatments had higher (P<0.05) a* values of 4.50, 4.21 and 3.97, respectively compared to other treatments. The a* values obtained were also inversely proportional to their respective L* values for every treatment. On the other hand, for b* values (indicating yellowness), no significant difference (P>0.05) was noted among the various physical stimulant treatments, with values ranging from 14.08 to 15.69. The insignificant values of b* might be due to the physical stimulants applied not having much effect on the color or pigments (yellowness) in the grey oyster mushroom pileus.
Conclusions and Recommendations
In conclusion, the effect of high light intensity and electrical shock treatments has proven to have a positive impact on the growth performance and yield of grey oyster mushrooms (P. sajor-caju). Spawn-running time was shown the shortest when stimulated by ES, alternate ES-HLI and combined ES-HLI treatments. Time taken for pinhead emergence was led by HLI meanwhile combined ES-HLI treatment was the fastest for overall spawning until the formation of fruiting bodies for first harvesting. Among all the treatments in this study, mushroom stimulated with electrical shock seems to be the most effective treatment compared to others due to the highest yield, shorter growth period and comparable postharvest quality. Nevertheless, the treatment of high light intensity appeared to have the largest diameter of pileus with the lightest color whereas the control treatment had the highest color a* value (redness). Mushroom growers can use these physical stimulants to improve the productivity of the mushroom industry. Further research is warranted to explore the optimal conditions, including intensity, duration, and treatment frequency, for each stimulant. Additionally, investigating the synergistic effects of combining these stimulants holds significant potential and merits further exploration.
Acknowledgements
The authors would like to express their gratitude to the financial support provided by the Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2016/WAB01/UNIMAP/03/5) and all the staff in the Faculty of Chemical Engineering and Technology and Institute of Sustainable Agrotechnology (INSAT) of Universiti Malaysia Perlis (UniMAP).
Novelty Statement
This study highlights the use of cost-effective and sustainable physical stimulants, namely electrical shock and high light intensity, to enhance the growth and yield of grey oyster mushrooms. The boosted productivity of mushrooms contributes to improved food security without the need for additional arable land or space.
Author’s Contribution
Roshita Ibrahim: Data curation, conceptualization, methodology, writing, review and editing, visualization.
Anis Nursyazwani Aminuddin: Data curation, methodology, writing,
Mohd Nizam Lani: Conceptualization, review and editing, visualization.
Conflict of interest
All authors declared no conflict of interest.
References
Abdullah, S.N.F., H. Yusoff and K.M. Hyie. 2023. Effects of various led light colours on yield and physical characteristics of white oyster mushrooms. ESTEEM Acad. J., 19: 23-36. https://doi.org/10.24191/esteem.v19iSeptember.22881
Ahmed, M., N. Abdullah and M.M. Nuruddin. 2016. Yield and nutritional composition of oyster mushrooms: An alternative nutritional source for rural people. Sains Malays., 45(11): 1609–1615.
Atri, N.S., S.K. Sharma and A. Gulati. 2012. Study on mycelial growth pattern of five wild Pleurotus species from North-West India. Am. Eurasian J. Sci. Res., 7(1): 12-15. https://doi.org/10.1615/IntJMedMushr.v15.i1.60
Ayimbila, F. and S. Keawsompong. 2023. Nutritional quality and biological application of mushroom protein as a novel protein alternative. Curr. Nutr. Rep., 12(2): 290–307. https://doi.org/10.1007/s13668-023-00468-x
Barman, P., and R. Bhattacharya. 2016. Impact of electric and magnetic field exposure on young plants. A review. Int. J. Curr. Res. Acad. Rev., 4(2): 182–192. https://doi.org/10.20546/ijcrar.2016.402.023
Bellettini, M.B., F.A. Fiorda, H.A. Maieves, G.L. Teixeira, S. Avila, P.S. Hornung, A.M. Junior and R.H. Ribani. 2019. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci., 26: 633–646. https://doi.org/10.1016/j.sjbs.2016.12.005
Ibrahim, R., 2023. Physical stimulations in mushroom cultivation. In: eds. Z. Zakaria and M.A. Nasir, Innovations and technology in oyster mushroom cultivation 1st edn. Universiti Malaysia Perlis Publisher, Malaysia. pp. 41-66.
Ibrahim, R., A.A.I.M. Jamil, S.M.Z. Hasan, A.M. Arshad and Z. Zakaria. 2017. Enhancing growth and yield of grey oyster mushroom (Pleurotus sajor-caju) using different acoustic sound treatments. MATEC Web Conf., 97: 01054. https://doi.org/10.1051/matecconf/20179701054
Ibrahim, R., K.M. Aziz, A. Mat Arshad and S.M.Z.S. Hasan. 2015. Enhancing mushroom production using physical treatments prior to fruiting body formation. Malays. Appl. Biol., 44(1): 69–73.
Ibrahim, R., L.S. Boon, M.N.I.H. Mazidi and N.D. Yaacob. 2020. Effects of electrical shock and blue LED treatments on the growth, yield and quality of grey oyster mushrooms (Pleurotus sajor-caju). IOP Conf. Ser. Mat. Sci. Eng., 932(1): 012003. https://doi.org/10.1088/1757-899X/932/1/012003
Khan, F. and R. Chandra. 2017. Effect of physiochemical factors on fruiting body formation in mushroom. Int. J. Pharm. Pharmac. Sci., 9(10): 33. https://doi.org/10.22159/ijpps.2017v9i10.20086
Khan, M.W., M.A. Ali, N.A. Khan, M.A. Khan, A. Rehman and N. Javed. 2013. Effect of different levels of lime and pH on mycelial growth and production efficiency of oyster mushroom (Pleurotus spp.). Pakistan J. Bot., 45(1): 297–302.
Kumar, A. and R. Chandra. 2020. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon, 6: e03170. https://doi.org/10.1016/j.heliyon.2020.e03170
Mandal, P. and S. Sadhukhan. 2019. Carbon dioxide the green-house gas and mushroom fruiting. Rev. Res., 8(4): 1-7.
Miyazaki, Y., K. Masuno, M. Abe, H. Nishizawa, T. Matsumoto, S. Kunitomo and T. Kamada. 2011. Light-stimulative effects on the cultivation of edible mushroom by using blue LED. In 7th International Conference on Mushroom Biology and Mushroom Product (ICMBP7). Japan: Agriculture, Forestry and Fisheries Research Council (AFFRC). pp. 58–67.
Naeem, M.Y., S. Ugur and S. Rani. 2020. Emerging role of edible mushrooms in food industry and its nutritional and medicinal consequences. Eur. J. Food Sci. Technol., 4(1): 6-23.
Riquelme, M., J. Aguirre, S. Bartnicki-García, G.H. Braus, M. Feldbrügge, U. Fleig, W. Hansberg, A. Herrera-Estrella, J. Kämper, U. Kück, R.R. Mouriño-Pérez, N. Takeshita and R. Fischer. 2018. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev., 82(2): e00068-17. https://doi.org/10.1128/MMBR.00068-17
Ryall, J., 2010. Lightning makes mushrooms multiply. For National Geographic News. Published April 10, 2010.
Sakamoto, Y., 2018. Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol. Rev., 32(4). https://doi.org/10.1016/j.fbr.2018.02.003
Sharma, N., S. Acharya, K. Kumar, N. Singh and O.P. Chaurasia. 2019. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv., 17(4): 364-371. https://doi.org/10.5958/2455-7145.2018.00056.5
Sharma, N.K., A. Rai, P.K. Rai and S. Singh. 2015. Environmental factors affecting edible and medicinal mushroom production. In: Production techniques of tropical mushrooms in India, 1st edn. Nirmal Publishers, New Delhi, India. pp. 231
Takaki, K., K. Yoshida, T. Saito, T. Kusaka, R. Yamaguchi, K. Takahashi and Y. Sakamoto. 2014. Effect of electrical stimulation on fruit body formation in cultivating mushrooms. Microorganisms, 2(1): 58–72. https://doi.org/10.3390/microorganisms2010058
Tesfaw, A., 2015. Optimization of oyster (Pleurotus ostreatus) mushroom cultivation using locally available substrates and materials in Debre Berhan, Ethiopia. J. appl. Biol. Biotechnol., 3(01): 015-020.
Torres-Martínez, B.D.M., R.D. Vargas-Sánchez, G.R. Torrescano-Urrutia, M. Esqueda, J.G. Rodríguez-Carpena, J. Fernández-López, J.A. Perez-Alvarez, and A. Sánchez-Escalante. 2022. Pleurotus genus as a potential ingredient for meat products. Foods, 11(6): 779. https://doi.org/10.3390/foods11060779
Tsukamoto, S., H. Kudoh, S. Oga, K. Yamamoto and H. Akiyama. 2005. Proceeding 15th Pulsed Power Conference. pp. 1437. https://doi.org/10.1109/PPC.2005.300675
Yi, J.Y., 2012. Effects of a low-voltage electric pulse charged to culture soil on plant growth and variations of the bacterial community. Agric. Sci., 3(3): 339–346. https://doi.org/10.4236/as.2012.33038
Zmitrovich, I.V. and S.P. Wasser. 2016. Is widely cultivated Pleurotus sajor-caju, especially in Asia, indeed an independent species? Int. J. Med. Mushr., 18(7): 583–588. https://doi.org/10.1615/IntJMedMushrooms.v18.i7.30
To share on other social networks, click on any share button. What are these?






