Phenotypic Reversion of Somaclonal Variants Derived from Inflorescence of Date Palm (Phoenix dactylifera L.) in the Open Field Trials
Phenotypic Reversion of Somaclonal Variants Derived from Inflorescence of Date Palm (Phoenix dactylifera L.) in the Open Field Trials
Abdul A. Mirani1,2*, Chee H. Teo2, Adel A. Abul-Soad3, Ghulam S. Markhand1, Tahira Jatt1, Ameer A. Mirbahar1,4, Najamuddin Solangi1
1Date Palm Research Institute (DPRI), Shah Abdul Latif University, Khairpur, Pakistan; 2Centre for Research in Biotechnology for Agriculture (CEBAR), University of Malaya, 50603 Kuala Lumpur, Malaysia; 3Horticulture Research Institute, Agricultural Research Center, Cairo, Egypt; 4Department of Botany, Shah Abdul Latif University, Khairpur, Sindh, Pakistan.
Abstract | The likelihood and degree of the reversion of somaclonal variations have not been reported for tissue-cultured date palm (Phoenix dactylifera) plants derived from immature inflorescences. In this study, three to four years old field grown tissue cultured date palm plants of cvs. Kashuwari and Gulistan derived from in vitro subculture 1-10 (block I) and 11-25 (block II) in multiplication stage were screened for type and nature of phenotypic abnormalities. Six phenotypic abnormalities were detected: 1) dwarfism, 2) excessive vegetative growth, 3) production of deformed offshoots and twisted inflorescence, 4) abnormal frond growth, 5) low levels of fruit setting and 6) inflorescence absent. Among the six different variant phenotypes, complete reversion of phenotypic abnormalities for the phenotypes of inflorescence absent, low level of fruit setting, production of deformed offshoots, and abnormal frond growth were observed in open field trial plants during the three years’ study period. An overall percentage of all the variations was higher (64.19%) in block II during the first season as compared to block I (2.42%). This study provides a good indicator for the date palm tissue culture industry where in vitro cultures with subculture number <11 cycles in multiplication stage are needed to obtain higher percentage of true-to-type regenerants.
Received | February 05, 2019; Accepted | April 05, 2019; Published | June 25, 2019
*Correspondence | Abdul A. Mirani, Date Palm Research Institute (DPRI), Shah Abdul Latif University, Khairpur, Pakistan; Email: [email protected]
Citation | Mirani, A.A., C.H. Teo, A.A. Abul-Soad, G.S. Markhand, T. Jatt, A.A. Mirbahar, N. Solangi. 2019. Phenotypic reversion of somaclonal variants derived from inflorescence of date palm (Phoenix dactylifera L.) in the open field trials. Sarhad Journal of Agriculture, 35(3): 719-726.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.719.726
Keywords | Abnormal phenotype, Date palm, Inflorescence, Micropropagation, Somaclonal variations
Introduction
Date palm is an important fruit crop for its high nutritional fruit and useful by-products (Khushk et al., 2009). It is the major food source for human and livestock in arid regions of the world. Its propagation through tissue culture technique has enabled date palm growers to cultivate both native and exotic cultivars at large scale (Abul-Soad and Mahdi, 2010). However, date palm plants produced through this method may exhibit various phenotypic variations in open field depending on type and concentration of plant growth regulator, in vitro subculture cycle number, type of explant and also genotype used. The variations may persist for short time (temporary, epigenetic variations) or remain adhered to the plants forever (permanent, genetic variations) (Faissal et al., 2013).
In vitro multiplication of date palm (Phoenix dactylifera L.) plants using inflorescence or flower explants (Abul-Soad et al., 2016) is the most preferred explant source for commercial purpose over leaf (Sharma et al., 1980), root (Smith, 1975), zygotic embryo (Zaid and Tisserat, 1984) and shoot tip from an offshoot (Abul-Soad et al., 2002). It is time saving and with minimal efforts to produce thousands of plants via somatic embryogenesis process. It takes 1-2 years for entire production as compared to 2-3 years with shoot tip explant from an offshoot (Abul-Soad, 2011). A high concentration of auxin, specially 2,4-dichlorophenoxyacetic acid (2,4-D), is required to induce callus formation from date palm inflorescence via this method (Alkhateeb, 2008). An increase in auxin concentrations has been reported to cause increased cytological and genetical variations not only in the micropropagation of date palm (Saker et al., 2006), but also in a number of other species such as banana, barley and cotton (Bairu et al., 2006; Jin et al., 2008; Ziauddin and Kasha, 1990). These spontaneous variations arise in somatic cells during the in vitro tissue culture process are known as somaclonal variations (Larkin and Scowcroft, 1981), which are generally considered as a drawback in commercial production of the clonal plants.
The likelihood and degree of phenotypic variations in tissue cultured plants increase with the age of initial cultures, or the number of subculture cycle, that maintained on nutrient medium (Bairu et al., 2006). The variation might be generated either from the actively mutating part of cultures that eventually lead to phenotypic variation (Orton, 1984) or from the increase in mutation rate accumulated over each generation and then consecutive accumulation of these mutations in the final cultures (Benzion and Phillips, 1988). Somaclonal variations may be expressed as permanent or temporary phenotypic abnormalities of vegetative and/or reproductive characters in date palm (Supplementary Table 1). The temporary phenotypic abnormalities such as delayed flowering, low level of fruit setting and parthenocarpy that caused by epigenetic changes have been reported to disappears, or reverse to explant phenotype, with increasing age of the plants in the fields (Gurevich et al., 2005).
The phenotypic variations and their recovery behavior from the variations during open field trial have not been reported previously in the date palm plants derived from immature inflorescence explant. The current study focused on the evaluation of 4-5-year-old open field grown tissue cultured plants of two Pakistani date palm cultivars that derived from in vitro cultures with different number of subcultures in multiplication stage. The percentage of various abnormalities during multiplication process and their phenotypic reversion behavior in the open field trial from season 2016 to season 2018 were documented and analyzed to investigate the effect of initial culture age on the occurrence of the somaclonal variations and also recovery behavior of the variations during open trials.
Materials and Methods
Plant material
The date palm tissue cultured plants that produced at Date Palm Research Institute (DPRI) were grown at two fields (i.e. two blocks): 1) Date Palm Tissue Culture experimental field of Shah Abdul Latif University, Khairpur (4-year-old) and 2) Tissue Cultured Date Palm Field at Taluka Salih Pat, District Sukkur, Sindh (3-year-old). The plants that derived from immature inflorescence through direct somatic embryogenesis pathway according to Abul-Soad and Mahdi (2010) are belong to two different Pakistani date palm cultivars (cv.) viz. P. dactylifera cv. Kashuwari (25 years old) and cv. Gulistan (30 years old). Based on the in vitro culture age (subculture number during the in vitro multiplication stage), plants in the block I were regenerated from the in vitro cultures of subculture cycle 1 to 10 and plants in the block II were from the cultures of subculture cycle 11 to 25. The plants were kept in a greenhouse for 2-3 years for adaptation before use for the open field trials. The plants from each cultivar were disease-free and no other physiological disorder symptoms were transferred for the open field trials in 2013 and 2014.
Detection of phenotypic abnormalities
A survey was conducted from season 2016 to season 2018 on 412 date palm tissue cultured plants in both blocks to detect any reported or unreported phenotypic abnormalities (Supplementary Table 1). Dwarf phenotypes in cultivar type were characterized on their smaller size compared to normal tissue cultured plants. The plant with less than 50% the height of the normal plants with the same age in each population was recorded as a dwarf phenotype. Low level of fruit setting was calculated based on the presence of less than four fruit bunches per plant and more than 50% parthenocarpic fruit per plant.
Comparison of phenotypic characters between tissue cultured and mother plants of P. dactylifera cultivars
The economically important bunch and fruit physical characters of 10 randomly selected (5 from each block) tissue cultured (TC) plants were evaluated and compared to their respective mother plants (MP) during 2017 season. The fruit physical characters were documented at four different stages of fruit development i.e. Kimri, Khalal, Rutab and Tamar stage.
Bunch stalk and spikelet length were recorded as an average length of the three bunches per plant and of three spikelets for the three bunches per plant, respectively. Number of spikelets per bunch was calculated as an average total number of spikelets for the three bunch per plant. Retained fruit per spikelet was recorded as an average fruit number per five spikelets for the three bunches per plant. Dropped fruit per spikelet was recorded as an average fruit number per five spikelets for the three bunches per plant.
Average bunch length at khalal stage for three randomly selected bunches per plant was calculated as per following formula:
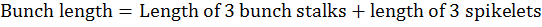
Retained fruit per bunch was calculated as per the following formula:
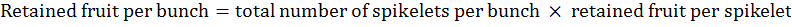
Total number of fruits per spikelet was calculated according to the following formula:
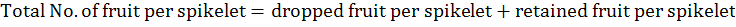
The fruit physical traits, fruit length and diameter were measured with Vernier caliper and recorded as average length and diameter of 10 randomly picked fruits per tree. Fruit and pulp weight (g) were recorded as average weight of 10 randomly picked fruits and pulp per tree, respectively. Pulp/fruit ratio (%) was calculated as per the following formula:
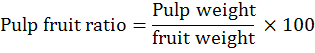
Data analysis
The obtained data was analyzed using three-way (for phenotypic variations) and one-way (for bunch and fruit physical characters) ANOVA in SPSS 20.0 software and the differences among the means were calculated through LSD at <0.05 level of significance (Steel and Torrie, 1980). The percentage of the phenotypic variations were based on the total number of observed plants in each block (block I, n = 27 and block II, n = 385) using Microsoft Excel 2016. The average variation percentage per cultivar and per block was calculated based on the total number of abnormal plants (n = 578) observed in three years trial seasons.
Results and Discussion
Phenotypic abnormalities
The effects of block (B), cultivar (C), year (C) and their interactions i.e. B × C, B × Y, C × Y and B × C × Y on phenotypic variations in field grown tissue cultured date palm plants are presented in Table 1. It can be seen from the ANOVA that except the significant effect of B (P ≤ 0.012) rest of the effects of C (P ≤ 0.494), Y (P ≤ 0.189) and interaction effects of B × C (P ≤ 0.529), B × Y (P ≤ 0.23), C × Y (P ≤ 0.766) as well as the overall interaction effect of B × C × Y (P ≤ 0.744) over phenotypic variations were non-significant.
Table 1: ANOVA of phenotypic variations in P. dactylifera cv. Gulistan and cv. Kashuwari at block I and block II observed during the season 2016, 2017 and 2018.
| Source of variability | Phenotypic variation1 |
| Block (B) |
0.012* |
| Cultivar (C) |
0.494ns |
| Year (Y) |
0.189ns |
| B × C |
0.529ns |
| B × Y |
0.23ns |
| C × Y |
0.766ns |
| B × C × Y |
0.744ns |
| Block (Mean ± SE2) | |
| Block I | 0.67 ± 4.34 |
| Block II | 15.39 ± 4.34 |
| Cultivar (Mean ± SE) | |
| Gulistan | 10.14 ± 4.34 |
| Kashuwari | 5.92 ± 4.34 |
| Year (Mean ± SE) | |
| 2016 | 16.04 ± 5.32 |
| 2017 | 4.58 ± 5.32 |
| 2018 | 3.46 ± 5.32 |
1ns, * nonsignificant or significant at P≤ 0.05; 2Standard errors.
Number of phenotypic abnormalities were documented throughout the study period and are accordance with numerous studies (Supplementary Table 1). The percentage of each variant varied between the two blocks and the two cultivars. In general, the percentage of the overall phenotypic variation in block II was higher (64.19%) than the block I (2.42%) at the end of first season of the open field trial in year 2016 (Figure 1). Figure 1 shows a significant reduction in the overall phenotypic variation for plants derived from block II cultures from 64.19% to 13.76% whereas for the plants from block I cultures, the variation percentage reduced from 2.42% to 0.69% at the end of third season in year 2018.
The total percentage of variant plants belonging to P. dactylifera cv. Gulistan was higher (44.29%) than cv. Kashuwari (22.32%) in both the block at the end of first season in year 2016. A significant reduction in the overall variation percentage were observed for both cv. Gulistan with reduction from 44.29% to 8.13% and cv. Kashuwari with reduction from 22.32% to 6.23% at the end of third season in year 2018 (Figure 1).
The most frequent variant phenotype among all the variant plants was “inflorescence absent phenotype”, however, the percentage of which decreased significantly in both the blocks and both cultivars (Figure 2). The observed percentage of variation for this phenotype was higher in cv. Kashuwari (25.93%) than cv. Gulistan (7.41%) at block I at the end of the first season in year 2016 (Figure 2). The observed percentage of variation decreased to 3.70% and 0.00% for cv. Kashuwari and cv. Gulistan, respectively. In contrast to plants from block I, the total percentage of inflorescence absent phenotype at block II was higher in cv. Gulistan (52.73%) than cv. Kashuwari (25.45%). The variation percentage decreased up to 3.64% in cv. Gulistan and 4.68% in cv. Kashuwari at the end of third season in year 2018 (Figure 2). It was observed that most of the plants of cv. Kashuwari that having a high number of offshoots per plant (10-13) produced no inflorescence in both the blocks (Figure 3I).
The phenotype “excessive vegetative growth” was only found in cv. Gulistan of block II (Figure 2). An overall percentage of this phenotype in the block was only 0.52% throughout the study period. This phenotype existed through broader leaves and leaflets (Figure 3B, C) as compared to the rest of the tissue cultured plants in the same population (Figure 3A). The average leaflet length and width of the variant phenotypes ranged from 50.5 to 60 cm and 4.1 to 4.2 cm respectively, as compared to 42 to 50 cm and 2.4 to 3.5 cm leaf length and width, respectively, of normal plants. In addition, both the plants also do not have any offshoot and inflorescence (Figure 3B).
The “dwarf phenotype” or plants with slow growth and development rate was found in both the date palm cultivars from block II (Figure 2). The incidence of this phenotype was 2.34% in cv. Kashuwari and 4.68% in cv. Gulistan, which remained the same throughout the study period. This phenotype of both the cultivars showed a retardation in growth and produced no inflorescence at all (Figure 3E).
Low level of fruit setting also reported as a “failure of fertilization” was found in both the blocks (Figure 2, Figure 3F). However, in block I only the plants belonging to cv. Gulistan revealed low level of fruit setting (11.11%), which was the highest and persistent variation percentage as compared to other variant types of cv. Gulistan in this block. In block II, the maximum low level of fruit setting of 5.71% was observed in cv. Gulistan at the end of first season in year 2016 as compared to 3.90% in cv. Kashuwari (Figure 2). The incidence of these variants decreased to 2.60% in cv. Gulistan and 2.08% in cv. Kashuwari at the end of third season in year 2018.
The production of deformed offshoots was observed only in cv. Gulistan of both the blocks (Figure 2). Generally, a single deformed offshoot that also produced a twisted inflorescence, in addition to other normal offshoots, was found per plant (Figure 3G, H). A twisted inflorescence is defined as the conversion of a deformed vegetative bud into a floral bud (Al-Mazroui et al., 2006). An overall percentage of this variation in block I was higher (7.41%) than the block II (0.52%) at the end of first season in year 2016. This variation was completely recovered at the end of third season in year 2018. The twisted inflorescence found on each deformed offshoot in cv. Gulistan produced 1-5 spikelets having few unfertilized fruits (Figure 3H).
The variant plants with abnormal frond growth were only found in cv. Gulistan of block II (Figure 2). An overall percentage of this variant type was 0.52% at the end of first season in year 2016 and the abnormal phenotype was complete recovered at the end of the third season in year 2018. This variant type revealed all the normal vegetative and reproductive characters except one-sided abnormal growth of the fronds (Figure 3D).
Comparison of phenotypic characters between tissue cultured and mother plants of P. dactylifera cultivars
Figure 4 show the mean variations between mother plants (MP) and tissue cultured (TC) plants of P. dactylifera cv. Kashuwari and cv. Gulistan for four bunch reproductive characters. In P. dactylifera cv. Kashuwari, the mean significant differences between MP and all TC plants were observed in all the characters examined except for the bunch length character (P ≤ 0.172) (Figure 4). Most of the TC plants in the block I and II showed non-significant mean variations among themselves (Figure 4). In contrast, TC plants of P. dactylifera cv. Gulistan revealed significant mean variations between MP and all TC plants except in the “retained fruit per bunch” character (P ≤ 0.204) (Figure 4).
For the fruit physical characters, Figure 5 shows the significant mean variations between MP and all TC plants of P. dactylifera cv. Kashuwari for all the fruit physical variables except the fruit length (P ≤ 0.286). Similar observation was found in P. dactylifera cv. Gulistan where the mean variations for all the fruit physical characters were significantly different at P ≤ 0.05 except the pulp-fruit ratio (P ≤ 0.888) (Figure 5).
The information of type and nature of somaclonal variations occur in P. dactylifera micropropagation using immature inflorescence is scarce. In the current study, six types of phenotypic variations were detected during the screening of the field grown tissue cultured date palm plants derived from immature inflorescence explants of P. dactylifera cv. Kashuwari and cv. Gulistan. Among the abnormal phenotypes detected, plants with inflorescence absent phenotype represented the highest proportion of the phenotypic variations. Others variant types such as low fruit setting, dwarfism, excessive vegetative growth phenotype, deformed offshoots/ twisted inflorescence and abnormal frond growth were in lower percentage. The dwarfism has been previously reported as permanent somaclonal variations in date palm (Alkhateeb, 2008) and in banana cv. Williams (Ramage et al., 2004).
A culture-age dependent variation was observed in the current study where the observed variations were higher in the plants from the block II compared to the plants from the block I. Some of the observed variations such as excessive vegetative growth, abnormal frond or leaf growth and dwarfism were absent in the plants, regardless genotype, from the block I. The results of current study are in agreement with the results reported by Saker et al. (2000) where the authors reported an increase in the percentage of overall variation with an increase in the number of in vitro subcultures. Similar observation was reported in banana where the likelihood and degree of variation in tissue cultured plants increased with the age of cultures (Bairu et al., 2006). In another study in banana, Rodrigues et al. (1997) reported that phenotypic variations increased the age of cultures increased from subculture 5 to more than 10 subcultures. The reason for this age-dependent increment in phenotypic variation could be due to the long period exposure of in vitro cultures to abiotic stresses or caused by proliferation and accumulation of mutant cells during tissue culture process (Orton, 1984).
A genotype-dependent variation was also observed in the current study where the percentage of variants in cv. Gulistan was significantly higher than in cv. Kashuwari. The low level of fruit setting phenotype was only observed in cv. Gulistan. This might be due to the highly sensitive nature of cv. Gulistan to in vitro stress conditions as it was similarly explained by Alkhateeb (2008). The degree of somaclonal variations in tissue cultured plants is generally vary depend on genotypes of a species used. Each genotype has its own capacity to respond to a specific stress condition, including in vitro abiotic stresses. In date palm, certain cultivars are relatively stable whilst others show a high level of inclination to variations (El Hadrami et al., 2011). The current findings are also in agreement with the results reported in tomato by Kurtz and Lineberger (1983) where the authors showed that the frequency of somaclonal variations varied depend on the cultivar used. Similar observation was reported in banana by Hwang and Ko (1987).
Interestingly, a reversion of abnormal phenotypes back to mother plants’ phenotypes was observed during the field trial period from year 2016 to 2018 except for “excessive vegetative growth”, “dwarfism” and “low level of fruit setting” phenotypes. The degree of phenotype reversion varied depend on the type of phenotype, age of in vitro culture and the genotype used. Most of the abnormal phenotypes appeared in both blocks were either disappeared totally or reduced to a significant extent. The reversion of the abnormal phenotypes might be due to the epigenetic changes in the genome of date palm (epigenetic variations). Epigenetic variations are caused by physiological imbalances found in cultured tissue, which alter DNA methylation pattern or activate transposable elements in the genome. In date palm, the rejuvenation, flower abnormalities and delay in flowering time phenotype variation in tissue cultured plants were reported to be triggered by an epigenetic mechanism (Smulders and De Klerk, 2011), which is relatively unstable and may disappear with the age of plant (Skirvin et al., 1994). The occurrence of deformed offshoot and twisted inflorescence in P. dactylifera cv. Barhee has also been reported as epigenetic variations (Al-Mazroui et al., 2006), which was associated with an imbalance of in vitro accumulated plant growth regulators (Varughese, 2000).
The evaluation of economically important phenotypic characters is also an important procedure for screening tissue cultured plants to ensure their production quantity and the quality of fruit. However, these characters are normally influenced by several ecological and physiological factors in the field or may vary with age and development of the plant (Haider et al., 2015). In current study, the similarities between the mother plants (MP) and tissue cultured plants (TC) from the block I and block II were found to be statistically non-significant in terms of the documented characters. However, TC plants of block I (5 years old) were more similar to their mother plants than TC plants of block II (4 years old), this phenomenon may be due to the juvenility of the plants in block II. In conclusion, the variation in fruit quality may be due to the differences in age of the MP plant (25-30 years) and TC plants (4-5 years) (Haider et al. 2015) or due to any other ecological factors (Hammadi et al. 2009).
Conclusions and Recommendations
This study showed that the percentage of the somaclonal variations of date palm increase with the increase in the age of initial cultures. The percentage of variations varied depend on the genotype of date palm used. The current study reported the permanent (genetic) and temporary (epigenetic) phenotypic variations. This study provides a good indication that cultures with subculture cycle less than 11 cycles are good source for generating “true-to-type” date palm. The current study also showed the importance of cultivar selection for date palm tissue culture industry.
Novelty Statement
This research manuscript is reporting on the occurrence and reversion of deformed phenotypes in micropropagated date palm that are planted in the open fields. The study is of a great importance to date palm industry especially in the production of true-to-type seedling materials from inflorescence explant source with subculture number <11 cycles in the multiplication stage. To our knowledge, this is the first report on tissue cultured date palms derived from immature inflores-cences.
Authors’ Contribution
Conceptualization of research (AA Abul-Soad); Designing of the experiments (AA Mirani); Contribution of experimental materials (GS Markhand); Execution of field experiments and data collection (AA Mirani, T Jatt, AA Mirbahar and N Solangi); Analysis of data and interpretation (AA Mirani and CH Teo); Preparation of the manuscript (AA Mirani, CH Teo and AA Abul-Soad)
There is supplementry material associated with this article. Access the material online at:
References
Abul-Soad, A. 2011. Micropropagation of date palm using inflorescence explants. In: Date Palm Biotechnology. Springer Int. Germany. 91-117. https://doi.org/10.1007/978-94-007-1318-5_6
Abul-Soad, A., K. Emara, A. Abdallah and S. Mahdi. 2016. Somatic embryogenesis in Phoenix dactylifera L. using floral bud explants. In: IX Int. Symp. In Vitro Cult. Hort. Breeding, Egypt. 1187: 13-28.
Abul-Soad, A., I. Ibrahim, N. El-Sherbeny and S. Baker. 2002. In vitro optimization for plant regeneration of date palm (Phoenix dactylifera L.). Minia. J. Agric. Res. Dev. 22: 2265-2282.
Abul-Soad, A.A. and S.M. Mahdi. 2010. Commercial production of tissue culture date palm (Phoenix dactylifera L.) by inflorescence technique. J. Genet. Eng. Biotechnol. 8: 39-44.
Al-Mazroui, H., A. Zaid and N. Bouhouche. 2006. Morphological abnormalities in tissue culture-derived date palm (Phoenix dactylifera L.). In: III Int. Date Palm Conf. UAE: 736: 329-335.
Alkhateeb, A.A. 2008. A review the problems facing the use of tissue culture technique in date palm (Phoenix dactylifera L.). Sci. J. King Faisal Univ. Basic Appl. Sci. 9: 85-104.
Bairu, M.W., C.W. Fennell and J.V. Staden. 2006. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv.‘Zelig’). Sci. Horti. 108: 347-351. https://doi.org/10.1016/j.scienta.2006.01.039
Benzion, G. and R.L. Phillips. 1988. Cytogenetic stability of maize tissue cultures: a cell line pedigree analysis. Genome. 30: 318-325. https://doi.org/10.1139/g88-056
El Hadrami, A., F. Daayf, S. Elshibli, S. Jain and I. El Hadrami. 2011. Somaclonal variation in date palm. In: Date Palm Biotechnol.. Springer, Germany. 183-203. https://doi.org/10.1007/978-94-007-1318-5_9
Faissal, F.A., M.A. Mohamed, A.A. Gobara and A. Ahmed. 2013. Evaluation of some dry date palm varieties propagated through seed and tissue culture technique under aswan region climatic conditions. Stem Cell. 4: 14-24.
Gurevich, V., U. Lavi and Y. Cohen. 2005. Genetic variation in date palms propagated from offshoots and tissue culture. J. Am. Soc. Hortic. Sci. 130: 46-53. https://doi.org/10.21273/JASHS.130.1.46
Haider, M.S., I.A. Khan, M.J. Jaskani, S.A. Naqvi, M. Hameed, M. Azam, A.A. Khan and J.C. Pintaud. 2015. Assessment of morphological attributes of date palm accessions of diverse agro-ecological origin. Pak. J. Bot. 47: 1143-1151.
Hammadi, H., R. Mokhtar, E. Mokhtar and F. Ali. 2009. New approach for the morphological identification of date palm (Phoenix dactylifera L.) cultivars from Tunisia. Pak. J. Bot. 41: 2671-2681.
Hwang, S. and W. Ko. 1987. Somaclonal variation of bananas and screening for resistance to Fusarium wilt. Banana and plantain breeding strategies. 21: 151-156.
Jin, S., R. Mushke, H. Zhu, L. Tu, Z. Lin, Y. Zhang and X. Zhang. 2008. Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep. 27: 1303-1316. https://doi.org/10.1007/s00299-008-0557-2
Khushk, A.M., A. Memon and K.M. Aujla. 2009. Marketing channels and margins of dates in Sindh. Pak. J. Agric. Sci. 47: 293-308.
Kurtz, S. and R. Lineberger. 1983. Genotypic differences in morphogenic capacity of cultured leaf explants of tomato (Lycopersicon esculentum). J. Am. Soc. Hortic. Sci. 108: 710-714.
Larkin, P.J. and W.R. Scowcroft. 1981. Somaclonal variation, A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60:197-214. https://doi.org/10.1007/BF02342540
Orton, T.J. 1984. Genetic variation in somatic tissues: method or madness?. Adv. Plant Path. 2: 153-189.
Ramage, C., A. Borda, S. Hamill and M. Smith. 2004. A simplified PCR test for early detection of dwarf off-types in micropropagated Cavendish bananas (Musa spp. AAA). Sci. Horti. 103: 145-151. https://doi.org/10.1016/j.scienta.2004.04.015
Rodrigues, P., N.A. Tulmann, N.P. Cassieri and B. Mendes. 1997. Influence of the number of subcultures on somaclonal variation in micropropagated nanicao (Musa spp., AAA Group). In: II Int. Symp. Banana: I Int. Symp. Banana Subtropics, Spain: 490: 469-474.
Saker, M., S. Adawy, A. Mohamed and H. El-Itriby. 2006. Monitoring of cultivar identity in tissue culture-derived date palms using RAPD and AFLP analysis. Biol. Plant. 50: 198-204. https://doi.org/10.1007/s10535-006-0007-3
Saker, M., S. Bekheet, H. Taha, A. Fahmy and H. Moursy. 2000. Detection of somaclonal variations in tissue culture-derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol. Plant. 43: 347-351. https://doi.org/10.1023/A:1026755913034
Sharma, D., R. Kumari and J. Chowdhury. 1980. In vitro culture of female date palm (Phoenix dactylifera L.) tissues. Euphytica. 29: 169-174. https://doi.org/10.1007/BF00037263
Skirvin, R.M., K.D. McPheeters and M. Norton. 1994. Sources and frequency of somaclonal variation. Hort. Sci. 29: 1232-1237. https://doi.org/10.21273/HORTSCI.29.11.1232
Smith, S. 1975. Vegetative propagation of the date palm by root tip culture. Bull d’Agron. Saharienne. 1: 67.
Smulders, M. and G. De Klerk. 2011. Epigenetics in plant tissue culture. Plant Growth Regul. 63: 137-146. https://doi.org/10.1007/s10725-010-9531-4
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics: a biometrical approach. McGraw-Hill, USA.
Varughese, A. 2000. Mass propagation of date palm through tissue culture: an efficient method by SAPAD. In: Proc. Date Palm Int. Symp. Windhoek, Namibia: 65-67.
Zaid, A. and B. Tisserat. 1984. Survey of the morphogenetic potential of excised palm embryos in vitro. Crop Res. 24: 1-9.
Ziauddin, A. and K. Kasha. 1990. Long-term callus cultures of diploid barley (Hordeum vulgare). II. Effect of auxins on chromosomal status of cultures and regeneration of plants. Euphytica. 48: 279-286. https://doi.org/10.1007/BF00037197
To share on other social networks, click on any share button. What are these?








