In vitro and In vivo Antifungal Activity of Selected Fungal Species against the Fusarium Wilt of Solanum lycopersicum
In vitro and In vivo Antifungal Activity of Selected Fungal Species against the Fusarium Wilt of Solanum lycopersicum
Zulqarnain and Zafar Iqbal*
Department of Agricultural Chemistry, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Fusarium wilt of tomato is a devastating fungal disease with several infectious strategies leading to the complete death of plant. None of the strategies in controlling the wilt have shown fruitful results. Dual culture assay was employed is this study to identify the fungal species with their potent antifungal activity against the Fusarium wilt of tomato. Six distinct fungal species including Penicillium sp., Aspergillus niger, Aspergillus flavus, Trichoderma harzianum, Alternaria solani and Pythium aphanidermatum showed growth inhibition (%) against the pathogen. Penicillium sp. showed maximum growth inhibition (63.85 ± 3.26 %) and its extract showed 9.20 ± 0.14 mm zone of inhibition at 1000 mg·L-1 against the Fusarium wilt. Based on its higher in vitro antifungal activity, in vivo screen house experiments were conducted. Plants given the treatment as Penicillium + Fusarium showed slight yellowing of the leaves after 60th days of cultivation. Present results may help in exploiting the Penicillium sp. extract for isolation and structural characterization of its antifungal compounds.
Received | May 19, 2018; Accepted | October 28, 2018; Published | November 20, 2018
*Correspondence | Zafar Iqbal, Department of Agricultural Chemistry, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: zafar.iqbal@aup.edu.pk
Citation | Zulqarnain and Z. Iqbal. 2018. In vitro and in vivo antifungal activity of selected fungal species against the fusarium wilt of Solanum lycopersicum. Sarhad Journal of Agriculture, 34(4): 834-839.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.4.834.839
Keywords | Antifungal activity, Dual culture assay, Fusarium wilt, Tomato, Pot experiments
Introduction
Several pathogenic species of Fusarium cause diseases in plants with great diversity. These fungal species can be air borne, soil borne or reside inside the plant and can be recovered from any part of the plant (Summerell et al., 2003). Fusarium oxysporum f.sp. lycopersici (Fol) is a pathogenic fungus that can cause Fusarium wilt in tomato and lead to economic loss (Agrious, 2005). Control of wilt diseases depends mainly on biological control or synthetic fungicides (Singha et al., 2011). Fungal bio-control agents have a narrow host range and they act slowly (Copping and Menn, 2000) whereas use of synthetic fungicides against phytopathogenic fungi has been questioned due to their environmental concern and several microorganisms have developed resistance to these chemical fungicides (Gaigole et al., 2011). Therefore, control of these pathogenic fungi has become important (Bernal et al., 2002) and there is a need to work on the development of natural product based fungicides.
Natural products, generally known as secondary metabolites show no harmful effects on the producing organisms while they have deterrent effects on predators and competitors (Schafer and Wink, 2009). The possible use of the natural products as pharmaceuticals has been extensively investigated, and it is estimated that 50% of the clinically approved drugs in today’s global market are derived from natural products (Butler, 2008). Fungal novel bioactive compounds have contributed advances in agriculture and human health with impacts on societal and economic growth (Goler, 2007). Therefore, present study was designed to isolate distinct fungal species and to test their antifungal efficacy against the Fusarium wilt of tomato. Moreover, in vivo screening of the bioactive extract was also conducted in pot experiments.
Materials and Methods
Isolation of fungal species from soil samples
Fungal species were isolated using soil serial dilution method (Warcup, 1950). The mixed fungal colonies were further purified by sub culturing the inoculum from each colony to new petri dish (Freitas et al., 2011).
Isolation of endophytes
Healthy plant segments (Stem and leaves) were first washed with a running tape water to remove dust and debris. Small pieces of the segments (0.5 to 1 cm in length) were surface sterilized with 75 % methanol and by dipping them in mercuric chloride (HgCl2) solution inside biosafety cabinet. The segments were then rinsed with sterile distilled water twice and were dried on blot paper. Finally, 5 to 6 segments were placed on agar plate and the dishes were incubated at 27 oC for 7-10 days. The plates with mixed fungal colonies were further purified by sub culturing the inoculum from each colony to new petri dish (Arnold et al., 2000).
Identification of fungal Species
Fungal species were identified on the basis of their morphological characteristics and microscopic examination (Diba et al., 2007). Length and width of the colony, presence and absence of mycelium, color and any pigment production were observed as morphological characteristics whereas spore structures were observed under the light microscope. The fungal species were identified with the help of Dr. Shaukat Husain (Mycologist) at the Department of Plant Pathology, The University of Agriculture, Peshawar, Pakistan and relevant literature (Ilhan et al., 2006).
Isolation of Fusarium oxysporum f.sp. lycopersici (Fol)
Infected tomato plants were selected to isolate Fol as described (Singha et al., 2011) . Fungal growth micro conidia were observed under the microscope (Agrios, 2005). The pure culture was stored at 4 °C. The fungus was re-cultured every second month.
Dual culture assays
Dual culture techique was used to test the antifungal efficacy of the isolated fungal species against Fol (Mishra, 2010). The petri dishes with PDA media were first incolucated with the mycelial plugs (5 mm in diameter) of the isolated fungal species and after 2 days of incubation at 28 °C, the same plates were inoculated with the mycelial plug (5 mm in diameter) of the Fol placed 5 cm away from the antagonists. The antagonist and the pathogen were incubated for additional 9 days at 28 °C. The growth of the pathogen in both the test and control experiments was recorded and % inhibition of the radial growth was calculated as:
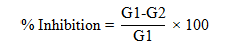
Where; G1 = growth of the pathogen in control and G2 = growth of the pathogen in dual culture.
Well diffusion method
Well diffusion method was used for in vitro antifungal assay of the crude extracts as described (Sonawane et al., 2015). Crude extracts (20 µL) dissolved in methanol (10 mg·mL-1) were poured in wells. Mancozeb and methanol were used as positive and negative control respectively. The plates were incubated at 27 °C for 7 days and zone of inhibition (mm) was measured.
In vivo screen house experiments of tomato
In vivo antifungal activity of the extract was carried out in screen house as described (Lee et al., 2001). The pots were filled with sterilized soil (1 kg) in each pot. After 10 days of cultivation, 5 mL of the treatment suspension was applied using hypodermic syringe to the plants as Fungal Extract (FE), Fusarium (Fus), Fungal Extract (FE) + Fusarium (Fus), Manzoceb + Fusarium (Man+Fus), Fungal Extract (Sprayed) + Fusarium (FEsp + Fus) and Mancozeb (Sprayed) + Fusarium (Mansp + Fus).
Statistical analysis
Means and standard deviations were calculated using Statistix 8.1.
Results and Discussion
Isolation of fungal species from soil and plants
Total nine fungal species including Penicillium sp., Aspergillus flavus, Aspergillus niger, Alternaria solani, Trichoderma harzianum, Rhizopus stolonifer and Paecilomyces lilacinus from soil samples were isolated whereas Pythium aphanidermatum was isolated from the plant samples. Fusarium oxysporum f.sp. lycopersici was isolated from the diseased tomato plant showing the symptoms of wilt. Geographical location and sampling methods are important factors in the isolation of fungal species (Gaddeyya et al., 2012). It has been reported that occurrence (%) of Aspergillus and Fusarium was more than other fungal species at Wazirabad and Mandi Bahudin, Punjab, Pakistan (Raza et al., 2013). The environmental factors of soil such as moisture, nutrients, temperature and pH affect the diversity of fungal population (Yu et al., 2007).
Soil microflora have an important role in various biochemical transformations and mineralization activities of the soil and plants. Fungi, a diversified kingdom plays an important role in soil ecosystem especially in agricultural soils for organic matter decomposition and release of minerals (Ainsworth and Bisby, 1995).
Dual culture assays
Isolated fungal species were tested for their antagonistic activities against Fusarium oxysporum f.sp. lycopersici. It was observed that out of the nine fungal species, six of them including A. solani, A. niger, Penicillium sp., P. aphanidermatum, T. harzianum and A. flavus showed antagonistic activity in dual culture assay. It is clear from Table 1 that the maximum % inhibition (63.85 ± 3.26) was shown by Penicillium sp. followed by A. niger (51.54 ± 1.09) and A. solani (46.15 ± 2.18). A. flavus showed minimum % inhibition (20.00 ± 2.18) against the pathogen while P. aphanidermatum and T. harzianum showed % inhibition 36.16 ± 1.09 and 33.85 ± 4.35 respectively.
Table 1: Inhibition (%) of six different fungal species against Fusarium oxysporum f.sp. lycopersici in dual culture assay.
| Fungal Species | Pathogen | Inhibition % ± SD |
| Aspergillus niger |
Fusarium oxysporum f.sp. lycopersici |
51.54 ± 1.09 |
| Alternaria solani | 46.15 ± 2.18 | |
| Penicillium sp. | 63.85 ± 3.26 | |
| Trichoderma harzianum | 33.85 ± 4.35 | |
| Pythium aphanidermatum | 36.15 ± 1.09 | |
| Aspergillus flavus | 20.00 ± 2.18 |
A zone of inhibition was observed during the dual culture assay of A. niger, Penicillium sp., T. harzianum, P. aphanidermatum and A. flavus against Fusarium oxysporum (Fol). This zone of inhibition is often produced by the antagonists without hyphae contact by releasing the inhibitory metabolites such as the secretion of diffusible non-volatile inhibitory substances known as extracellular exochitinases (Brunner et al., 2003; Kullning et al., 2000). Hajieghrari et al. (2008) carried out dual culture assay of T. harzianum against Fusarium oxysporum (Fol) and zone of inhibition between them was suggested as the secretion of exochitinases. Cell fragments of the hosts are dissolved by diffusion of these enzymes and which in turn stimulate further enzymes for rapid growth of Trichoderma sp. (Zeilinger et al., 1999). Alwathnani et al. (2012) reported in his study that P. citrinum inhibited F. oxysporum f. sp. phaseoli with 53.5 % inhibition. This may be due to the production of inhibitory substances such as viridian, terricin and terric acid etc. (Mondal et al., 2000; Xiao Yan et al., 2006).
In vitro antifungal activity
Based on highest antifungal activity of Penicillium sp. in dual culture assay, in vitro antifungal activity of the crude extract of Penicillium sp. was carried out at three different concentrations i.e. 1000, 500 and 250 mg·L-1 (Table 2). The extract showed maximum zone of inhibition (9.20 ± 0.14 mm) against the wilt pathogen at 1000 mg·L-1. Mancozeb, a commercial fungicide showed 11.70 ± 0.57 mm zone of inhibition. Mancozeb is a well-known agricultural non-systemic dithiocarbamate fungicide, which is a mixture of Maneb and Zineb (Gullino et al., 2010). Its higher antifungal activity at 250 mg·L-1 shows that antifungal compounds in the crude extract of Penicillium sp. might be in lower concentration and therefore showed less activity than Mancozeb even at 1000 mg·L-1. Saadabi and El Amin (2007) investigated the effects of cultural filtrates of different fungi in vitro against F. oxysporum.
Table 2: Mean zone of inhibition (mm) ± SD of Penicillium sp.
|
Fungal Species
|
Media | Concentration (mg·L-1) | Zone of Inhibition (mm) ± SD |
|
Penicillium sp. |
GPYB | 1000 | 9.20 ± 0.14 |
| 500 | 4.45 ± 0.21 | ||
| 250 | 2.40 ± 0.28 | ||
| Positive Control | Mancozeb | 250 | 11.70 ± 0.57 |
*Whereas GPYB stands for Glucose Peptone Yeast Extract Broth.
In vivo screen house pot experiment
Due to the higher in vitro antifungal activity of the crude extract of Penicillium sp. against the pathogen, in vivo screen house pot experiments were carried out. The experiment was conducted twice.
First growing season: Penicillium sp. was selected for pot experiments based on its higher in vitro antifungal activity against Fol. The efficacy of its crude extract was tested in pot experiment. The Roma variety of tomato was selected for pot experiment. The plant started showing symptoms of wilting after 20 days of cultivation. The disease severity was calculated as (Grattidge and O’ Brien, 1982): 0 = no symptom, 1 = slight yellowing or wilting, 2 = moderate yellowing or wilting, 3 = complete wilting and 4 for 100% leaves yellowed and wilted (Table 3). The plants were given scale 0 (no symptom) to all the treatments after 20 days. After 30 days of cultivation scale 0 was given to control and Penicillium (Pen) crude whereas Penicillium + Fusarium (Pen+Fus), Mancozeb + Fusarium (Man+Fus) while Fusarium (Fus), Penicillium crude (Sprayed) + Fusarium, Mancozeb (Sprayed) + Fusarium, Penicillium (Filtrate) + Fusarium showed scale 1 (slight yellowing). After 60 days of cultivation, all of the treatments showed 100 % wilting of the leaves (Figure 1). Pot experiments showed mixed results in replicates and no clear idea was drawn from the trail. The same experiment was repeated in 2nd growing season of tomato for clear outcomes.
Table 3: Average disease severity scale given to different treatments in pot experiments during the first growing season.
| Treatments | Given Scale | ||||
|
20th Day |
30th Day |
40th Day |
50th Day |
60th Day |
|
| Control | 0 | 0 | 0 | 0 | 0 |
|
Penicillium (Crude) |
0 | 0 | 0 | 0 | 0 |
| Penicillium + Fusarium | 0 | 1 | 2 | 3 | 4 |
| Fusarium | 0 | 1 | 2 | 3 | 4 |
|
Mancozeb + Fusarium |
0 | 1 | 2 | 3 | 4 |
|
Penicillium (Sprayed) + Fusarium |
0 | 1 | 2 | 3 | 4 |
|
Mancozeb (Sprayed) + Fusarium |
0 | 1 | 2 | 3 | 4 |
|
Penicillium (Filtrate) + Fusarium |
0 | 1 | 2 | 3 | 4 |
0: no symptom; 1: slight yellowing or wilting; 2: moderate yellowing or wilting; 3: complete wilting; 4: 100% leaves yellowed and wilted.
Second growing season: The experiment was repeated in second season due to its inconsistent results in first season to get reliable and reproducible results. The pot experiments of tomato plants were carried out during the month of March-April. The plant started to show symptoms of Fusarium after 20 days of cultivation. The disease severity was calculated according to the scale described by Grattidge and O’ Brien (1982): 0 = no symptom, 1 = slight yellowing or wilting, 2 = moderate yellowing or wilting, 3 = complete wilting and 4 for 100% leaves yellowed and wilted.
It was observed that control plants and the plants given the treatment as only Penicillium (Extract) did not show symptoms even after 60th day of cultivation (Table 4). On the 20th day of cultivation all the other treated plants did not show wilting symptoms. On the 30th, 40th, 50th and 60th day of cultivation, the plant infested with Fusarium showed slight yellowing or wilting, moderate yellowing, complete yellowing and 100% leaves yellowed and wilted respectively. The plants given the treatments as Penicillium + Fusarium (Pen+Fus) did not show wilting symptoms till 40th day however it showed slight yellowing/wilting on the 50th and 60th day. The plants given the treatments as Penicillium (Sprayed) + Fusarium and Mancozeb (Sprayed) + Fusarium showed complete wilting of the plants after 60th of cultivation while Mancozeb + Fusarium showed moderate yellowing/wilting.
The in vivo pathogenicity test showed yellowing of the older leaves as an early symptoms of the tomato wilt disease. Most of the foliage are affected by turning yellow and ultimately leads to wilting. The wilting continues until the plant dies. It has been commonly reported that tomato plants are attacked by Fusarium wilt within a growth period of 60 days during flower initiation. The pathogen proliferates in vascular tissues through the roots of the plant and breakdown the water and food supply of the infected plants (Agrios, 2005).
Table 4: Average disease severity scale given to different treatments in pot experiments during the second growing season.
| Treatments | Given Scale | ||||
|
20th Day |
30th Day |
40th Day |
50th Day |
60th Day |
|
| Control | 0 | 0 | 0 | 0 | 0 |
|
Penicillium (Extract) |
0 | 0 | 0 | 0 | 0 |
| Penicillium+ Fusarium | 0 | 0 | 0 | 1 | 1 |
| Fusarium | 0 | 1 | 2 | 3 | 4 |
|
Mancozeb + Fusarium |
0 | 0 | 0 | 1 | 2 |
|
Penicillium (Sprayed) + Fusarium |
0 | 0 | 1 | 2 | 3 |
|
Mancozeb (Sprayed) + Fusarium |
0 | 0 | 1 | 2 | 3 |
0: no symptom; 1: slight yellowing or wilting; 2: moderate yellowing or wilting; 3: complete wilting; 4: 100% leaves yellowed and wilted.
Conclusions and Recommendations
Nine fungal species were isolated from soil and plant samples. Dual culture assays revealed six antifungal species against Fusarium oxysporum f.sp. lycopersici. Penicillium sp. showed maximum % inhibition against the wilt pathogen. The crude extract of the Penicillium sp. showed 9.20 ± 0.14 mm zone of inhibition at 1000 mg·L-1. In vivo pot experiments showed mixed results during the first growing season. During the second season even after 60 days of cultivation the tomato plant showed slight yellowing or wilting with the application of Penicillium extract. These in vitro and in vivo experiments may help in exploiting the crude extract for the identification and isolation of antifungal compounds in future.
Acknowledgements
We are thankful to Pakistan Science Foundation (PSF) for funding the overall research studies under Project No. PSF/NSLP/KP-AU (421). We are also thankful to Dr. Muhammad Sajid, Associate Professor, Department of Horticulture, The University of Agriculture, Peshawar, Pakistan for his assistance in pot experiments. Dr. Jawad Anwar is thanked for proof reading of the article.
Author’s Contribution
The present research is a part of doctoral study of Zulqarnain who performed experiments and wrote the article under the supervision of Zafar Iqbal.
References
Agrios, G. 2005. Plant pathology. 5th ed. Academic Press, New York, USA.
Ainsworth, G. and G. Bisby. 1995. Dictionary of the Fungi. 8th ed. Common Wealth Mycological Institute Kew, Surrey.
Alwathnani, H.A., K. Perveen, R. Tahmaz and S. Alhaqbani. 2012. Evaluation of biological control potential of locally isolated antagonist fungi against Fusarium oxysporum under in vitro and pot conditions. Afr. J. Microbiol. Res. 6: 312-319. https://doi.org/10.5897/AJMR11.1367
Arnold, A.E., Z. Maynard, G.S. Gilbert, P.D. Coley and T.A. Kursar. 2000. Are tropical fungal endophytes hyperdiverse? Ecol. Lett. 3: 267-274. https://doi.org/10.1046/j.1461-0248.2000.00159.x
Bernal, G., A. Illanes and L. Ciampi. 2002. Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. Electro. J. Biotechnol. 5: 7-8. https://doi.org/10.2225/vol5-issue1-fulltext-4
Brunner, K., C.K. Peterbauer, R.L. Mach, M. Lorito, S. Zeilinger and C.P. Kubicek. 2003. The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr. Genetics 43: 289-295. https://doi.org/10.1007/s00294-003-0399-y
Butler, M.S. 2008. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 25: 475-516. https://doi.org/10.1039/b514294f
Copping, L.G. and J.J. Menn. 2000. Biopesticides: a review of their action, applications and efficacy. Pest Manage. Sci. 56: 651-676. https://doi.org/10.1002/1526-4998(200008)56:8<651::AID-PS201>3.0.CO;2-U
Diba, K., P. Kordbacheh, S. Mirhendi, S. Rezaie and M. Mahmoudi. 2007. Identification of Aspergillus species using morphological characteristics. Pak. J. Med. Sci. 23: 867.
Freitas, R.S., K.C. Dantas, C.N. Pereira, J.E. Levi and J.E.C. Martins. 2011. Preservation methods of fungi in 35 years old stock culture storages: A comparative study. Afr. J. Microbiol. Res. 5: 555-561.
Grattidge, R. and R. O’Brien. 1982. Occurrence of a third race of Fusarium wilt of tomatoes in Queensland. Plant Dis. 66: 165-166.
Gaddeyya, G., P.S. Niharika, P. Bharathi and P.R. Kumar. 2012. Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Adv. Appl. Sci. Res. 3: 2020-2026.
Gaigole, A., G. Wagh and A. Khadse. 2011. Antifungal activity of Trichoderma species against soil borne pathogen. Asiat. J. Biotech. Resour. 2: 461-465.
Gloer, J. 2007. 15 Applications of Fungal Ecology in the Search for New Bioactive Natural Products. Environ. Microb. Relat. 4: 257. https://doi.org/10.1007/978-3-540-71840-6_15
Gullino, M.L., F. Tinivella, A. Garibaldi, G.M. Kemmitt, L. Bacci and B. Sheppard. 2010. Mancozeb: past, present, and future. Plant Dis. 94: 1076-1087. https://doi.org/10.1094/PDIS-94-9-1076
Hajieghrari, B., M. Torabi-Giglou, M.R. Mohammadi and M. Davari. 2008. Biological potantial of some Iranian Trichoderma isolates in the control of soil borne plant pathogenic fungi. Afr. J. Biotechnol. 7: 967-972.
İlhan, S., R. Demirel, A. Asan, C. Bayçu and E. KINACI. 2006. Colonial and Morphological Characteristics of Some Microfungal Species Isolated from Agricultural Soils in Eskişehir Province (Turkey). Turk. J. Bot. 30: 95-104.
Kullnig, C., R.L. Mach, M. Lorito and C.P. Kubicek. 2000. Enzyme diffusion from Trichoderma atroviride (= T. harzianum P1) to Rhizoctonia solani is a prerequisite for triggering of Trichoderma ech42 gene expression before mycoparasitic contact. Appl. Environ. Microbiol. 66: 2232-2234. https://doi.org/10.1128/AEM.66.5.2232-2234.2000
Lee, S.E., B.S. Park, M.K. Kim, W.S. Choi, H.T. Kim, K.Y. Cho, S.G. Lee and H.S. Lee. 2001. Fungicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum L., against phytopathogenic fungi. Crop Prot. 20: 523-528. https://doi.org/10.1016/S0261-2194(00)00172-1
Mishra, V. 2010. In vitro antagonism of Trichoderma species against Pythium aphanidermatum. J. Phytol. 2: 28-35.
Mondal, G., P. Dureja and B. Sen. 2000. Fungal metabolites from Aspergillus niger AN27 related to plant growth promotion. Ind. J. Exp. Biol. 38: 84-87.
Raza, M., M.U. Ghazanfar, M. Hussain, Z. Iqbal and Y. Iftikhar. 2013. Pathogenic and non-pathogenic fungi isolated from soil and roots of tobacco. Pak. J. Phytopathol. 25: 95-100.
Saadabi, A.M. and Z.N. El-Amin. 2007. In vitro Studies on the Toxicity of Culture Filtrates of Different Fungi on the Growth of Fusarium oxysporum f. sp. vasinfectum. J. Biol. Sci. 7: 429-432. https://doi.org/10.3923/jbs.2007.429.432
Schafer, H. and M. Wink. 2009. Medicinally important secondary metabolites in recombinant microorganisms or plants: progress in alkaloid biosynthesis. Biotechnol. J. 4: 1684-1703. https://doi.org/10.1002/biot.200900229
Singha, I.M., Y. Kakoty, B.G. Unni, M.C. Kalita, J. Das, A. Naglot, S.B. Wann and L. Singh. 2011. Control of Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici using leaf extract of Piper betle L.: a preliminary study. World J. Microbiol. Biotechnol. 27: 2583. https://doi.org/10.1007/s11274-011-0730-6
Sonawane, A., M. Mahajan and S. Renake. 2015. Antifungal activity of a fungal isolates against Pomegranate wilt pathogen Fusarium. Int. J. Curr. Microbiol. Appl. Sci. 2: 48-57.
Summerell, B.A., B. Salleh and J.F. Leslie. 2003. A utilitarian approach to Fusarium identification. Plant Dis 87: 117-128. https://doi.org/10.1094/PDIS.2003.87.2.117
Warcup, J. 1950. The soil-plate method for isolation of fungi from soil. Nat. 166: 117-118. https://doi.org/10.1038/166117b0
Xiao-Yan, S., S. Qing-Tao, X. Shu-Tao, C. Xiu-Lan, S. Cai-Yun and Z. Yu-Zhong. 2006. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 260: 119-125. https://doi.org/10.1111/j.1574-6968.2006.00316.x
Yu, C., D. Lü, S. Qin, G. Du and G. Liu. 2007. Microbial flora in Cerasus sachalinensis rhizosphere. J. Appl. Ecol. 18: 2277-2281.
Zeilinger, S., C. Galhaup, K. Payer, S.L. Woo, R.L. Mach, C. Fekete, M. Lorito and C.P. Kubicek. 1999. Chitinase Gene Expression during Mycoparasitic Interaction ofTrichoderma harzianumwith Its Host. Fungal Genet. Biol. 26: 131-140. https://doi.org/10.1006/fgbi.1998.1111
To share on other social networks, click on any share button. What are these?








