Organochlorine Residues in Some Egyptian Fish: Detection and Improvement Trials
Research Article
Organochlorine Residues in Some Egyptian Fish: Detection and Improvement Trials
Nahla Hamada Magd Khalil1*, Ihab Mahmoud Helal2, El-Desoky Hassan Ibrahim Dorrah1, Soad Ahmed Soliman Ismail3
1Food Inspection Laboratory, Animal Health Research Institute, ARC, Egypt; 2Food Hygiene Department, Animal Health Research Institute, ARC, Egypt; 3Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Suez Canal University, Egypt.
Abstract | Sixty fish samples consisted of (20 tilapia fish, 20 mullets, and 10 basses and 10 shrimps) were randomly collected from various regions at Damietta governorate, Egypt. The samples were analyzed for detection of organochlorine pesticide residues (OCP) by gas chromatography. The results revealed presence of different types of OCP residues (Dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethane (DDD), chlorodiphenyldichloroethylene (DDE), Alderin, Dieldrin, Heptachlor, Heptachlor epoxide, Alpha hexachlorocyclohexanes (αHCH), Gamma hexachlorocyclohexanes (γHCH), Endosulfan and Gamma chlordane (γ-chlordane) in the examined fish species at different concentrations. Mullet fish samples had significantly (P < 0.05) the highest concentrations of alderin, dieldrin and heptachlor epoxide with mean ± SE concentration of 89±0.85, 36.7±0.66, and 38.9 ±0.49 ppb, respectively. Tilapia fish had the highest concentrations of DDD, DDE and γ-chlordane with a mean ± SE concentration of 80.5±8.61, 46.6±9.77, and 79.5±17.37 ppb, respectively. Bass had the lowest concentration of DDT and DDE with mean ± SE concentration of 3.17±0.73, and 4.17±0.44 ppb while most of the OCP were not detected in bass samples. The mean values ± SE of DDT, DDD, DDE, Heptachlor, Heptachlor epoxide, in shrimp samples were 9.75±0.52, 29.63± 0.55, 13.3± 0.32, 17.2±0.56 and 30.4±0.57 ppb, respectively, but the other types were not detected. Overall, OCP residues in all examined raw fish samples were below the maximum permissible limits (MPL). The effect of heat treatment (microwaving, roasting and boiling) for DDD residues showed reduction percentages of 81.13%, 60.38%, and 79.25%, respectively. While DDE residues showed reduction percentages of 100%, 57.14%, and 71.43%, respectively. On the other hand, DDT residue was completely reduced in all cooking methods (100%). Heptachlor reduction by roasting was 86.36%, and completely reduced by microwaving and boiling. Heptachlor epoxide reduction was 94.88%, 79.49% after microwaving and roasting, respectively, and completely reduced by boiling. Concerning α-HCH, the reduction percentage was 95.83% for roasting, and 100% by microwaving and boiling while γ-HCH was completely reduced (100%) by all cooking methods. Alderin reduction was 82.02%, 67.42% and 100% after microwaving, roasting and boiling, respectively. Dieldrin residue was completely reduced (100%) by all cooking methods. Endosulfan and γ-chlordane reduction was 82.02%, 67.42 % and 100% after microwaving, roasting and boiling, respectively.
Keywords | Organochlorine, Fish, Microwaving, Roasting, Boiling
Received | February 06, 2022; Accepted | March 18, 2022; Published | April 15, 2022
*Correspondence | Nahla Hamada Magd Khalil, Food Inspection Laboratory, Animal Health Research Institute, ARC, Egypt; Email: drnh14124@gmail.com
Citation | Khalil NHM, Helal IM, Dorrah E-DHI, Ismail SAS (2022). Organochlorine residues in some Egyptian fish: Detection and improvement trials. Adv. Anim. Vet. Sci. 10(5): 1119-1126.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.5.1119.1126
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Fish is a desirable and highly nutritive food source in Egypt; Fish as human food considered as a good source of protein, omega-3 fatty acids, calcium, zinc, and iron (Morshdy et al., 2013). Hence aquaculture is the primary source of fish production in Egypt; therefore, the safety of fish and fish products from aquaculture is of public health interest. Pesticides are toxic chemical substance or a combination of substances or biological agents, which are intentionally released into the atmosphere to avoid, discourage, monitor and/or kill and destroy insect populations, weeds, rodents, fungi, or other harmful pests (Lah, 2011).
Organochlorine pesticides (OCPs) have been used for decades around the world, particularly in Egypt for the purpose of insect control (Saber et al., 2018; Morshdy et al., 2018). Such OCPs are released into the environment including water bodies (Thompson et al., 2017). Fish is then exposed to pesticides in four main dermal ways: (1) Direct absorption by swimming through the skin in pesticide-contaminated waters, (2) Respiration, by the direct absorption of pesticides by the gills, (3) Orally, through swallowing pesticide-contaminated aquatic Water, and (4) feeding on dead insects that have been killed by insecticides (Louis et al., 2009).
Consumption of such contaminated fish represent a great health hazard. As OCPs have several deleterious effects on human health including reproductive failures and birth defects, malfunctioning of the immune system, endocrine diseases, carcinogenic effects (Afful et al., 2010). In addition, OCPs are linked to the occurrence of type 2 diabetes, nervous disorders, hepatic, and renal dysfunctions (Polder et al., 2014; Thompson and Darwish, 2019).
In sight of the previous facts, this study aimed at investigation of the occurrence of different OCPs in four fish and shellfish species commonly consumed in Egypt, namely tilapia, mullet, bass, and shrimp. As fish is consumed cooked in Egypt, therefore, the effects of different cooking methods on OCPs content in the examined fish species were further examined. Estimated daily intakes and potential human health risks associated with consumption of such examined fish were further calculated and discussed.
Materials and Methods
Collection of samples
Sixty local fish samples were randomly collected from various regions at Damietta governorate, Egypt. The examined fish species consisted of 20 tilapia fish, 20 mullets, 10 bass and 10 shrimp, which individually packed in a clean polyethylene bag marked and stored frozen at approximately - 20°C until transferred to laboratory.
Preparation of samples
According to Association Official Analytical Chemists (AOAC, 1996), fish samples were washed several times with deionized water to clean them from sediments and other adhesive materials. The fish samples were identified scaled, and the heads were removed using a stainless-steel knife, the flesh and other edible portions removed from the bone and entrails. Fifty grams from the dorsal muscle were ground in meat chopper and frozen until analysis.
Analytical procedure
OCPs extraction and analysis were conducted according to AOAC (1996).
Extraction
Fifty grams of the sample +100 g anhydrous sodium sulphate +150 ml petroleum ether were blended for 2 minutes, then the extract was pored through filter funnel into a suction flask. The flask was put in rotary evaporator until complete evaporation and formation of a dry fat film.
Clean up by acetonitrile partitioning
The dry fat film + 15 ml petroleum ether + 30 ml acetonitrile saturated with petroleum ether were transferred into 125 ml separatory funnel. The content was vigorously shaken until formation of two layers. Then the lower layer was drained into 1L separatory funnel containing 650 ml distilled water +40 ml NaCl solution +100 ml petroleum ether and then the content was vigorously shaken till separation of two layers. The upper one was then drained into 250 ml flask through a filter funnel containing sodium sulphate anhydrous and conditioned with petroleum ether. Then, the flask was put in a rotary evaporator until complete dryness.
Clean up by florisil column
The activated florisil was put in a chromatography column to the height of 10 cm then topped with 1 cm anhydrous sodium sulphate and conditioned with 50 ml petroleum ether. Two grams of sodium sulphate anhydrous + 20 ml petroleum ether was added to the obtained dry film, then passed via the prepared florisil column at a rate of 5 ml/min. The column was eluted 3 times at the same rate, with 20 ml of 6, 15 and 50% diethyl ether in petroleum ether, respectively and the eluate was concentrated in a rotary evaporator till obtaining a dry film. 2 ml n-hexane was added to the dry film and transferred into 2 ml auto sampler vial and transported to GC for analysis.
Agilent GC model 6890 with ECD adjusted as follow: The oven temperature was programmed from an initial temperature 160°C (2 min hold) to 280°C at a rate of 5°C /min and was maintained at 280°C for 10 min. Injector and detector temperatures were maintained at 280°C and 320°C, respectively. Nitrogen was used as a carrier at a flow rate of 4 ml /min and an injection volume of 1µl.
Experimental part
To reduce OCPs content in the fish samples, the contaminated fish samples with OCPs were heat treated (microwaving, roasting and boiling) according to Attia et al. (2019), then 50 g of the heat-treated fish muscle were prepared, extracted, and analyzed for their OCPs content as mentioned before. Reduction rates (RR) in OCPs content were calculated according to the following equation:
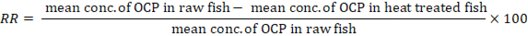
Human health risk assessment
Human health risks associated with the consumption of such fish types were determined through the following parameters:
Estimated daily intake (EDI): The estimated daily dietary intake of OCPs from fish consumption was calculated according to (WHO, 1987) using the following equation: EDI = (C × DR) / BW. Where C is the concentration of the OCPs (mg/kg) in raw fish, DR is the daily consumption of fish (Kg/day) and BW is the average body weight set at 60 kg (WHO, 2010). The estimated daily consumption rate in Egypt was conservatively set at 55.9 g/day per person in Egypt (FAO, 2003).
Carcinogenic risk: Both cancer risk (CR) and hazard ratios (HR) were calculated according to USEPA (2005) from the following equation:
CR= EDI× CSF
Where; CSF was cancer slope factor (mg/kg per day). Its value was 1.6 for HCB, 0.35 for ΣCHL 16 for dieldrin, 9.1 for heptachlor epoxide, 1.3 for lindane and 0.34 for ΣDDT (USEPA, 2000). If the CR is more than 10-6 it was considered ‘acceptable risk’, between 10-6 and 10-4 are considered ‘levels of concern’ and a CR less than than 10-4 is considered Unacceptable risk’ (USEPA, 2005).
The hazard ratios (HR): To evaluate the potential non-carcinogenic health risk, it was assessed by calculating the hazard index (HI) (USEPA, 1991).
HI = EDI / ADI
Where, EDI is the estimated daily intake (mg/ kg bw) and ADI is acceptable daily intake (mg/ kg bw). For a preliminary quantitative risk assessment, a HI ≤ 0.2 is considered as negligible adverse health effects as a result of exposure, while HI values exceeding this threshold require a further detailed risk assessment or risk management measures to be undertaken (Health Canada, 2004). For carcinogenic effects evaluation of the hazard ratio (HR) was calculated using the equation suggested by USEPA (2005).
HR= EDI/ BMC
Where the BMC is the benchmark concentration derived by USEPA (2005).
BMC = (Risk × BW) / (Fish consumption × CSF)
Where; the risk was set at one in a million chances for lifetime exposure, and fish consumption was the fish consumption per day (Kg/d) relative to body weight (kg). An HR greater than one indicated a potential risk to human health.
Statistical analysis
Data obtained from the current study was statistically analyzed by using the Statistical Package for the Social Sciences (SPSS) software. A value of P <0.05 was considered significant (Significant at P < 0.05).
Results and Discussion
Organochlorine pesticides residues in the examined fish species
The data recorded in Table 1 showed that DDT residues were detected in 25%, 60%, 30%, and 40% of the examined mullet, tilapia, bass, and shrimp samples, respectively. Concerning the concentration of DDT, the mean values of DDT (± SE) was 5.1±0.64, 18.3±2.54, 3.17±0.73, and 9.75± 0.52 ppb in mullet, tilapia, bass, and shrimp, respectively, indicated that all values were within Permissible Limit (5000 ppb) according to US-FDA (2008). Nearly similar findings in mullet were obtained by Salem (2003) who revealed that concentration of DDT was 7.46 ppb in mullet; higher findings in mullet were estimated by Morshdy et al. (2018) as 98.11ppb, while lower findings were reported by Kamel et al. (2015) as 0.036±0.009 ppb. Nearly similar findings in tilapia were obtained by El-Nemr and Abd-Allah (2004) as 15.1 ppb. Higher findings were recorded by Abd El-Halim (2020) as 50.62±5.13 ppb. Nearly similar findings in bass were estimated by Yim et al. (2005) as 4.8ppb in samples from Island. Degradation resistance and high lipid solubility of DDT residues and metabolites (DDD and DDE) made them soluble in fats and lipids of animals, therefore, when water was contaminated, fish and other aquatic organisms had the capacity to absorb them from water and concentrate them in their fatty tissues, in addition, DDT is oxidized to DDE in aerobic or oxidation environment and deoxidize to DDD under anaerobic or reducing environment (WHO, 2010).
Table 1: Organochlorine Pesticides residues (µg/kg=ppb) in analyzed fish samples.
| Pesticides | Mullet (Mugil cephalus) n=20 | Tilapia (Oreochromis niloticus) n=20 | Bass n=10 | Shrimp n=10 | Permissible limits (ppb) | ||||||||
| Positive samples | Mean ± SE | Positive samples | Mean ± SE | Positive samples | Mean ± SE | Positive samples | Mean ± SE | ||||||
| No. | % | No. | % | No. | % | No. | % | ||||||
| DDT | 5 | 25 | 5.1± 0.64 | 12 | 60 | 18.3±2.54 | 3 | 30 | 3.17±0.73 | 4 | 40 | 9.75±0.52 | 5000 |
| DDD | 5 | 25 | 52.7±0.97 | 12 | 60 | 80.5± 8.61 | 3 | 30 | 4.17±0.44 | 4 | 40 | 29.63±0.55 | 5000 |
| DDE | 5 | 25 | 7.56±0.75 | 12 | 60 | 46.6±9.77 | N.D | 4 | 40 | 13.3±0.32 | 5000 | ||
| Alderin | 5 | 25 | 89± 0.85 | 14 | 70 | 40.3±3.72 | ND | ND | 300 | ||||
| Dieldrin | 5 | 25 | 36.7± 0.66 | 14 | 70 | 35.75±5.51 | ND | ND | 300 | ||||
| Heptachlor | 7 | 35 | 20±0.59 | 13 | 65 | 8.38±1.62 | ND | 6 | 60 | 17.2±0.56 | 300 | ||
| Heptachlor epoxide | 7 | 35 | 38.9±0.49 | 13 | 65 | 13.1±2.38 | ND | 6 | 60 | 30.4±0.57 | 300 | ||
|
α -HCH |
10 | 50 | 15.3±2.44 | 12 | 60 | 48±10.75 | ND | ND | 200 | ||||
|
γ-HCH |
ND | 3 | 15 | 2.8±.44 | ND | ND | 2000 | ||||||
| Endosulfan | ND | 8 | 40 | 12.8±1.21 | ND | ND | 100 | ||||||
|
γ-chlordane |
ND | 8 | 40 | 79.5±17.37 | ND | ND | 300 | ||||||
Concerning, DDD residues were found in 25%, 60%, 30%, and 40% of the examined mullet, tilapia, bass, and shrimp samples, respectively. The recorded concentrations of DDD were 52.7±0.97, 80.5± 8.61, 4.17±0.44, and 29.63±0.55 ppb, respectively. All recorded values were within the Permissible Limit (5000 ppb) set by FDA (2008). Nearly similar findings were recorded by Morshdy et al. (2018), higher findings were estimated by Hassanen et al. (2016), while lower findings were estimated by Said et al. (2008). Nearly similar findings in bass were recorded by Salem (2003) (9.93 ppb). However, lower values were reported in sea bass by Serrano et al. (2003) (1.9 ppb). El-Nemr and Abd-Allah (2004) recorded nearly similar findings in shrimp.
DDE was detected in 25%, 60%, 40%, of mullet, tilapia, and shrimp samples, respectively, but it was not detected in bass samples. The recorded mean ± SE values of DDE residues were 7.56±0.75, 46.6±9.77, and 13.3±0.32 ppb in mullet, tilapia, and shrimp samples, respectively. All recorded values were within the established Permissible limit according to FDA (2008). Ahmed and El-Gammal (2010), and Gad (2010) reported nearly similar findings. While higher levels were recorded by Mohamed et al. (2016), but lower residual contents were recorded by Marzouk et al. (2016). Pesticides enter the aquatic ecosystem through the run-off from farmland, animal dips and factory effluent into the river Nile then get access into the drainage system (Sherma and Zweig, 1983).
Alderin was detected in 25%, and 70% of the examined mullet and tilapia, respectively with mean values of 89 ± 0.85, and 40.3±3.72 ppb in the two species, respectively. Aldrin was not detected in bass and shrimp tissues. Nearly similar findings in the mullet were estimated by Talab and Ghannam (2015), while lower findings were reported by Kamel et al. (2015) (0.06 ppb). Mohamed et al. (2016) recorded relatively similar aldrin concentrations in tilapia (63.86 ppb).
Similarly, dieldrin was not detected in bass and shrimp. The mean dieldrin residual concentrations in mullet and tilapia were 36.7± 0.66, and 35.75±5.51 ppb, respectively. Nearly similar findings in mullet were estimated by Mohamed et al. (2016) , While lower records were detected by Morshdy (1992) as 0.013 ppb in samples collected from Manzala Lake. Nearly similar findings in tilapia were estimated by Abd El-Halim (2020) as 35.66 ppb in 400-600gm weighted tilapia. Aldrin may convert into Dieldrin by the action of sunlight and bacteria, which is resistant to bacterial and chemical breakdown (Afful et al., 2013). While Endrin residues were not detected in all analyzed samples in this study. Heptachlor was detected in mullet, tilapia, and shrimp samples at 35%. 65%, and 60^, respectively with mean concentrations of 20±0.59, 8.38±1.62, and 17.2±0.56 ppb, respectively. Nearly similar findings were estimated by Mansour et al. (2001), and Morshdy et al. (2018), who detected 14 ppb and 18.55 ppb in fish samples from Qarun Lake and Damietta, respectively. Our results were lower than the results detected by Abd El-Gawad and Abou El-Ella (2014), they reported concentration of 206.6 ppb, while our findings were higher than Abd-Allah et al. (1998) as they reported 0.2 ppb in mullet samples collected from Gamasa markets. On the other hand, higher contents were detected by Gad (2010) as 800 ppb from tilapia from El-Qalubia.
Heptachlor epoxide residues were 38.9±0.49, 13.1±2.38, and 30.4±0.57 ppb in mullet, tilapia, and shrimp samples, respectively with detection rates of 35%, 65%, and 60% in these species, respectively. Nearly similar findings were estimated by Abd El-Halim et al. (2020).
The occurrence of OCPs residues differ significantly among the examined fish species. This could be attributed to the level of environmental contamination, level of exposure among fish species, nature of the pesticide, its solubility, the fish species, and its ability to excrete these compounds, and the lipid content in each fish species (Mwevura et al., 2002; Hassan et al., 2020).
α-HCH residues were detected in 50%, and 60%, of mullet, and tilapia samples, the recorded concentrations were 15.3±2.44, and 48±10.75 ppb, respectively, while it could not be detected in bass and shrimp tissues. Nearly similar findings in mullet were reported by Talab and Ghannam (2015), and Morshdy et al. (2018); lower results were detected by Kamel et al. (2015). γ-HCH residues was detected in 15% of tilapia samples with a mean value of 2.8±0.44 ppb. However, this contaminant was not detected in mullet, bass, and shrimp. β-HCH, and δ-HCH could not be detected in this study. Nearly similar findings in tilapia were estimated by Said et al. (2008) (2.1 ppb) from El Berka eastern part. Higher levels were recorded by Morshdy et al. (2018) (121 ppb). In spite of, endosulfan residues were not detected in mullet, bass, and shrimp tissues, it was detected in 40% of tilapia samples at a concentration of 12.8±1.2 ppb. Nearly similar findings were estimated by Abd El-Halim (2020) in tilapia; higher value was recorded by Marzouk et al. (2016) and lower result was recorded by Abd El-Halim (2020). Endosulfan remained in the environment for longer periods and bioaccumulated in plants and animals, which lead to contamination of food consumed by humans. Also, γ-chlordane residues were not detected in mullet, bass, and shrimp, while found at 40% of tilapia samples by 79.5±17.37 ppb. Nearly similar findings were estimated by Hassanen et al. (2016); lower findings were recorded by AbdEl Halim (2020). Levels of OCPs contaminants could vary significantly within the same fish species depending on the area where the fish was caught, the age and the fat content (Pandelova et al., 2008).
Effect of heat treatment on OCPs content
Experimental work was done to investigate the effect of different cooking methods (microwaving, roasting, and boiling) on the concentration of OCPs in fish flesh. The results illustrated in Table 2, revealed that there were highly significant differences (P<0.01) between different means recovered from different cooking methods. For DDD residues, the reduction percentage was 81.13%, 60.38%, and 79.25% by microwaving, roasting, and boiling respectively. For DDE residues, the reduction percentage were 57.14%, 71.43%. While DDT could not be detected after any cooking method as the reduction percentage was 100%. Heptachlor residues were completely reduced 100% after microwaving and boiling. For heptachlor epoxide residues, the reduction percentage was 94.88% for microwaving, and 79.49% for roasting. Aldrin reduction percentage was 82.02% for microwaving, and 67.42% for roasting. The obtained results were higher than the results reported by Witczak (2009) who found that frying reduced levels of OCP in fish meat by 10-32%. The results detected by Morshdy et al. (2018) demonstrated the reduction percentages of 80%, 75%, 85.5, and 90% on the total DDTs, HCHs, HPTs and drins (aldrin and endrin) respectively. Reduction rate in OCPs varied among the examined fish species and its locality according to Hassanen et al. (2016) who reported that reduction rate in tilapia samples from Manzala lake were 100%, 90%, 11%, 8%, 67%, 67%, 67% for α-BHC, β-BHC, γ-chlordane, HCB, heptachlor, aldrin, DDE, respectively. It is proved that heat treatment is an effective method for the reduction of OCPs in fish meat.
Table 2: Organochlorine Pesticides residues (µg/kg=ppb) in positive fish sample safter different heat treatments (Microwaving, Roasting, and Boiling) Significant at P < 0.01 among different fish species.
| Items | Microwaved samples | Roasted samples | Boiled samples | |||
| Mean ± SE | RR % | Mean ± SE | RR % | Mean ± SE | RR % | |
| DDT | 0* | 100 | 0 | 100 | 0* | 100 |
| DDD | 10.7±1.34* | 81.13 | 22.46±2.82* | 60.38 | 11.76±1.48* | 79.25 |
| DDE | 0* | 100 | 8.86±1.95* | 57.14 | 13.27±2.92* | 71.43 |
| Alderin | 9.55±1.03* | 82.02 | 17.3±1.87* | 67.42 | 0* | 100 |
| Dieldrin | 0* | 100 | 0* | 100 | 0* | 100 |
| Heptachlor | 0* | 100 | 1.84±0.18* | 86.36 | 0* | 100 |
| Heptachlor epoxide | 1.23±0.13* | 94.88 | 4.93±0.52* | 79.49 | 0* | 100 |
|
α –HCH |
0* | 100 | 1.36±0.28* | 95.83 | 0* | 100 |
|
γ-HCH |
0* | 100 | 0* | 100 | 0* | 100 |
| Endosulfan | 2.3±0.22* | 82.02 | 4.2±0.4* | 67.42 | 0* | 100 |
|
γ-chlordane |
14.31±3.13* | 82.02 | 25.92±5.66* | 67.42 | 0* | 100 |
R.R= reduction rate of OCP; RR%= reduction rate of OCP. * There were highly significant differences (P<0.01) between different means.
Table 3: Estimated daily intake (EDI) and Carcinogenic risk (CR) and Human risk (HR) of Organochlorine pesticides in fish samples.
| OCPS | ADI | CSF |
EDI |
CR |
HR |
|||||||||
| Mullet | Tilapia | Bass | Shrimp | Mullet | Tilapia | Bass | Shrimp | Mullet | Tilapia | Bass | Shrimp | |||
Total DDT |
10000 | 0.34 | 6.09E-05 | 1.35E-04 | 6.8 3E- 06 |
4.90E-05 | 2.07E-05 | 4.59E-05 | 2.32E-06 | 1.67E-05 | 1.93E-08 | 4.28E-08 | 2.1 6E- 02 |
1.55E-01 |
| Dieldrin | 10000 | 16 | 3.42E-05 | 3.33E-05 | ND | ND | 5.47E-04 | 5.33E-04 | ND | ND | 5.10E-07 | 4.96E-07 | ND | ND |
|
γ chlordane |
500 | 0.35 | ND | 7.41E-05 | ND | ND | --- | 2.59E-05 | ND | ND | ND | 2.42E-08 | ND | ND |
|
α –HCH |
100 | 1.6 | 1.43E-05 | 4.47E-05 | ND | ND | 2.29E-05 | 7.15E-05 | ND | ND | 2.13E-08 | 6.66E-08 | ND | ND |
Hept achlor epoxide |
5000 | 9.1 | 3.62E-05 | 1.22E-05 | ND | 2.83E-05 | 3.29E-04 | 1.11E-04 | ND | 2.58E-04 | 3.07E-07 | 1.03E-07 | ND | 3.34E-03 |
|
Total heptachlor |
100 | 4.5 | 1.86E-05 | 7.81E-06 | ND | 1.60E-05 | 8.37E-05 | 3.51E-05 | ND | 7.20E-05 | 7.80E-08 | 3.27E-08 | ND | 3.82E-03 |
ADI: accepted daily intake (ng/kg bw/day) according to WHO (2010); CSF: cancer slope factor according to USEPA (2000); EDI: estimated daily intake (mg/kg bw/day); CR: carcinogenic risk; HR: human risk; ND: not detected.
Public health hazard from OCP residues in fish samples
Regarding OCPs hazards on humans, they cause, restlessness, dizziness, trouble breathing, headache, vomiting, itching and skin irritation, in addition to neurotoxicity, cancer and death. A strong link existed between DDTs and breast adenocarcinoma, reproductive disorders, and fetal abnormalities. Moreover, type-2 diabetes was firmly associated with primarily exposure to OCPs. β-HCH, HCB and DDT residues impaired the thyroid hormone levels in the newborn, which can move from mothers to babies by placenta, while endosulfan causing adverse effects on the immune system, on the production of semen, on the sperm count, sperm morphology and on other male sex hormone defects (Afful et al., 2010).
Human health risk from consumption of fish tissue
The ADI, EDI, HR, and CR were estimated for each fish species. Data obtained in Table 3 showed that the hazard ratio of all studied OCPs in the examined raw fish species was less than 1 that ensure consumption of these fish has no potential hazards to human health. The calculated carcinogenic risks for the examined raw fish species represented a level of concern (between 10-6 and 10-4) for total DDTs, dieldrin, chlordane, α-HCH, heptachlor epoxide, and total heptachlor. While recorded levels in bass samples were considered acceptable risk (more than 10-6). Similarly, HR of OCPs due to consumption of tilapia in Ethiopia was less than one and did not pose ant risks for Ethiopian adults (Deribe et al., 2014). However, consumption of Mozambique tilapia and African sharp tooth catfish in South Africa indicated potential dietary risk associated with exposure to heptachlor, heptachlor epoxide and dieldrin (Buah-Kwofie et al., 2018). In Egypt, risk assessment due to consumption of tilapia from Edko lake in Egypt revealed that dieldrin had a health risk index of more than one, which indicates a health risk to adults associated with the consumption of fish. However, heptachlorepoxide, p,p-DDE, p,p-DDD, and endrin did not show any direct health risk (Abbasy et al., 2021).
Conclusions and Recommendations
From the current study, it could be concluded that most of the examined fish samples contained OCPs levels within the established maximum permissible limits. However, future studies should be conducted with a larger sample size and more fish species to make more accurate image about the occurrence of OCPs in the study area. The use of pesticides in catching fishes should be forbidden. People should only eat the fillet of the fish. Throw away the fatty parts such as the head, fat, and skin. Efficient heat treatment of fish is of value in reducing their OCPs content.
Acknowledgements
This work was supported by Department of Meat Hygiene and Control, Faculty of Veterinary Medicine, Suez Canal University, Egypt; Food Safety, Reference Laboratory, Animal Health Research Institute, (Dokki, Egypt) and Central Laboratory at Faculty of Veterinary Medicine, Benha University, Egypt.
Novelty Statement
Organochlorine pesticides were found to posses multiple health risks and their presence in high amounts in fish has become a major environmental and health concern.
Author’s Contribution
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbassy MA, Khalifa MA, Nassar AMK, El-Deen EEN, Salim YM (2021). Analysis of organochlorine pesticides residues in fish from Edko Lake (North of Egypt) using eco-friendly method and their health implications for humans. Toxicol. Res., 37(4): 495-503. https://doi.org/10.1007/s43188-020-00085-8
Abd-Allah AM, Ali HA, El-Sebae A (1998). Level of chlorinated hydrocarbons in a teleost fish and a bivalve from the Egyptian Mediterranean Coast and Nile Estuary. Z. Lebensm Unters Forsch A., 206: 25-28. https://doi.org/10.1007/s002170050207
Abd-El-Halim SRK (2020). Some chemical residues and their potential health hazards in tilapia fish. Ph. D.V.M. Sc. thesis (Meat Hygiene), Fac. Vet. Med., Benha Univ., Egypt.
Afful S, Anim AK, Serfor-Armah Y (2010). Spectrum of organochlorine pesticide residues in fish samples from the Densu basin. Res. J. Environ. Earth Sci., 2(3): 133-138. https://doi.org/10.5539/ijc.v2n2p234
Afful S, Awudza JAM, Osae S, Twumasi SK (2013). Persistent organochlorine compounds in the water and sediment samples from Lake Bosomtwe in Ghana. Am. Chem. Sci. J., 3(4): 434-448. https://doi.org/10.9734/ACSJ/2013/3913
Ahmed NS, El-Gammal OM (2010). Effect of heat treatment on the nutritive value and residues of some synthetic pesticides in fresh bolti fish. Arab Universities. J. Agric. Sci., 18(2): 329-334. https://doi.org/10.21608/ajs.2010.14900
Atia AS, Darwish WS, Zaki MS (2018). Monitoring of heavy metal residues, metal-metal interactions, and the effect of cooking on the metal load in shellfish. J. Anim. Plant Sci., 28(3): 732-743.
Buah-Kwofie A, Humphries MS, Pillay L (2018). Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso Wetland Park, South Africa. Sci. Total Environ., 621: 273-281. https://doi.org/10.1016/j.scitotenv.2017.11.212
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Skipperud L, Eklo OM (2014). Organochlorine pesticides and polychlorinated biphenyls in fish from Lake Awassa in the Ethiopian Rift Valley: human health risks. Bull. Environ. Contam. Toxicol., 93(2): 238-244. https://doi.org/10.1007/s00128-014-1314-6
El-Nemr A, Abd-Allah AM (2004). Organochlorine contamination in some marketable fish in Egypt. Chemosphere, 54: 1401-1406. https://doi.org/10.1016/j.chemosphere.2003.10.041
FDA (Food and Drug Administration) (2008). U.S. Department of Health and Human Services, Pesticides chemical residues in food enforcement criteria (CPG 7141.01). Draft Guidance, January 2008.
Gad NS (2010). Organochlorine pesticides and trace metals contamination in some marketable fish in Egypt. Egypt. J. Aquat. Res., 36(4): 633-642.
Hassan MAE, Ahmed AM, Marzouk NM, Maky MA (2020). Detection of organochlorine pesticides residues in Nile fish and its risks in Qena city. SVU- Int. J. Vet. Sci., 3(1): 51-65. https://doi.org/10.21608/svu.2020.19713.1036
Hassanen FS, Kamel EA, Gaafar AM, Shaheen AA (2016). Effect of grilling on pesticides residues in O. niloticus muscles. Benha Vet. Med. J., 30(1): 834‐357. https://doi.org/10.21608/bvmj.2016.31405
Kamel E, Moussa S, Abonorag M, Konuk M (2015). Occurrence and possible fate of organochlorine pesticide residues at Manzala Lake in Egypt as a model study. Environ. Monitor. Assess., 187: 4161-4171. https://doi.org/10.1007/s10661-014-4161-3
Lah K (2011). Effects of pesticides on human health. In: Toxipedia. http://www.toxipedia.org/display/toxipedia/Effects+of+Pesticides+on+ uman+Health. Accessed Jan 16, 2022.
Louis AH, Weigmann DL, Hipkins P, Stinson ER (2009). Pesticides and aquatic animals: A guide to reducing impacts on aquatic systems, Virginia State University www.ext.vt.edu, Publication May 1st., 420: 1-24.
Marzouk NM, Shoukry HM, Ali H, Naser GA, Fayed AM (2016). Detection of harmful residues in some fish species Egypt. J. Chem. Environ. Health, 2(2): 363-381.
Mohamed AA, Tharwat AE, Khalifa HA (2016). Estimation of organochlorine pesticides and heavy metal residues in two species of mostly consumed fish in Sharkia Governorate, Egypt. Jpn. J. Vet. Res., 64(2): 233-241.
Morshdy AE (1992). Pesticide evaluation of fishes of Manzala Lake. Zagazig Vet. J., 20: 705-708.
Morshdy AE, Darwish WS, Daoud JR, Hussein MA, Sebak MA (2018). Monitoring of organochlorine pesticide residues in Oreochromis niloticus collected from some localities in Egypt. Slov. Vet. Res., 55(20): 303-311.
Morshdy AE, Hafez AE, Darwish WS, Hussein MA, Tharwat AE (2013). Heavy metal residues in canned fishes in Egypt. Jpn. J. Vet. Res. 61(Suppl): S54-47.
Mwevura H, Othman OC, Mehe GL (2002). Organochlorine pesticide residues in edible biota from the coastal area of Dares Salaam city. J. Mar. Sci., 1: 91-96. https://doi.org/10.1016/S0025-326X(01)00331-9
Pandelova M, Henkelmann B, Roots O, Simm M, Schramm KW (2008). Levels of PCDD/F and dioxin-like PCB in Baltic fish of different age and gender. Chemosphere, 71: 369-378. https://doi.org/10.1016/j.chemosphere.2007.08.050
Polder A, Muller MB, Lyche JL, Lie E (2014). Levels and patterns of persistent organic pollutants (POPs) in tilapia (Oreochromis sp.) from four different lakes in Tanzania: Geographical differences and implications for human health. Sci. Total Environ., 488(489): 252-260. https://doi.org/10.1016/j.scitotenv.2014.04.085
Saber TM, Khedr MH, Darwish WS (2018). Residual levels of organochlorine pesticides and heavy metals in shellfish from Egypt with assessment of health risks. Slov. Vet. Res., 55(2): 101-113. https://doi.org/10.26873/SVR-453-2017
Said TO, El Moselhy KM, Rashad AA, Shreadah MA (2008). Organochlorine contaminants in water, sediment and fish of Lake Burullus, Egyptian Mediterranean Sea. Bull. Environ. Contam. Toxicol., 81: 136-146. https://doi.org/10.1007/s00128-008-9422-9
Salem DA (2003). Survey of some environmental pollutants in freshwater fishes in Assiut Governorate, Egypt. Ass. Univ. Bull. Environ. Res., 6(2): 15-28. https://doi.org/10.21608/auber.2003.150959
Serrano R, Juliaan A, Hernandez F (2003). Biomagnification Study on organochlorine compounds in marine aquaculture: The sea bass (Dicentrarchus labrax) as a Model. Environ. Sci. Technol., 37: 3375-3381. https://doi.org/10.1021/es020229+
Sherma J, Zweig G (1983). Pesticides. Am. Chem. Soc., 55: 57-70. https://doi.org/10.1021/ac00256a005
Thompson LA, Darwish WS (2019). Environmental chemical contaminants in food: Review of a global problem. J. Toxicol., 2019: 2345283. https://doi.org/10.1155/2019/2345283
Thompson LA, Darwish WS, Ikenaka Y, Nakayama SM, Mizukawa H, Ishizuka M (2017). Organochlorine pesticide contamination of foods in Africa: incidence and public health significance. J. Vet. Med. Sci., 79(4): 751-764. https://doi.org/10.1292/jvms.16-0214
USEPA (United States Environmental Protection Agency) (2000). Guidance for assessing chemical contaminant data for use in fish advisories volume 2, risk assessment and fish consumption limits third edition.
USEPA United States Environmental Protection Agency (2005). Guidelines for carcinogen risk assessment. Risk Assessment Forum U.S. Environmental Protection Agency Washington, DC. EPA/630/P-03/001F. March 2005.
US-FDA (U.S. Department of Health and Human Services, Food and Drug Administration) (2008). Pesticide chemical residues in food enforcement criteria (CPG 7141.01). Draft Guidance, January 2008.
WHO (World Health Organization) (1987). Evaluation of certain food additives and contaminants (Technical Report No. 751). Cambridge University Press.
WHO (World Health Organization) (2010). Inventory of IPCS and other WHO pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPRs).
Witczak A (2009). Effect of heat treatment on organochlorine pesticide residues in selected fish species. Polish J. Food Nutr. Sci., 59(3): 231-235.
Woodwell GM, Craig PP, Johnson HA (1971). DDT in the biosphere: where does it go? Science, 174: 1101-1107. https://doi.org/10.1126/science.174.4014.1101
Yim UH, Hong SH, Shim WJ, Oh JR (2005). Levels of persistent organochlorine contaminants in fish from Korea and their potential health risk. Arch. Environ. Contam. Toxicol., 48: 358-366. https://doi.org/10.1007/s00244-004-0085-1
To share on other social networks, click on any share button. What are these?






