New-HPLC-Method-for-Determination-of-Cefquinome
Ijaz Ahmad1,5*, Haihong Hao1, Pascal Sanders2, Zafar Iqbal3, Saeed Ahmed4, Farhan Anwar Khan5, Zahir Shah5, Muhammad Ibrahim6 and Lingli Huang1
1National Reference Laboratory of Veterinary Drug Residues (HZAU) and MAO Key Laboratory for Detection of Veterinary Drug Residues, Huazhong Agriculture University Wuhan, China.
2Laboratory of Fougères, French Agency for Food, Environmental and Occupational Safety, Maisons-Alfort, France.
3Department of Agricultural Chemistry, The University of Agriculture, Peshawar, Pakistan
4Department of Biological Sciences, National University of Medical Sciences, Islamabad, Pakistan
5College of Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan
6Animal Biotechnology Division, Institute of Biotechnology and Genetic Engineering, The University of Agriculture, Peshawar, Pakistan
Ijaz Ahmad and Haihong Hao contribute equally to this work.
* Corresponding author: drijaz@aup.edu.pk
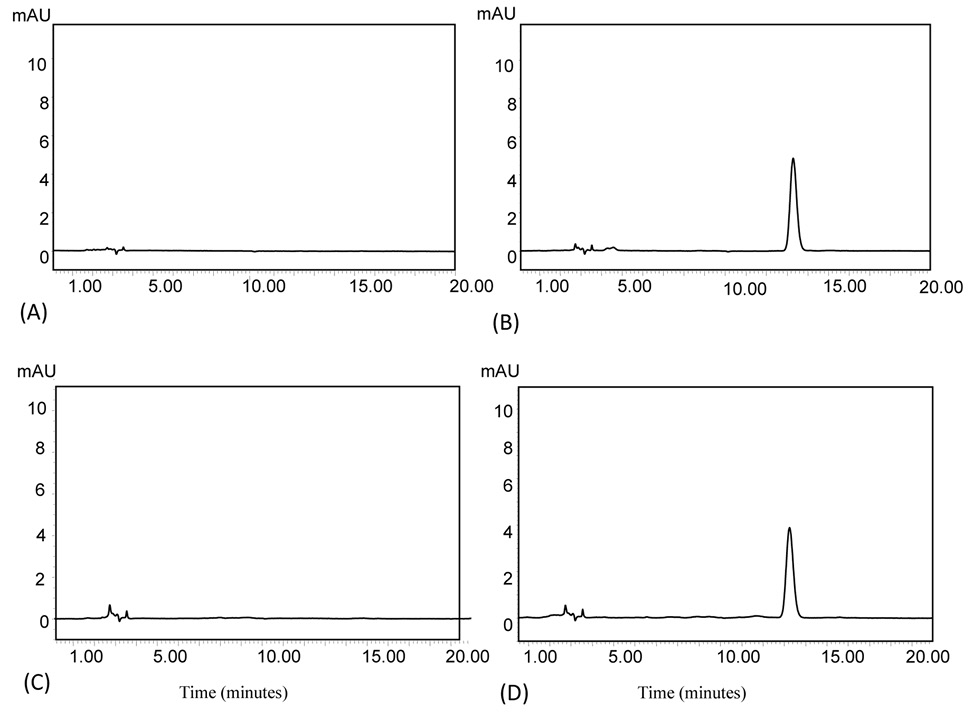
Fig. 1.
Chromatograms of blank standard (A), 1µg/ml of cefquinome standard solution (B), blank plasma (C) and 1µg/ml of cefquinome in plasma (D).
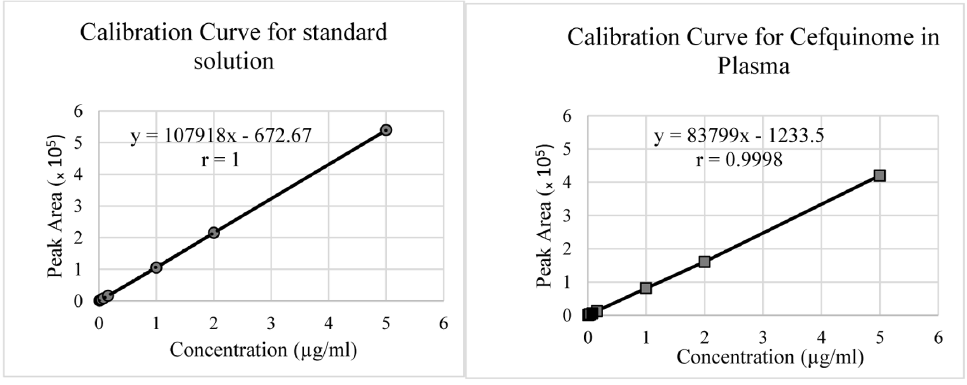
Fig. 2.
Calibration curve for cefquinome is standard (A) and for plasma (B).
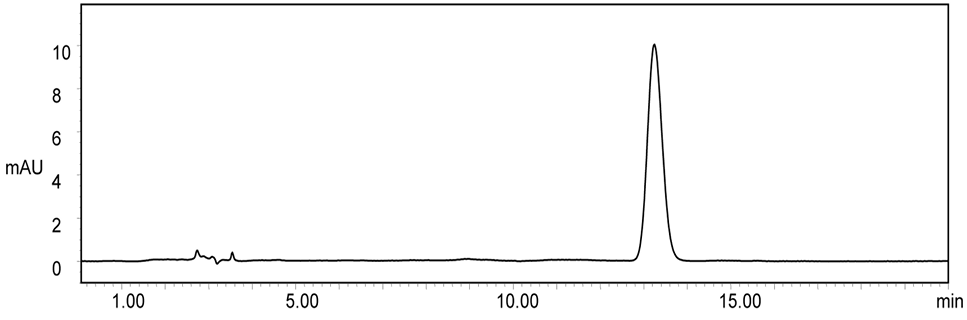
Fig. 3.
Chromatogram of cefquinome in cattle plasma after 1.5 h of intramuscular drug administration of 1.25mg/kg.
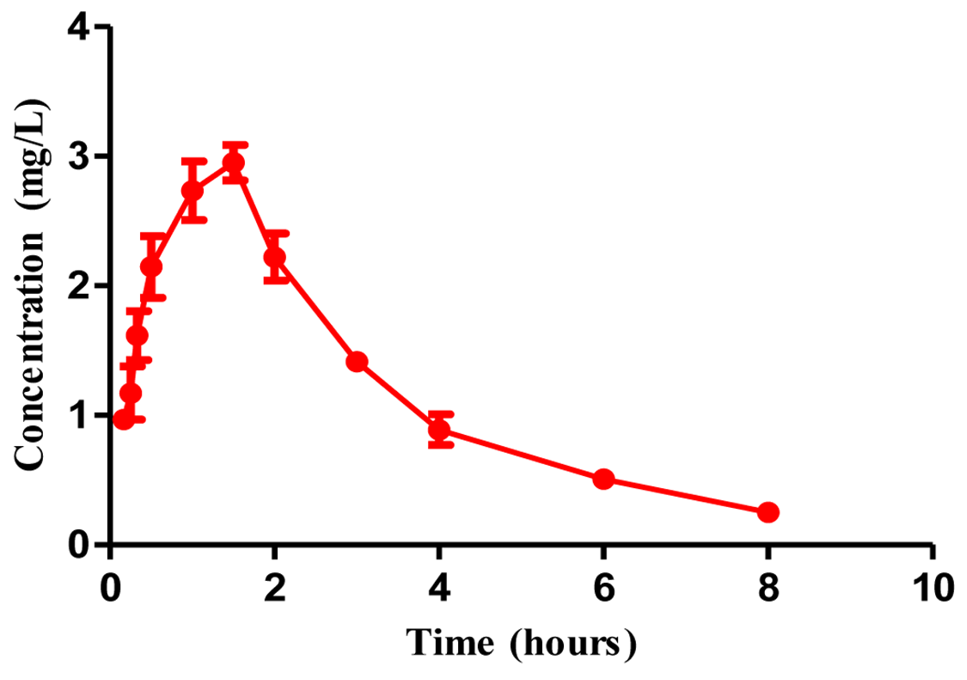
Fig. 4.
Plasma concentration of cefquinome (Mean±SD)versus time curve after I/M administration (1.25mg/kg) in cattle (n=4).