Nematoxic Effect of (Polar and Non-Polar) Chemical Constituents of Cumin (Cyminum Spp.) Against Meloidogyne javanica
Nematoxic Effect of (Polar and Non-Polar) Chemical Constituents of Cumin (Cyminum Spp.) Against Meloidogyne javanica
Saima Mehar1, Amtul Sami2 and Erum Iqbal3*
1Sardar Bahadur Khan Women University, Chemistry Department, Quetta, Balochistan, Pakistan; 2Deptartment of Health Biotechnology, Women University Swabi, Khyber Pakhtunkhwa, Pakistan; 3National Nematological Research Centre, University of Karachi, Karachi, Pakistan.
Abstract | The nematoxic effect was determined for the extracts of Cyminum spp. (Cuminum cyminum and Nigella sativa) in methanol, chloroform, ether, butanol, and hexane on egg hatching and mortality of the juveniles of root-knot nematode Meloidogyne javanica. After 72 hours, C. cyminum (MeOH) demonstrated the maximum nematicidal activity in egg hatching (15%±2.10) of M. javanica, followed by N. sativa (Ether), 22.33±6.86%, at the lowest administered dose of 0.25 % concentration. After 72 hours of treatment, C. cyminum (MeOH) and N. sativa (BeOH) had a non-significant difference in nematotoxic impact (90.0±10.3, 88.0±7.2 mortality %, respectively) in 1% concentration. According to decreasing order, MeOH>BuOH>Chloroform>ether >Hexane, all fractionates showed positive reactivity and potential.
Received | December 27, 2021; Accepted | April 18, 2023; Published | May 04, 2023
*Correspondence | Erum Iqbal, National Nematological Research Centre, University of Karachi, Karachi, Pakistan; Email: erum_i@yahoo.com
Citation | Mehar, S., A. Sami and E. Iqbal. 2023. Nematoxic effect of (polar and non-polar) chemical constituents of cumin (Cyminum Spp.) against Meloidogyne javanica. Pakistan Journal of Nematology, 41(1): 24-30.
DOI | https://dx.doi.org/10.17582/journal.pjn/2023/41.1.24.40
Keywords | Nematicidal potential, Root-knot nematode, Cumin seeds, Cuminum cyminum, Nigella sativa
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Root-knot nematodes (RNK) belongs to the genus Meloidogyne and are important crop pests because they infect a wide range of plant types. It is an endo-parasite that resides in the root system’s secondary roots. This pest is distinguished by its formation of enormous cells, often known as gall cells. RNK has the potential to develop over 2,000 different plant species (Sasser and Freckman, 1987). They are responsible for half of the total damage to plants caused by all types of nematodes (Sasser, 1980). Several extracts of spices, including Eucalyptus spp., Aegiceras corniculatum, Avicennia marina, Ceriopstagal, and Rhizophora mucronata have been reported to kill these nematodes (Mehdi and Dawar, 2008; Samreen et al., 2009).
Spices are dried fruits, seeds, bark, roots, and other vegetative material that are used as a food additive in nutritionally insignificant amounts to enhance the flavour of food by eradicating or inhibiting the spread of dangerous bacteria (Burkill, 1985). Cumin seeds come from the herb Cuminum cyminum, a member of the Apiaceae family that includes parsley and is native to the South Asia and East Mediterranean. Cumin seeds are yellow–grey in colour and oblong in shape. Black seed (also known as black cumin; Nigella sativa) is from Ranunculaceae family, annual flowering plant native to Southern Europe, North Africa, and Southwest Asia. Cumin seeds are nutrient-dense, containing a high amount of fat (especially monounsaturated fat), dietary fibre, and protein. Cumin seeds are abundant in B and E vitamins, as well as a range of other nutrients, including iron. Cuminaldehyde, cymene, and terpenoids are the primary volatile components of cumin (Bettaieb et al., 2011). Commercial insecticides were found less efficient than essential oil from cumin and it was proposed in innovative green formulations in crop protection against E. fetida and H. axyridis (Dawar et al., 2007). Cumin cyminum is used to keep harmful bacteria at bay by either inhibiting their growth or killing them (Burkill, 1985). Pmentha-1, 4-dien-7-al, gamma-terpinene, cumin aldehyde, and beta-pinene are all found in C. cyminum essential oil (Lacobellis et al., 2005). Cumin’s biological activities have been related to the active components, such as terpenes, phenols, and flavonoids, and their concentration and activity (Burkill, 1985; Bettaieb et al., 2011).
Materials and Methods
Collection of plants material
Cumin seeds were purchased from open market of Quetta city. Collected seeds were washed with distilled water and shade dried for two week. The step was followed by crushing and grinding of sample to convert into fine powderand stored in airtight bottles.
Preparation of extracts
To make the ethanol extract, 100 ml of methanol was used to extract 10g of dried powder on a rotary shaker at 190-220 rpm for 24 hours. The methanol extracts were fractionated on the basis of polarity of various solvents such as Methanol, Butanol, Chloroform, Hexane, and, Ether and then concentrated in a rotary evaporator and dried in a vacuum oven at 45 °C, using 200 g of seeds per extract.
It was then passed through five layers of muslin fabric and centrifuged at 5000 g for 15 minutes. The solvent was evaporated, and the supernatant was collected, resulting in a final volume that was one-fourth that of the original (Parekh et al., 2005). For future investigation, it was maintained in sealed vials at 6ºC. To make 1 percent, 0.50 percent, and 0.25 percent concentrations, a suitable amount of extract was dissolved in DMSO (Faizi et al., 2011).
Nematode culture preparation
Roots of infected root-knot nematodes plants were obtained in Balochistan’s Quetta/Jaffarabad city. The perenialpattern that Taylor and Netscher (1974) described was used to identify root-knot nematodes. The isolated root-knot nematodes were treated to single egg-mass culture preparation. Using a 3 %NaOCl solution, RKN eggs were extracted from infected plants. With minor modifications, the acquired egg samples were preserved according to the procedure given in McClure et al. (1973). The eggs suspension was placed onto cotton-wool filter paper and kept at 28±2°C for 48 hours to yield freshly hatched juveniles (J2).
Egg hatching test
Two ml of cumin seed extract was poured into glass cavity blocks (diameter 2.5 cm) after which two egg-masses of M. javanica were placed to evaluate the influence of different cumin seed extracts for egg hatching activity of M. javanica. The control group consisted of egg-masses kept in distilled water. Each treatment was put thrice to the bioassay. A stereomicroscope at (4X) magnification was used to count the number of hatched juveniles after 72 hours of exposure. The treatments were done in triplicate on the glass cavity blocks, which were randomized at room temperature (28ºC). The toxicity of cumin seeds extract was determined using the average percentage of hatching eggs.
Mortality test
To measure nematicidal activity, different extracts of cumin seeds were made at the concentrations of 1%, 0.50% and 0.25%, then placed on 2.5 cm diam. glass slides, and permitted to evaporate the organic solvent for 48 hours. To determine juvenile mortality after 24 hours, 48 and 72 hours, 2ml of the juvenile suspension (100 juveniles/ml) was put to each glass slide and kept at room temperature (28º C). Each treatment was tested three times. The negative control was only water whereas carbofuran served as positive control. The number of deceased juveniles was counted under a low power stereomicroscope after 72 hours of exposure. Toxicity of different extracts of cumin spp. was determined by calculating the average proportion of dead nematodes. If nematodes did not move when probed with a fine needle, they were declared dead.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) for which Least Significance Difference (LSD) and Duncan’s Multiple Range Test (DMRT) (Sokal and Rohlf, 1995) were carried out.
Reversible effects were not expected. Mortality percent was calculated by the following formula (Cayrol et al., 1989).
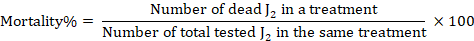
Calculating lethal concentrations and toxicity index for M. javanica J2, the following formula to calculate the toxicity index of each plant extract was used (Sun, 1950).
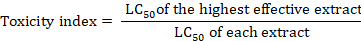
Inhibition rates were calculated according to the following formula:
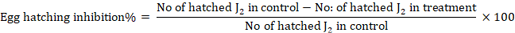
Graphs were plotted from the reading of egg hatching inhibition % using Microsoft excel.
Result and Discussion
Cumin seeds have potential source of phytochemicals as nematoxins (Samreen et al., 2009). This information has been re-examining by using two species of cumin against root-knot nematodes. Their fractionates of varied concentrations in polar and non-polar solvents at different time intervals, were tested against root-knot nematode in this study.
Data obtained from the experiment had shown lowest egg hatching and also capable of causing significant mortality of M. javanica juveniles by C. cyminumas as compared to Nigella sativa. Among all the examined fractionates of both cumin spp., C.cyminum (MeOH) showed maximum nematicidal activity by obtaining (85.00%±12.10) egg hatching inhibition% of M. javanica eggs followed by N. sativa (Ether), 78.3%±12.23 at lowest applied dose i.e 0.25% concentrations after 72 hours (Table 1). Similarly mortality test was also significantly high for C. cyminum (MeOH) as compared to Nigella sativa (MeOH). Aqueous extract of C. cyminum had the best nematicidal action against M. javanica eggs, entirely reduced eggs at 100% w/v (Samreen et al., 2009). Other spices, such as black pepper, were found to control M. javanica hatching and mortality. Parmer et al. (1997), identified Pipernonaline, a piperolein alkaloid. The presence of 18 components in the acetone extract of pepper contributed for 75.59 % of the total amount. The main chemicals were piperine (33.53 %), piperolein B (13.73 %), piperamide (3.43 %), and guineensine (3.23 %). The volatile oil in black seed (Nigella sativa), oil contains about 0.5-1.5 percent nigellone and thymochinone, which have anti-histamine, anti-oxidant, and anti-infective properties. Present observation showed that C.cyminum (MeOH) and N. sativa (BuOH) have non-significant difference and maximum nematoxic effect (90.0±10.3, 88.0±7.2 mortality percentage, respectively) in 1% concentration after 72 hours of treatment. Damasius et al. (2007) calculated the antioxidant activity of ethanolic and aqueous cumin extracts and it was shown that the aqueous extract had comparatively higher DPPH activity than the ethanolic extract which is consistent with the findings of our investigation. Previous researches have shown similar results, indicating that cumin’s phenolic components play a key role in its nematicidal capability.
M. javanica was likewise killed by ethyl acetate and hexane fractions at varied concentrations, according to Siddiqui et al. (2000). Similarly, Mehdi et al. (2001) on Avicennia marina and R. mucronata and Tariq et al. (2007) on Rhizophora mucronata found that water, methanol, and chloroform extracts killed M. javanica significantly.
Aqueous extracts of Z. officinale and C. carvi displayed the most nematicidal activity against M. javanica eggs, while an ethanol extract of F. vulgare at 1000 ppm killed the most juveniles. At 1000 l/liter concentrations, the essential oils of F. vulgare, C. carvi and menthe rout displayed the highest nematicidal activity, according to Oka et al. (2000). Phellandrene and 78 % eugenol are found in this essential oil, which are similar to a cinnamon leaf. Spices with nematicidal activity showed promise in the control of root-knot nematode (M. javanica). The interesting finding of the present study was that the extracts in ethanol, chloroform, hexane, butanol and ether had different levels of nematoxic effect (Figure 1) Least activity was observed in hexane fraction of both type of seed C.cyminum and N. sativaat 1% after 72 hours
(55.0±5.98, 45±9.96 mortality %), respectively. The essential oil of C. cyminum included various quantities of monoterpenes and terpenes. The oil contained 23.6 percent hydrocarbons and 75.6 percent oxygenated monoterpenes (HMs, OMs), respectively (Johri, 2011). In comparison to the polar chemical elements of cumin spp., these non-polar molecules exhibited less promise. All other fraction also showed positive response but significance potential was in extract obtained from methanol. The positive potential according to decreasing order is mentioned below MeOH>BuOH> ether ≥Chloroform> Hexane.
Plants have a variety of secondary metabolites that might improve their efficiency. Phenolic molecules are one of them. Terpenes, monoterpenes, and sesquiterpenes are some of the other phytochemical elements (Skendi et al., 2017). Compounds containing sugars per aglycone are found in a range of point conjugated forms, resulting in a wide variety of phenolic forms (Martinez et al., 2015; Pimpao et al., 2013). Phenolic compounds such as carvacrol, thymolhave been reported in many studies as being responsible for antimicrobial properties (Tajkarimia et al., 2010; Burt, 2004). Such as cinnamic acid, catechol, caffeic acid, quinones have potential antimicrobial effects (Cowan, 1999). Flavonoids reported to have antimicrobial activity, form complexes with cell wall proteins (Cowan, 1999). Cumin polar fraction has been reported flavonoids, saponin, glucosinolatesand thiosulfinates showed antimicrobial effect. Numerous investigations on the antioxidant activity (Burits and Bucar, 2000), antibacterial activity (Hanafy and Hatem, 1991), antihyperglycemic impact, anticancer effect (Salomi et al., 1992), and antinociceptive action of the essential oil of black cumin and thymoquinone have been undertaken (Abdel-Fattah and Kinzo, 2000; Shivakumar et al., 2010). Cumin is a powerful antioxidant that suppresses radical-mediated lipid peroxidation by scavenging peroxy, hydroxy, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals (Manjree et al., 2001). Cumin aldehyde, cuminal, -pinene, -terpinene, and safranal, which are found in essential oils, are responsible for antioxidant action (Surveswaran et al., 2007). Pinocarveol, identified in the volatile cumin oil by El-Ghorab et al. (2010), may have antioxidant properties. Bettaieb et al. (2011) discovered terpinene, terpinene, and bornyl acetate as antioxidant components in cumin oils from stems, roots, leaves, and flowers, respectively. The nematode control potential of C. cyminum and N. sativa were found to be promising in the control of root-knot nematode (M. javanica). The results showed that plant has significant potential and thus can be a milestone in pesticide synthesis to protect plant crop.
Conclusions and Recommendations
It has been determined that the extracts of Cyminum spp. (Cuminum cyminum and Nigella sativa) in different polar and non-polar solvents such as methanol, chloroform, ether, butanol, and hexane has shown promising results to inhibit egg hatching and mortality of the juveniles of root-knot nematode Meloidogyne javanica. The findings demonstrated that plants have tremendous potential and can thus mark a turning point in the development of pesticides to safeguard plant crops.
Novelty Statement
The present research is the first time investigation of polar and non-polar chemical constituents of Cumin spp. against Meloidogyne javanica.
Author’s Contribution
S.M. did the extraction of compounds and drafted the manuscript, A.S. did statistical analysis and reviewed the manuscript; E.I. did egg hatching test and mortality test.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdel-Fattah, M., and Kinzo, M., 2000. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur. J. Pharmacol., 400: 89–97. https://doi.org/10.1016/S0014-2999(00)00340-X
Bettaieb, I., Bourgou, S., Sriti, J., Msaada, K., Limam, F., and Marzouk, B., 2011. Essential oils and fatty acids composition of Tunisian and Indian cumin (Cuminum cyminum L.) seeds: A comparative study. J. Sci. Food Agric., 91: 2100–2107. https://doi.org/10.1002/jsfa.4513
Burits, M. and Bucar, F., 2000. Antioxidant activity of Nigella sativa essential oil. Phytother. Res., 14: 323-328. https://doi.org/10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q
Burkill, H.M., 1985. The useful plants of west tropical Africa. Vol. 5. Royal Botanical Garden, pp. 2445.
Burt, S., 2004. Essential oils: Their antibacterial properties and potential applications in foods. A review. Int. J. Food Microbiol., 94(3): 223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Caryrol, J.C., Djian, C. and Pijarowski, I., 1989. Studies on the nematicidal properties of the culture filtrate of the nematophagous fungus Paecilomyceslilacinus. Rev. Nematol., 12: 331-336.
Cowan, M.M., 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev., 12(4): 564-582. https://doi.org/10.1128/CMR.12.4.564
Damasius, J., Skemaite, M., Kirkilaite, G., Vinauskiene, R., and Venskutonis, P.R., 2007. Antioxidant and antimicrobial properties of caraway (Carumcarvi L.) and Cumin (Cuminumcyminum L.) extracts. Vet. IrZootech., 40: 62.
Dawar, S., Younus, S.M., Tariq, M. and Zaki, M.J., 2007. Use of Eucalyptus sp., in the control of root infecting fungi on mung bean and chick-pea. Pak. J. Bot., 39(3): 975-979.
De, M., Krishna, De A. and Banerjee, A.B., 1999. Antimicrobial screening of some Indian spices. Phytother. Res., 13: 616-618. https://doi.org/10.1002/(SICI)1099-1573(199911)13:7<616::AID-PTR475>3.0.CO;2-V
El-Ghorab, A.H., Nauman, M., Anjum, F.M., Hussain, S. and Nadeem, M., 2010. A. comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminumcyminum). J. Agric. Food Chem. 58: 8231-8237. https://doi.org/10.1021/jf101202x
Faizi, S., Shahina, F., Samina, B., Erum, I., Lubna, Humaira, S., and Aneela, N., 2011. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: structure-activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem., 59(17): 9080-9093. https://doi.org/10.1021/jf201611b
Hanafy, M.S.M. and Hatem, M.E., 1991. Studies on the antimicrobial activity of Nigella sativa seed (black cumin). J. Ethnopharmacol., 34(2): 275-278. https://doi.org/10.1016/0378-8741(91)90047-H
Johri, R.K. 2011. Cuminum cyminum and Carum carvi: An update. Pharmacognosy Review. 5(9): 63-72. doi: 10.4103/0973-7847.79101. PMID: 22096320; PMCID: PMC3210012.
Lacobellis, N.S., Cantore, P.L., Capasso, F. and Senatore, F., 2005. Antimicrobial activity of Cuminum cyminum L. And Carumcarvi L. essential oil. J. Agric. Food Chem., 53(1): 57-61. https://doi.org/10.1021/jf0487351
Manjree, A., Walia, S., Dhingra, S. and Khambay., B.P.S., 2001. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag. Sci., 57(3): 289-300. https://doi.org/10.1002/ps.263
Martinez, C.G., Bermudez, C.A.G., Valcarcel, A.M.C., Pascual, M.S. and Saseta, C.F., 2015. Use of herbs and spices for food preservation: Advantages and limitations. Food Sci., 6: 38-43. https://doi.org/10.1016/j.cofs.2015.11.011
McClure, M.A., Kruk, T.H. and Misaghi, I., 1973. A method for obtaining quantities of clean Meloidogyne eggs. J. Nematol., 5: 230.
Mehdi, F.S. and. Dawar, S., 2008. Use of mangrove of Indus Delta in the control of root rot disease and growth promotion of crop plant. Technical report of HEC. Department of Botany, University of Karachi. pp. 173.
Mehdi, F.S., Siddiqui, I.A., Zia, T. and Ali, N.I., 2001. Use of mangrove for the control of M. javanica in tomato. Nematol. Medit., 29: 127-129.
Oka, Y., Nacar, S., Putievsky, E., Ravid, V., Yaniv, Z. and Spiegal, Y., 2000. Nematicidal activity of essential oils and their components against root knot nematode. Phytopathology, 90(7): 710-715. https://doi.org/10.1094/PHYTO.2000.90.7.710
Parekh, J., Jadeja, D. and Chanda, S., 2005. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol., 29: 203-210.
Parmer, V.S., Jains, S.C.K., Bisht, S., Jain, R., Taneja, P., Jha, O.D., Tyagi, A.K., Prasad, J., Wengel, C.E., Oisen, E. and Boll, P.M., 1997. Phytochemistry of the genus piper. Phytochem, 46: 597-673. https://doi.org/10.1016/S0031-9422(97)00328-2
Pimpao, R.C., Dew, T., Oliveira, P.B., Williamson, G., Ferreira, R.B., and Santos, C.N., 2013. Analysis of phenolic compounds in portuguese wild and commercial berries after multienzyme hydrolysis. J. Agric. Food Chem., 61: 4053-4062. https://doi.org/10.1021/jf305498j
Salomi, N.J., Nair, S.C., and Jayawardhanan, K.K., 1992. Antitumour principles from Nigella sativa seeds. Cancer Lett., 63(1): 41-46. https://doi.org/10.1016/0304-3835(92)90087-C
Samreen, A., Dawar, S., Marium, T. and Zaki, M.J., 2009. Nematicidal activity of spices against Meloidogyne javanica (Treub) Chitwood. Pak. J. Bot., 41(5): 2625-2632.
Sasser, J.N., 1980. Root-knot nematode. A global menace to crop production. Plant Dis., 104: 36-41. https://doi.org/10.1094/PD-64-36
Sasser, J.N. and Freckman, D.W., 1987. A world perspective on nematology: The role of the society, in vistas on nematology (eds. J.A. Veech and D.W. Dickson), Society of Nematologists, Hyattsville, Maryland, pp. 7–14.
Shivakumar, S.I., Shahapurkar, A.A., Kalmath, K.V., and Shivakumar, B., 2010. Antiinflammatory activity of fruits of Cuminumcyminum Linn. Der Pharmacia Lett., 2(1): 22–24.
Siddiqui, M.A., Shamin, A.Q., Sultana, V., Ehteshamul-Haque, S. and Ghaffar, A., 2000. Biological control of root rot and rot knot disease of tomato. Plant Soil, 27: 163-169.
Skendi, A., Irakli, M. and Chatzopoulou, P., 2017. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants, 6: 62-69. https://doi.org/10.1016/j.jarmap.2017.02.001
Sokal, R. and Rohlf, F.J., 1995. Biometry: The principals and practices of statistical in biological research. Freeman, New York, pp. 887.
Sun, Y.P., 1950. Toxicity index an improved of comparing the relative toxicity of insecticides. J. Econ. Entomol., 43(1): 45–53. https://doi.org/10.1093/jee/43.1.45
Surveswaran, S., Cai, Y.Z., Corke, H. and Sun, M., 2007. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem., 102: 938-953. https://doi.org/10.1016/j.foodchem.2006.06.033
Tajkarimia, M.M., Ibrahima, S.A. and Cliver, D.O., 2010. Antimicrobial herb and spice compounds in food. Food Contr., 21: 1199–1218. https://doi.org/10.1016/j.foodcont.2010.02.003
Tariq, M., Dawar, S., Mehdi, F.S. and Zaki, M.J., 2007. Use of Rhizophoramucronatain the control of Meloidogyne javanicaroot knot nematode on okra and mash bean. Pak. J. Bot., 39: 265-270.
Taylor, D.P. and Netscher, C., 1974. An improved technique for preparing perennial pattern of Meloidogyne spp. Nematology, 20: 268. https://doi.org/10.1163/187529274X00285
To share on other social networks, click on any share button. What are these?





