Nematicidal Activity of Pyrolisate of Bambusa vulgaris on Root-Knot Nematode; Meloidogyne incognita of Lettuce
Nematicidal Activity of Pyrolisate of Bambusa vulgaris on Root-Knot Nematode; Meloidogyne incognita of Lettuce
Nusirat Aderinsola Sadiku1, Oluwatoyin Adenike Fabiyi2* and Tesleem Taye Bello3
1Department of Forest Resources Management, Faculty of Agriculture, University of Ilorin, Nigeria; 2Department of Crop Protection, Faculty of Agriculture, University of Ilorin, Nigeria; 3Department of Agricultural Education, Federal College of Education PMB 2096, Abeokuta, Nigeria.
Abstract | Pyroligneous Liquors are obtained from diverse biomasses and have been found to contain important biologically active components with high phenolic, carbonyl and organic acid contents which have several pest control properties. Bamboo Pyroligneous Liquor (BPL) has proved effective in controlling several insect pests of many crops but its nematicidal potential is yet to be fully determined. We, therefore, investigated the nematicidal activities of Bamboo Pyroligneous Liquor in managing root-knot nematodes of Lactuca sativa in greenhouse trials. Bambusa vulgaris biomass was carbonised at 500 oC in a locally fabricated pyrolizer with automatic temperature control to obtain the Bamboo Pyroligneous Liquor (BPL). Two weeks old lettuce plants were inoculated with 1,000 juveniles of Meloidogyne incognita. BPL was applied at three concentrations of 100, 200 and 400 mg/ml. Results revealed that nematode infected lettuce plants treated with BPL recorded significantly higher (P< 0.05) mean number of leaves, plant height and yield compared to the untreated control. In addition, BPL at 400 mg/ml had substantially higher nematicidal effects on both the soil and root population of nematodes of L. sativa and compared favourably with the carbofuran treatments. We concluded that BPL could serve as a very potent eco-friendly alternative to synthetic nematicides in managing M. incognita of lettuce.
Received | November 03, 2023; Accepted | November 28, 2023; Published | December 12, 2023
*Correspondence | Fabiyi, O.A., Department of Crop Protection, Faculty of Agriculture, University of Ilorin, Nigeria; Email: fabiyitoyinike@hotmail.com
Citation | Sadiku, N.A., Fabiyi, O.A. and Bello, T.T., 2023. Nematicidal activity of pyrolisate of Bambusa vulgaris on root-knot Nematode; Meloidogyne incognita of lettuce. Pakistan Journal of Nematology, 41(2): 170-181.
DOI | https://dx.doi.org/10.17582/journal.pjn/2023/41.2.170.181
Keywords | Meloidogyne incognita, Lactuca sativa, Pyroligneous liquor, Bioactivity, Nematicide, Bamboo
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Pyroligneous acids or liquors which are generally light red or reddish brown in colour have been produced from diverse biomasses ranging from forest, agricultural and household wastes. Pyrolysis is a thermo-chemical process of decomposition of lignocellulosic materials into smaller molecules by thermal energy (Demirbas, 2007). The yields of Pyroligneous liquor (PL) depend on the pyrolysis procedures, moisture contents of feeding materials (Antal and Gronli, 2003), type of feeding materials (Fapetu, 1994; Oladeji, 2013); heating rate (Putun et al., 2007); and temperature (Oladeji, 2012; Yaman, 2004; Weerachanchai et al., 2011).
The bioactivities and possible applications of Pyroligneous liquor (PL) have been extensively explored (Souza et al., 2012; Ma et al., 2013, 2014; Wu et al., 2015). PL has been reported to contain various types of important biologically active components such as; organic acids, phenolic, alkane, furan derivatives, esters, alcohol, sugar derivatives, and nitrogen compounds (Wei et al., 2010; Pimenta et al., 2018). The diversity of functions of PL has attracted attention due to its environmental friendliness. PL has been widely used in medicine, food and agriculture (Cai et al., 2012; Dissatian et al., 2018). In agriculture, PL specifically from bamboo has been used in controlling insects pests such as termites, flies, ticks and fleas (Yatagai, 2004; Hagner et al., 2018); ameliorate soil, aids crop germination, used as fertilizer (Mohan et al., 2006; Jung, 2007; Wei et al., 2009, 2010); feed additive to improve animal performance (Samanya and Yamauchi, 2001; Yoo et al., 2007; Watarai and Koiwa, 2008; Choi et al., 2009; Wang et al., 2012); antibacterial, antifungal and antivirus for eliminating non-beneficial soil microbes (Mu et al., 2004, 2006), Herbicide (Tworkoski, 2002; Salonen et al., 2008; Ruuttunen, 2007); simulate plant growth (Mu et al., 2004, 2006); mushroom fruit-body formation stimulant (Yoshimura et al., 1995) and many more. Bamboo has been one of the popular sources of PL, however, there are still limited studies available on the bioactivities of PL obtained from bamboo against nematode pests.
Plant parasitic nematodes (PPNs) are regarded as a major threat to agricultural crop production causing extensive significant yield losses to farmers worldwide (Javad et al., 2006; Fabiyi, 2022a). Meloidogyne species also called the root-knot nematodes (RKN) are described as the most devastating due to their wide host range (Bello et al., 2020; Fabiyi, 2021a) and being responsible for yearly crop loss reaching approximately hundred million US dollars worldwide (Chitwood, 2003). Characteristic symptoms of RKN infection include visible galling of the roots, stunting, wilting and nitrogen deficiency signs (Siddiqui et al., 2001; Fabiyi, 2021b). Control of RKN is made difficult due to their wide host range, high rate of reproduction and short lifecycle (Natarajan et al., 2006).
Lettuce (Lactuca sativa L.) is an important crop that is widely grown in many home gardens and almost sometimes cooked, it is exclusively eaten as a fresh vegetable in salads (Lebeda et al., 2007). Lettuce is also produced on a commercial scale in different countries worldwide (Lebeda et al., 2007; Mou, 2008). In Nigeria, Lettuce is generally considered an important exotic vegetable crop and its increased production was attributed to the increasing awareness about its health and nutritional benefits (Ogbodo et al., 2010; Shuaibu and Mohammed, 2018). Lettuce like many other vegetable crops is susceptible and severely affected by the root-knot nematodes causing substantial yield losses thereby posing a significant threat to its production worldwide (Raid, 2004; Fabiyi, 2022). Growers most times resort to the use of synthetic chemical nematicides in managing this economically important pest (Fabiyi and Bello, 2023). Chemical control, aside being expensive, poses a potential hazard to the environment, crops and human health (Tsay et al., 2004; Fabiyi and Olatunji, 2021; Fabiyi et al., 2023). Due to the health and environmental hazards posed by continuous use of these synthetic nematicides, the search for other eco-friendly alternatives has been on the increase. The antibacterial properties and the effects of PL specifically bamboo PL as bacterial growth suppressors have been investigated by various researchers (Kim et al., 2005; Jankowsky et al., 2018). Pyroligneous liquid from different species of wood has been used to control birds (Strong, 1973) and insect pests (Pangnakorn, 2009; Regnault-Roger, 1997). They have also exhibited high insecticidal activities against termites (Yatagai et al., 2002), aphids (Myzus persicae) and psyllids (Trioza apicalis) (Lindqvist et al., 2010). Hagner et al. (2010) reported positive results on harmful soil microbes but no significant effects on nematodes when used at concentrations between 500-1360 Lha. Of all these reports, there is an inadequacy of scientific evidence to prove the efficacy of bamboo pyroligneous liquor in eradicating root-knot nematodes. In this study, we investigated the nematicidal activity of Bamboo Pyroligneous Liquor (BPL) against Meloidogyne incognita parasitizing lettuce plants.
Materials and Methods
Sample preparation and pyrolysis of the bamboo sample
Three to four-years old culm of Bambusa vulgaris were collected from Forestry Research Institute of Nigeria, Ibadan, Oyo state. The air-dried bamboo sample used was 1 kg which was 1.219 kg when collected fresh. The samples were cut into smaller sizes of about 4cm by 2cm and were air dried to constant weight. Slow pyrolysis was performed using an electric tube furnace. One kg of the dried bamboo biomass was fed into a locally fabricated laboratory scale pyroliser and was pyrolysed at a controlled temperature of 500 °C. The heating chamber was heated to 500 °C at a rate of 20 °C/min and then maintained for 150 min. The pyroligneous liquor was obtained by condensation of the volatile gases within 2 hours of reaching the desired pyrolytic temperature. Bio-oil was collected from the reactor outlet. The obtained BPL was analysed with Perkin–Elmer SPECTRUM-2000 spectrometer.
Oil property determination
The oil property of the liquor was determined using pH value, colour, odour, density, dissolved tar content, percentage ignition residue, yield and transparency through visual examination and the use of formulae.
Yield
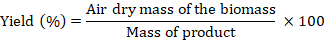
Density
This was determined using the formula:
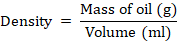
Percentage ignition residue
This was known using the formula:
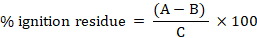
Where A is the weight of crucible and residue after heating; B is the weight of empty crucible; C is the weight of sample (crude bio-oil).
Dissolved tar content
This was determined by oven drying already weighed crucible and sample (crude bio-oil) at 60℃ for 24 hrs. The black residue was then regarded as the dissolved tar content and the dissolved tar content was determined by subtracting the weight of crucible with oil after oven drying from the weight of crucible and oil before oven drying.
Fourier transform infra-red spectroscopy (FTIR)
The functional groups of BPL constituents were analysed using FTIR.
Nematode inoculum collection
Galled roots of Celosia argentea plants containing a pure culture of M. incognita eggs were collected from the International Institute of Tropical Agriculture, IITA, Ibadan, Nigeria and multiplied on a susceptible tomato cultivar (Tropimech) using the protocol of Hussey and Baker (1973) so as to obtain the required quantity of nematode inoculum for the experiment. This was done by washing the galled roots thoroughly under running tap water to release the debris and sand attached to the roots. They were then cut into small bits between 1-1.5 cm lengths and poured into a litre glass jar containing a solution of 0.05% sodium hypochlorite. The jar was mixed thoroughly for four minutes after which the content was poured out and passed through stack of sieves: 75 µm, 55 µm, and 25 µm aperture, respectively. The eggs were retained inside the 25 µm sieve and were collected by using water to rinse the sieve off into a beaker and left in the laboratory at ambient temperature.
Bioactivity of the bamboo pyroligneous liquor on Meloidogyne incognita
The nematicidal effect of BPL on M. incognita infected lettuce plants was conducted at the screen-house of the Department of Crop Protection, University of Ilorin, Nigeria. Thirty-five 10-litre plastic buckets were filled with steam-sterilized loamy soil. Three weeks old lettuce plants were transplanted from nursery into each bucket. Each lettuce plant was inoculated with 1,000 active second stage juveniles (J2s) of M. incognita after a week of transplanting. The juveniles were introduced using a syringe to inject four 3 cm deep holes encircling each lettuce plant per container. The lettuce plants were treated two weeks after nematode inoculation with 100, 200 and 400 mg/ml of BPL and also, carbofuran at 1.0, 1.5 and 2.0 kg/a-i/ha. Lettuce growth was monitored for 5 weeks after treatment and at harvest.
Data collection and analysis
Data were taken on yield, nematode count in root, nematode count in 200 cc soil, number of leaves, and plant height. Additionally, 200 cc of soil was taken around each lettuce plant in the experimental pot and nematodes (J2s) were extracted using the modified Baermann tray (pie-pan) process (Coyne et al., 2007). Data were subjected to a factorial analysis of variance (ANOVA) using R software version, significant means were separated with Tukey’s HSD at P< 0.05.
Result and Discussion
Physical properties of the pyroligneous liquor
Liquor yield: The product of pyrolysis is three; the solid (char), the liquid (pyroligneous liquor) and the gas. Although, in this study, emphasis was placed on the liquid part of the products. Bamboo presents a very different product yield from agricultural waste and forestry residue that has been used for oil production. The results showed that the yield of non-condensable gases out of the three bamboo products (solid, liquid, gas) has the highest percentage of (89%), followed by that of char (7.5%) while the yield of the liquid (BPL) was lowest with (3.5%) Table 1. These results are contrary to earlier findings on wood pyrolysis. Dobele et al. (2007) reported 63% liquid product when dried mixture of hardwood was pyrolysed at 550oC. Şensoz et al. (2000) reported 46% bio-oil weight pyrolysis of Brassica napus conducted at 500oC running at 40oC/min. Pütün et al. (1999) reported 23.1% for hazelnut shells pyrolysed in a fixed-bed tubular reactor under nitrogen at 500 °C and a heating rate of 7K/min. Pyrolysed sugarcane bagasse involving a laboratory scale vacuum condition produced more oil; 34.4% vs 30.1% and less charcoal 19.4 wt% vs 25.7 wt% compared to pilot scale runs (Garcia-Perez et al., 2002). According to Mohan et al. (2006) paper, wood and other biomass depending on the composition of the feedstock, yielded bio-oil within the range of 60-99%. The researchers concluded that high lignin contents have a tendency to give lower liquid yields. Likewise, Sarkar and Wang (2010) recorded 48.7% yield at 600oC indicating that temperature played a vital role in the product yield, as well as having a vital effect on the characteristics of the PL.
Table 1: Yield of pyroligneous liquor.
|
Products |
Yield (g) |
|
Char |
7.5 |
|
Bio oil |
3.5 |
|
Gas |
89 |
Bamboo pyroligneous liquor characteristics
The main physical properties of bio-oil include heating value, water content, density, flash point, and so on. Table 2 shows the properties of the BPL based on some selected criteria such as pH value, moisture content, density, colour, odour, etc. It was discovered that bamboo has lower moisture content (18%) compared to between 15-35% reported by Bridgwater et al. (2001) and 48-56wt% by Weerachanchai et al. (2011).
Table 2: The physical properties of the liquor.
|
Physical properties |
Results |
|
pH |
2.39 |
|
Density |
1.04g/ml |
|
colour |
Dark brown |
|
odour |
smoky |
|
Dissolve tar content |
0.03 |
|
Transparency |
Not transparent has no suspended matter |
|
% Moisture |
18 |
The moisture content is usually derived from both the original moisture in the feedstock and from dehydration reactions that occur during biomass pyrolysis and bio-oil storage (Lu et al., 2009). The pH value was 2.39 which corroborated Mohan et al. (2006) that the pH of bio-oil can be as low as 2 or 3 as well as Weerachanchai et al. (2011) who recorded 2.98 and 2.95 for palm shell and cassava pulp residue. However, this was extremely low to 5.62 recorded for palm kernel oil (Weerachanchai et al., 2011). The pyrolysis liquid products consisted of the water content, this pointed to the acidic content of the BPL. The majority of acidic compounds in bio-oil are carboxylic acids, acetic acid and formic acid which according to Piskorz et al. (1988) represent around 5 wt% and 3 wt% of bio-oil. This may account for the acidic pH of 2.39 that is exhibited by the BPL and the acidity may be a factor of antimicrobial activities.
The density of the BPL was 1.04 g/cm3. This is in line with the range of 1.1-1.3 g/cm3 reported in literatures (Xu et al., 2011; Weerachanchai et al., 2011). The bulk density of the BPL in this study falls in the range recorded by Weerachanchai et al. (2011) for palm kernel (1.01g/cm3), palm shell (1.11g/cm3) and cassava pulp residues (1.10g/cm3) and higher than 0.78g/cm3 and 0.94g/cm3 recorded for fast diesel oil (Islam et al., 1999) and heavy fuel oil (Zhang et al., 2007), respectively. The density of BPL may be attributed to the high content of cellulose, hemicellulose and other macromolecules like phenolic and oligomeric compounds (Oasmaa and Czernik, 1999) in the bamboo raw material.
According to Weerachanchai et al. (2011), the types of biomass have an influence on the bio-oil appearances depending on the biomass sources. Transparent, dark red-brown colour was recorded for pyrolysis liquids from cassava pulp residue while palm kernel oil had opaque dark colour but with separation of oil phase and
Table 3: Band position (cm-1), peak assignment and structural polymer present in Bamboo Pyroligneous liquor as determined by FT-IR analysis.
|
S. No |
Band (cm-1) |
Functional group |
|
|
1. |
3395.26 |
O-H stretching from cellulose |
Phenols and Alcohols |
|
2. |
2955.15 |
C-H |
Alkanes |
|
3. |
1732.98 |
C=O Stretch |
Ester, six member lactone (ketone) |
|
4. |
1593.67 |
Aromatic skeletal vibration (C=C) |
Olefinic compounds |
|
5. |
1511.35 |
Aromatic skeletal vibration (C=C), guaiacyl > 5 |
Phenolic compounds |
|
6. |
1460.69 |
CH deformation, asymmetry in CH3 and CH2 |
Olefinic compounds |
|
7. |
1362.53 |
CH2 and CH3 bending |
Aldehyde and Ketones |
|
8. |
1223.22 |
C–O of guaiacyl ring |
Phenolic |
|
9. |
1099.74 |
O–H deformation vibration |
Alcohols and Phenolics |
|
10. |
1045.91 |
C–O stretch |
Alcohols |
aqueous phase (Weerachanchai et al., 2011). However, BPL obtained had a distinct dark-black viscous appearance with no suspended matter in it. This is similar to those reported in the literatures for bio-oil (Bridgwater et al., 2001; Bridgwater, 2004; Pollard et al., 2012). Aside black viscous appearance, Pollard et al. (2012) observed different colours ranging from extremely viscous black which on cooling turned to resinous solids at room temperature; honey-like flow characteristics; watery, red-tinted liquid similar to the characteristic of the BPL obtained in this study.
Fourier-transform infrared spectroscopy (FTIR) analysis
Infrared spectrum of the liquor obtained from pyrolysis of Bambusa vulgaris is presented in Figure 1. The characteristic peaks are presented in Table 3. The BPL contained diverse functional groups which were revealed at different wave numbers (Table 1). There were seven classes of compounds which are; alcohols, alkanes, esters, ketone, olefine, phenol and aldehyde. The BPL has O-H stretching vibration at 3395.26 cm-1 indicating the presence of polymeric O-H group that exists in water, phenol, alcohol, and/or carboxylic acids in the liquor. This indicates the presence of water/hydroxyl group, impurities and other polymeric O-H in the BPL (Lee et al., 2010; Islam et al., 2003). BPL is associated with highly oxygenated peak assignments with pronounced functional groups and aromatic compounds which makes them highly acidic (pH = 2.39). This result is in accordance with study conducted by Didem and Filiz (2004) and Natarajan and Ganapathy (2009) that the liquid composition from biomass of rice husk and rape cake, respectively are dominated by oxygenated compounds coupled with the C-O and aromatic compounds which showed the potential as a chemical feedstock.
The propensity of BPL to be utilised as fuel is largely due to the presence of alcohol and hydrocarbon (C-H; C-C) groups (Islam et al., 2012). The C=O stretching vibration at 1732.98cm-1 displayed aldehydes, carboxylic acids and ketonic compounds (Islam et al., 2012). The band of C-H stretching at 2955.15 cm-1 wave number shows the presence of alkanes in the BPL (Islam et al., 2003; Tsai et al., 2007). The C=C aromatic skeletal vibration, guaiacyl > 5 at 1511.35 cm-1 and C–O of guaiacyl ring at 1223.22 signifies the presence of phenolic compounds C=C aromatic skeletal vibration at 1593.67 cm-1 and the CH deformation, asymmetry in CH3 and CH2 vibration at 1460.69 cm-1 explains the presence of alcohol and olefinic (alkenes) compounds in the liquor. C-H deformation also occurred at 1362.53 cm-1. The Absorption bands of 1045.91 and 1099.74 cm-1 were due to the presence of primary, secondary and tertiary alcohols, ethers and esters due to the C–O stretching and O–H deformation vibration of these functional groups (Qian et al., 2007).
FTIR analysis of the BPL showed the presence of characteristic functional groups of alcohol, phenol, alkane, aldehyde and ester compound derivatives which were responsible for the nematicidal properties of the BPL. The obtained Pyroligneous liquor from B. vulgaris had typical infrared spectra that are comparable with Pyroligneous liquor from different lignocellulosic biomass from previous studies (Fuwape et al., 2011; Şensӧz, et al., 2006). Phenolic compounds were identified as the main organic components in the BPL followed by alcohols, olefins and ketonic compounds. Other components are esters, alkanes and aldehydes. The presence of phenol and ketone groups is attributed to its nematicidal activity. Most phenolic compounds have disinfectant properties. Several reports from literature suggested that high levels of organic and phenol contents in pyroligneous liquids had a positive correlation with bacterial growth inhibition (Ma et al., 2011; Wei et al., 2010a).
Table 4: Variations in the weekly height of L. sativa after the application of BPL and carbofuran treatments.
|
Treatment concentration |
Mean plant height |
||||
|
1 WAT |
2 WAT |
3 WAT |
4 WAT |
5 WAT |
|
|
CBFN0 |
11.03d |
12.13d |
12.93e |
15.00de |
16.33e |
|
BMBO0 |
9.93d |
10.60d |
12.43e |
13.67e |
17.00e |
|
CBFN1 |
14.43b |
13.57cd |
15.17d |
17.00d |
21.67d |
|
CBFN2 |
16.97b |
16.93c |
18.53cd |
20.33c |
26.67c |
|
CBFN3 |
21.63ab |
22.17b |
23.70b |
26.33b |
32.00b |
|
BMBO1 |
13.97bc |
15.43c |
18.50cd |
20.53c |
26.17c |
|
BMBO2 |
15.17b |
16.77c |
20.27c |
21.93c |
31.67b |
|
BMBO3 |
23.23a |
24.73a |
27.73a |
29.33a |
42.33a |
WAT = weeks after treatment was added; BMBO0 = PL 0% (control); BMBO1 = PL 100mg/ml; BMBO2 = PL 200mg/ml); BMBO3 = PL 400mg/ml; CBFN0 = Carbofuran 0g (control); CBFN1 = Carbofuran 5g; CBFN2 = Carbofuran 10g; CBFN3 = Carbofuran 15g. Means in the same column with same alphabets are not significantly different at P≤ 0.05.
Effect on L. sativa agronomic parameters
Tables 4 and 5 show the plant height and number of leaves of L. sativa across the growing weeks after transplanting (WAT). There were significant differences observed in the mean heights and mean number of leaves of L. sativa (Tables 4 and 5). Likewise, at different concentrations of BPL and carbofuran, the height of L. sativa at week 1 was statistically similar for both BPL and carbofuran at highest treatment concentrations of 400 mg/ml and 15 g respectively even though 400 mg/ml BPL gave the highest plant height (23.23 cm) compared to that of carbofuran (21.63 cm). However, at 2, 3, 4 and 5 WAT; BPL at 400 mg/ml gave the highest plant height and number of leaves which were followed by carbofuran treatment at 15 g concentration. However, the control treatments for BPL and carbofuran both showed decreased plant height and number of leaves (Tables 4 and 5). The results showed that the higher the concentration of BPL and carbofuran, the higher the number of leaves and plant height. However, BPL treated plants perform best even compared to the conventional nematicide (carbofuran). Although, it has been discovered that conventional carbofuran promotes plant growth (Adegbite and Agbaje, 2007; Tanimola, 2008). However, in this study, treating root-knot nematode (M. incognita) in lettuce with BPL was more effective than carbofuran, although, the increase in the yield (Figure 2C) was immediately followed by that of lettuce treated with carbofuran compared to the control proving that BPL displayed higher nematicidal potential than carbofuran.
Table 5: Variations in the number of leaves of L. sativa after the application of BPL and Carbofuran treatment.
|
Treatment concentrations |
Mean number of leaves |
||||
|
1 WAT |
2 WAT |
3 WAT |
4 WAT |
5 WAT |
|
|
CBFN0 |
5.00c |
5.67d |
6.33e |
5.33f |
6.00f |
|
BMBO0 |
5.33c |
9.00c |
7.33e |
4.33f |
5.67f |
|
CBFN1 |
7.00b |
7.67cd |
8.00d |
9.00e |
8.67e |
|
CBFN2 |
7.67b |
8.00c |
11.00c |
13.00cd |
14.00c |
|
CBFN3 |
12.67a |
11.67b |
13.67bc |
15.67c |
18.00b |
|
BMBO1 |
7.00b |
8.33c |
11.33c |
12.00d |
12.33d |
|
BMBO2 |
8.67b |
10.00b |
14.67b |
17.00b |
18.67b |
|
BMBO3 |
13.67a |
15.67a |
18.67a |
23.33a |
24.67a |
WAT = weeks after treatment was added; BMBO0 = PL 0% (control); BMBO1 = PL 100mg/ml; BMBO2 = PL 200mg/ml); BMBO3 = PL 400mg/ml; CBFN0 = Carbofuran 0g (control); CBFN1 = Carbofuran 5g; CBFN2 = Carbofuran 10g; CBFN3 = Carbofuran 15g. Means in the same column with same alphabets are not significantly different at P≤ 0.05
The increase in L. sativa height and number of leaves due to the addition of BPL may be a result of high organic acid content which is an essential component in the different compounds of BPL (Mungkunkamchao et al., 2013). Furthermore, the presence of some alcohols, aldehydes and acids has made BPL a viable energy and carbon substrate for some soil microbial agents that help to promote crop growth (Yang et al., 2014). Our findings agree with the previous reports of Mahmud et al. (2016) where 2% (v/v) pyroligneous acid used to fertilize okra (Abelmuscus esculentus) resulted in the highest mean number of fruits and leaves as well as studies of Mu et al. (2004, 2006), where bamboo vinegar showed an increase in vegetative growth, germination and radicle growth for several seeds. Thus, this study successfully demonstrated that BPL has great potential as an organic plant growth promoter with no detrimental effect on the environment.
Nematicidal effect of BPL on yield and nematode population in root and soil of lettuce plants
The results for nematode populations at harvest and yield of lettuce are presented in Figure 2A, B and C, respectively. There were significant variations in the root and soil nematode population across the BPL and carbofuran treatments applied (Figure 2). The nematode population in the root of L. sativa was highest for the control treatments which contained no BPL or carbofuran (Figure 2B). Generally, BPL had marked killing effect on both the soil and root nematodes compared to the carbofuran treatments and invariably an increase in yield of treated plants. The highest nematode population in the roots was 36 in BPL treatment as against 122 in carbofuran at 200 mg/ml and 5 g/ml, respectively. 11 nematodes were counted in 300 mg/ml of BPL as against 46.67 nematodes in 10 g/ml carbofuran. While no nematode was recorded at 400 mg/ml BPL treatments whereas, 16.33 were recorded for 15 g/ml carbofuran (Figure 2). The results of root nematode population follow a similar pattern to that of soil nematode population. These results showed that BPL was very effective in the control of M. incognita. Over the years, PL has shown interesting antimicrobial and disinfectant properties. PL has been used to control diversities of crop insect pests and diseases. For root-knot nematodes, Hagner et al. (2010) reported no significant effect on root-knot nematodes when used at concentrations between 500-1360 Lha-1. However, this study showed the killing effects of BPL on M. incognita even at low concentrations with complete eradication at higher dosage of 400 mg/ml. Whereas, carbofuran was able to lower the population of both the root and soil nematodes without complete killing. The action of BPL is attributed to the presence of compounds such as phenolic, carbonyls and organic acids (Lee at al., 2010; Loo et al., 2008).
Conclusions and Recommendations
Our findings have proved the efficacy of Bamboo Pyroligneous liquid both as a promising growth promoter and a potent nematicidal agent against root-knot nematode: M. incognita. This interesting nematicidal effect could be attributed to the presence of several compounds in the Pyroligneous liquor. BPL compared favourably to carbofuran (conventional nematicide) and the fact that it is an organic material makes it a viable alternative to the use of chemical nematicides in managing root-knot nematode pests of lettuce.
Acknowledgement
Dr. O. Adewuyi of IITA Ibadan, Nigeria for providing inoculum. Dr. I.A. Adegoke of FRIN Ibadan Nigeria for pyrolysis and Dr. R. Ibia of Labstock Lagos Nigeria for GCMS analysis.
Novelty Statement
This study has proved the efficacy of Bamboo Pyroligneous liquid as a potent nematicidal material which could serve as a substitute to chemical nematicides in managing M. incognita.
Author’s Contribution
Sadiku, N.A. and Fabiyi A.O. Designed the study.
Bello T.T. Analysed data.
Sadiku, N.A. Wrote the first draft of the manuscript.
Fabiyi A.O. and Bello T.T. Edited the final draft of the manuscript.
All authors approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Adegbite, A.A. and Agbaje, G.O., 2007. Efficacy of furadan (Carbofuran) in control of root-knot nematode (Meloidogyne incognita) in Hybrid Yam in South-Western Nigeria. World J. Agric. Sci., 3: 256-262.
Antal, M.J. and Gronli, M., 2003. The art, science and technology of charcoal production. Ind. Eng. Chem. Res., 42(8): 1619-1640. https://doi.org/10.1021/ie0207919
Appropriate Technology Association (ATA), 2006. Wood vinegar production and utilization. First Publication.
Bello, T.T., Coyne, D.L., Rashidifard, M. and Fourie, H., 2020. Abundance and diversity of plant parasitic nematodes associated with watermelon in Nigeria, with focus on Meloidogyne spp. Nematology, 22(7): 781-797. https://doi.org/10.1163/15685411-00003340
Bridgwater, A.V., 2004. Biomass fast pyrolysis. Ther. Sci., 8(2): 21–49. https://doi.org/10.2298/TSCI0402021B
Bridgwater, A.V., Czernik, S. and Piskorz, J., 2001. An overview of fast pyrolysis. Prog. Thermochem. Biomass Conv., 2: 977. https://doi.org/10.1002/9780470694954.ch80
Cai, K., Jiang, S., Ren, C., and He, Y., 2012. Significant damage-rescuing effects of wood vinegar extract in living Caenorhabditis elegans under oxidative stress. Sci. Food, 92: 29–36. https://doi.org/10.1002/jsfa.4624
Chitwood, D.J., 2003. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Manage. Sci., 59: 748–753. https://doi.org/10.1002/ps.684
Choi, J.H., Shinde, P.L., Kwon, I.K., Song, Y.H., and Chae, B.J., 2009. Effect of wood vinegar on the performance, nutrient digestibility and intestinal microflora in weanling pigs. Asian Aust. J. Anim. Sci., 22(2): 267-274. https://doi.org/10.5713/ajas.2009.80355
Coyne, D. L., Nicol, J. M., and Claudius-Cole, B. 2007. Practical plant nematology: A field and laboratory guide. Cotonou: SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA).
Demirbas, A., 2007. Biodiesel: A realistic fuel alternative for diesel engines. Springer-Verlag London Limited, ISBN 978-1-84628-994-1, London, UK.
Didem, O. and Filiz, K., 2004. Production and characterization of bio-oil and biochar from rapeseed cake. Renewable Energy, 29: 779-787. https://doi.org/10.1016/j.renene.2003.09.006
Dissatian, A., Sanitchon, J., Pongdontri, P., Jongrungklang, N., and Jothityangkoon, D., 2018. Potential of wood vinegar for enhancing seed germination of three upland rice varieties by suppressing malondialdehyde production. J. Agric. Sci., 40: 371–380. https://doi.org/10.17503/agrivita.v40i2.1332
Dobele, G., Urbanovich, I., Volpert, A., Kampars, V., and Samulis, E., 2007. Fast pyrolysis of wood for bio-oil. Biol. Resour., 2(4): 699-706. https://doi.org/10.15376/biores.2.4.699-706
Eisenback, J.D., Hirchmann, H., Sasser, J.N. and Triantaphylou, A.C., 1981. A guide to the four most common species of root-knot nematodes (Meloidogyne spp. with a pictorial key). North Carolina State Univ. Graphics, Raleigh, NC, pp. 48.
Fabiyi, O.A. and Olatunji, G.A., 2021. Environmental sustainability: Bioactivity of Leucaena leucocephala Leaves and Pesticide Residue analysis in Tomato Fruits. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis, 69(4): 473-480. https://doi.org/10.11118/actaun.2021.042
Fabiyi, O.A., 2021a. Application of furfural in sugarcane nematode pest management. Pak. J. Nematol., 39(2): 151-155. https://doi.org/10.17582/journal.pjn/2021.39.2.151.155
Fabiyi, O.A., 2021b. Evaluation of Nematicidal activity of Terminalia glaucescens fractions against Meloidogyne incognita on Capsicum chinense. J. Hortic. Res., 29(1): 67-74. https://doi.org/10.2478/johr-2021-0006
Fabiyi, O.A., 2022a. Cytotoxicity and Nematicidal Potential of Leaf Extracts of Adansonia digitata and Khaya senegalensis on root knot nematode (Meloidogyne incognita) associated with cabbage (Brassica oleracea). J. Agric. Sci. Sri Lanka, 17(3): 425-436. https://doi.org/10.4038/jas.v17i3.9922
Fabiyi, O.A., 2022b. Evaluation of weeds against root-knot nematode (Meloidogyne incognita) in vegetables. Sarhad J. Agric., 38(4): 1289-1299. https://doi.org/10.17582/journal.sja/2022/38.4.1289.1299
Fabiyi, O.A. and Bello, T.T., 2023. Nematode management in crops, limitations and challenges to meet future food demands. In: Novel biological and biotechnological applications in plant nematode management Khan, M.R. (Ed). Published by Springer. pp. 47-60. https://doi.org/10.1007/978-981-99-2893-4_2
Fabiyi, O.A., Adebisi, O.O., Falore, S.O. and Claudius-Cole, A.O., 2023. Potential inhibition of entomopathogenic nematodes and plant growth-promoting bacteria with exposure to selected herbicides and insecticides. Vegetos. https://doi.org/10.1007/s42535-023-00688-0
Fapetu, P.O., 1994. Evaluation of the thermo-chemical conversion of some forestry and agricultural biomass fuel and chemicals. An Unpublished Ph.D. Thesis, University of Ibadan, Nigeria.
Fuwape, J.A., Fabiyi, J.S. and Adegoke, O.A., 2011. Fourier transform-infrared analysis of pyrolysis oil from selected wood residues. For. For. Prod. J., 4: 16-20.
Garcı ̀a-Pèrez, M., Chaala, A., and Roy, C., 2002. Vacuum pyrolysis of sugarcane bagasse, J. Anal. Appl. Pyrolysis., 65: 111–136. https://doi.org/10.1016/S0165-2370(01)00184-X
Hagner M, Pasanen T, Lindqvist B., Lindqvist, I., Tiilikkala, K., Penttinen, O., and Setälä, H., 2010. Effects of birch tar oils on soil organisms and plants. Agric. Food Sci., 19: 13-23. https://doi.org/10.2137/145960610791015096
Hagner, M., Tiilikkala, K., Lindqvist, I., Niemelä, K. and Rasa, K., 2018. Performance of liquids from slow pyrolysis and hydrothermal carbonization in plant protection. Waste Biomass Valoriz pp. 1–12. https://portal.mtt.fi/portal/page/portal/mtt_en/mttps/07HerbP2.pdf.
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp. Plant Dis. Rep., 57: 1025-1028.
Islam, M.N., Zailani, R. and Ani, F.N., 1999. Pyrolytic oil from fluidized bed pyrolysis of oil palm shell and its characterization. Renewable Energy, 17: 73-84. https://doi.org/10.1016/S0960-1481(98)00112-8
Islam, M.R., Nabi, M.N. and Islam, M.N., 2012. The fuel properties of pyrolytic oils derived from carbonaceous solid wastes in Bangladesh. J. Teknol., 38(1): 75-89. https://doi.org/10.11113/jt.v38.484
Islam, M.R., Nabi, M.N., and Islam. 2003. The fuel properties of pyrolytic oil derived from carbanaceous solid waste in Bangladesh. T. Teknol., 38: 75-89. https://doi.org/10.11113/jt.v38.484
Jankowsky, L., Possedente de Lira, S., Ossamu Tanaka, F.A., Jankowsky, I.P., and Brito, J.O., 2018. Antimicrobial activity of the methanolic fraction of bamboo pyroligneous liquor. J. Pharm. Pharmacol., 6: 924-934. https://doi.org/10.17265/2328-2150/2018.10.005
Javad, N., Gowmen, S.R., Ulhaq, M.I., Abdullah, K. and Shahina, F., 2006. Systemic and persistent effect of neem (Azardirachta indica) formulations against root knot nematodes, Meloidogyne javanica and their storage life. Crop Prot., 26: 911-916. https://doi.org/10.1016/j.cropro.2006.08.011
Jung, K., 2007. Growth inhibition effect of pyroligneous acid on pathogenic fungus, Alternaria mali, the agent of Alternaria blotch of apple. Biotechnol. Bioprocess Eng., 12: 318-322. https://doi.org/10.1007/BF02931111
Kadota, M., and Niimi, Y., 2004. Effects of charcoal with pyroligeneous acid and barnyard manure on bedding plants. Sci. Hortic., 101: 327-332. https://doi.org/10.1016/j.scienta.2004.01.002
Kim, J.S., Park, S.W., Ham, Y.S., Jung, S.K., Lee, S.H., and Chung, S.K., 2005. Antimicrobial activities and phenolic compounds of pyroligneous liquor. Korean J. Food Preserv., 12(5): 470-475.
Lebeda, A., Doležalová, I., Křístková, E., Dehmer, K. J., Astley, D., Van de Wiel, C. C. M., and Van Treuren, R. 2007. Acquisition and ecological characterization of Lactuca serriola L. germplasm collected in the Czech Republic, Germany, the Netherlands and United Kingdom. Gen. Resour. Crop Evolut., 54: 555-562.
Lee, S.H., H’ng, P.S., Lee, A.N., Sajap, A.S., Tey, B.T. and Salmiah, U., 2010. Production of Pyroligneous acid from lignocellulosic biomass and their effectiveness against biological attacks. J. Appl. Sci., 10: 2440-2446. https://doi.org/10.3923/jas.2010.2440.2446
Lindqvist, I., Lindqvist, B., Tuovinen, T., Nissinen, N., Korpela, S., Tiilikkala, K., Hoppula, K., Kauppinen, S., Setälä, H. and Perdikis, D., 2009. In: Tiilikkala K, Segerstedt M, Eds. The potential of botanical birch tar oil for insect pest control. Koivutisle – kasvinsuojelun uusi innovaatio; 143: 1-129. ISBN 978-952-487-226-3 (Verkkojulkaisu) and ISSN 1458-5081 (Verkkojulkaisu). Available from: http://www.mtt.fi/met/pdf/met143.pdf.
Loo, A.Y., Jain, K. and Darah, I., 2008. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chemistry 107(3): 1151-1160. https://doi.org/10.1016/j.foodchem.2007.09.044
Lu, Q., Li, W.Z., Zhu, X.F., 2009. Overview of fuel properties of biomass fast pyrolysis oils, Energy Conversion and Management 50 (5):1376–1383. https://doi.org/10.1016/j.enconman.2009.01.001
Ma, C., Li, W., Zu, Y., Yang, L. and Li, J., 2014. Antioxidant properties of pyroligneous acid obtained by thermochemical conversion of Schisandra chinensis Baill. Mol., 19(12): 20821-20838. https://doi.org/10.3390/molecules191220821
Ma, C., Song, K., Yu, J., Yang, L., Zhao, C., Wang, W., Zu, G. and Zu, Y., 2013. Pyrolysis process and antioxidant activity of pyroligneous acid from Rosmarinus officinalis leaves. J. Anal. Appl. Pyrol., 104: 38-47. https://doi.org/10.1016/j.jaap.2013.09.011
Ma, X., Wei, Q., Zhang, S., Shi, L. and Zhao, Z., 2011. Isolation and bioactivities of organic acids and phenols from walnut shell pyroligneous acid. J. Anal. Appl. Pyrol., 91(2): 338-343. https://doi.org/10.1016/j.jaap.2011.03.009
Mahmud, K.N., Yahayu, M., Md. Sarip, S.H., Rizan, N.H., Min, C.B., Mustafa, N.F., Ngadiran, S., Ujang, S. and Zakaria, Z.A., 2016. Evaluation on efficiency of pyroligneous acid from Palm Kernel shell as antifungal and solid pineapple biomass as antibacterial and plant growth promoter. Sains Malaysiana 45(10): 1423–1434.
Mohan, D., Pittman, C.U., and Steele, P.H., 2006. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels, 20: 848-889. https://doi.org/10.1021/ef0502397
Mou, B. 2008. Lettuce. In Vegetables I: Asteraceae, brassicaceae, chenopodicaceae, and cucurbitaceae (pp. 75-116). New York, NY: Springer New York.
Mu, J., Uehara, T. and Furuno, T., 2004. Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants II: Composition of moso bamboo vinegar at different collection temperature and its effects. J. Wood Sci., 50: 470−476. https://doi.org/10.1007/s10086-003-0586-y
Mu, J., Yu, Z., Wu, W. and Wu, Q., 2006. Preliminary study of application effect of bamboo vinegar on vegetable growth. For. Stud. Chin., 8: 43−47. https://doi.org/10.1007/s11632-006-0023-6
Mungkunkamchao, T., Kesmala, T., Pimratch, S., Toomsan, B. and Jothityangkoon, D., 2013. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic., 154: 66-72. https://doi.org/10.1016/j.scienta.2013.02.020
Natarajan, N., Cork, A., Boomathi, N., Pandi, R., Velavan, S., and Dhakshnamoorthy, G. 2006. Cold aqueous extracts of African marigold, Tagetes erecta for control tomato root knot nematode, Meloidogyne incognita. Crop Prot., 25(11): 1210-1213.
Natarajan, E. and Ganapathy, S.E., 2009. Pyrolysis of rice husk in a fixed bed reactor. World Acad. Sci., Eng. Technol., 56: 504-508.
Oasmaa, A. and Czernik, S., 1999. Fuel oil quality of biomass pyrolysis oils state of the art for the end users. Energy and Fuels, 13(4): 914-921. https://doi.org/10.1021/ef980272b
Ogbodo, E.N., Okorie, P.O. and Utobo, E.B., 2010. Growth and yield of lettuce (Lactuca sativa L.) at Abakaliki agro-ecological zone of southeastern Nigeria. World J. Agric. Sci., 6(2): 141-148.
Oka, Y., Koltai, H., Bareyal, M., Mor, M., Sharon, E., Chet, I. and Spiegel, Y., 2000. New strategies for the control of plant-parasitic nematodes. Pest Manage. Sci., 56: 983–988. https://doi.org/10.1002/1526-4998(200011)56:11<983::AID-PS233>3.0.CO;2-X
Oladeji, J.T., 2013. Comparative study of products of pyrolysis of cow dung and poultry litter. Innov. Syst. Design Eng., 4(9): 98-102.
Oladeji, J.T., 2012. Utilization of potential of melon shells for pyrolysis as biomass fuels. World Rural Observ., 4(2): 60-64.
Pangnakorn, U., 2009. Utilization of wood vinegar by-product from Iwate kiln for organic agricultural system. Technology and innovation for sustainable development conference (TISD2008) Faculty of Engineering, Khon Kaen University, Thailand, January 28-29, 2009.
Pimenta, A.S., Fasciotti, M., Monteiro, T.V.C. and Lima, K.M.G., 2018. Chemical composition of pyroligneous acid obtained from eucalyptus gg100 clone. Molecules, 23: 426. https://doi.org/10.3390/molecules23020426
Piskorz, J., Scott, D.S. and Radlien, D., 1988. Composition of oils obtained by fast pyrolysis of different woods. In: Pyrolysis oils from biomass: Producing analyzing and upgrading; american chemical society: Washington, DC, pp. 167-178. https://doi.org/10.1021/bk-1988-0376.ch016
Pollard, A.S., Rover, M.R. and Brown, R.C., 2012. Characterization of bio-oil recovered as stage fractions with unique chemical and physical properties. J. Anal. Appl. Pyrol., 93: 129–138. https://doi.org/10.1016/j.jaap.2011.10.007
Pütün, A.E., Ozcan, A. and Putun, E., 1999. Pyrolysis of hazelnut shells in a fixed-bed tubular reactor: Yields and structural analysis of bio-oil. J. Anal. Appl. Pyrol., 52(1): 33-49. https://doi.org/10.1016/S0165-2370(99)00044-3
Pütün, E., Uzun, B.B. and Putun, A.E., 2007. Fixed-bed catalytic pyrolysis of cotton seed cake: Effects of pyrolysis temperature, natural zeolite content and sweeping gas flow rate. Bio-Resour. Technol., 97: 701-710. https://doi.org/10.1016/j.biortech.2005.04.005
Qian, Y.C., Tan, Z.J. and He, J., 2007. Structural analysis of bio-oils from sub and supercritical water liquefaction of woody biomass. Energy, 32: 196-202. https://doi.org/10.1016/j.energy.2006.03.027
Raid, R.N., 2004. Lettuce diseases and their management. In: Diseases of Fruits and Vegetables: Volume II: Diagnosis and Management. Dordrecht: Springer Netherlands. pp. 121-147. https://doi.org/10.1007/1-4020-2607-2_5
Regnault-Roger, C., 1997. The potential of botanical essential oils for insect pest control. Integr. Pest Manage. Rev., 2: 25-34. https://doi.org/10.1023/A:1018472227889
Ruuttunen, P., 2007. Evaluation of birch oil distillate for weed control in potato. MTT Agrifood Res. Trial Rep., Herbicides, pp. 1-10.
Salonen, J., Tiilikkala, K., Ruuttunen, P., Lindqvist, I. and Lindqvist, B., 2008. Birch tar oil: A potential herbicide from the forests of Finland. In: Abstracts of the 5th International Weed Science Congress. Weeds local problems/global challenge. Vancouver, British Co-lumbia, Canada: IWSS, June 23-27.
Samanya, M. and Yamauchi, K., 2001. Morphological changes of the intestinal villi chickens fed the dietary charcoal powder including wood vinegar compounds. J. Poult. Sci., 38: 289-301. https://doi.org/10.2141/jpsa.38.289
Sarkar, J.K. and Wang, Q., 2010. Different pyrolysis process conditions of South Asian waste coconut shell and characterization of gas, bio-char, and bio-oil. Energies, 13: 1970. https://doi.org/10.3390/en13081970
Sensoz, S., Angin, D. and Yorgun, S., 2000. Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): Fuel properties of bio-oil. Biomass Bioenergy, 19: 271-279. https://doi.org/10.1016/S0961-9534(00)00041-6
Şensӧz, S., Demiral, İ. and Gercęl, H.F., 2006. Olive bagasse (Olea europea L.) pyrolysis. Bioresour. Technol., 7: 429-436. https://doi.org/10.1016/j.biortech.2005.03.007
Şensöz, S., Angin, D., Yorgun, S., and Koçkar, Ö. M. 2000. Biooil production from an oilseed crop: fixed-bed pyrolysis of rapeseed (Brassica napus L.). Energy Sources. 22(10): 891-899.
Shuaibu, A.U. and Mohammed, A.B., 2018. Economic potentials and threats to vegetable amaranth (Amaranthus cruentus) and Lettuce (Lactuca sativa) production using wastewater of metropolitan Jakara River in Kano, Nigeria. Int. J. Appl. Phys. Sci., 4(1): 12-20. https://doi.org/10.20469/ijaps.4.50003-1
Siddique, Z.A., Iqbal, A. and Mahmood, I., 2001. Effect of Pseudomonas fluorescens and fertilizers on the reproduction of Meloidogyne incognita and growth of tomato. Appl. Soil Ecol., 16(2): 179-185. https://doi.org/10.1016/S0929-1393(00)00083-4
Souza, J.B.G., Re-Poppi, N. and Raposa Jr., J.L., 2012. Characterization of pyroligneous acid used in agriculture by gas chromatography-mass spectometry. J. Braz. Chem. Soc., 23(4): 610-617. https://doi.org/10.1590/S0103-50532012000400005
Strong, R.G., 1973. Protection of wheat seed with hardwood tar oil in a dust formulation. Environ. Entomol., 2(6): 1126-1127. https://doi.org/10.1093/ee/2.6.1126
Tanimola, A.A., 2008. Comparison of the effect of carbofuran and poultry manure in the management of Meloidogyne incognita race 2 on the growth and root-knot infected cowpea (Vigna unguicuilata L. Walp). J. Agric. Soc. Res., 8(1): 15-17. https://doi.org/10.4314/jasr.v8i1.2880
Tsai, W.T., Lee, C.Y. and Chang, C.Y., 2007. Fast pyrolysis of rice husk: Product yiled and compositions. Bioresour. Technol., 98: 22-28. https://doi.org/10.1016/j.biortech.2005.12.005
Tsay, T.T., Wu, T.S. and Lin, Y.Y., 2004. Evaluation of asteraceae plant for control of Meloidogyne incognita. J. Nematol., 36: 36-41.
Tworkoski, T., 2002. Herbicide effects of essential oils. Weed Sci., 50: 425–431. https://doi.org/10.1614/0043-1745(2002)050[0425:HEOEO]2.0.CO;2
Wang, H.F., Wang, J.L., Wang, C., Zhang, W.M., Liu, J.X. and Dai, B., 2012. Effect of bamboo vinegar as an antibiotic alternative on growth performance and fecal bacterial communities of weaned piglets. Livest. Sci., 144: 173–180. https://doi.org/10.1016/j.livsci.2011.11.015
Watarai, S. and Koiwa, M., 2008. Feeding activated charcoal from bark containing wood vinegar liquid (Nekka-Rich) is effective as treatment for Cryptosporidiosis in calves. J. Dairy Sci., 91: 1458-1463. https://doi.org/10.3168/jds.2007-0406
Weerechanchai, P., Tanggsathitkulchai, C. and Tanggsathitkulchai, M., 2011. Characterization of products of pyrolysis of palm kernel cake and cassava pulp residue. Korean J. Chem. Eng., 28(2): 2262-2272. https://doi.org/10.1007/s11814-011-0116-3
Wei, Q., Ma, X., and Dong, J., 2010. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrol., 87(1): 24-28. https://doi.org/10.1016/j.jaap.2009.09.006
Wei, Q.Y., Liu, G.Q., Wei, X.M., Ma, X.X.X., Dong, L. and Dong, R.J., 2009. Influence of wood vinegar as leaves fertilizer on yield and quality of celery. J. China Agric. Univ., 14(1): 89-92.
Whitehead, A.G. and Hemming, J.R., 1965. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol., 55: 25-38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Wu, Q., Zhang, S., Hou, B., Zheng, H., Deng, W., Liu, D. and Tang, W., 2015. Study on the preparation of wood vinegar from biomass residues by carbonization process. Bioresour. Technol., 179: 98–103. https://doi.org/10.1016/j.biortech.2014.12.026
Xu, Y., Hu, X., Li, W. and Shi, Y., 2011. Preparation and characterization of bio-oil from biomass, progress in biomass and bioenergy production, Dr. Shahid Shaukat (Ed.), ISBN: 978-953-307-491-7, InTech. pp. 197-222. http://www.intechopen.com/books/progress-in-biomass-and-bioenergy-production/preparation-and-characterization-of-bio-oil-from-biomass, https://doi.org/10.5772/16466
Yaman, S., 2004. Pyrolysis of biomass to produce fuels and chemical feed stocks. Energy Conv. Manage. 45: 651-671. https://doi.org/10.1016/S0196-8904(03)00177-8
Yang, X.J., Lin, Z.K., Chen, J., Wu, J.H., Si, H.P. and Lin, K.Y., 2014. Research progress of biochar, pyroligneous acid and organic fertilizer mixture and its components in agricultural production. Appl. Mech. Mater., 448: 680-687. https://doi.org/10.4028/www.scientific.net/AMM.448-453.680
Yatagai, M., 2004. Biological activity of wood vinegar and its recent applications. New Food Ind., 46: 5–16.
Yatagai, M., Nishimoto, M., Hori, K., Ohira, T. and Shibata, A., 2002. Termiticidal activity of wood vinegar, its components and homologues. J. Wood Sci., 48(4): 338-342. https://doi.org/10.1007/BF00831357
Yoo, J.H., Ji, S.C. and Jeong, G.S., 2007. Effect of dietary charcoal and wood vinegar mixture (CV82) on body composition of olive flounder Paralichthys alivaceus. J. World Aquac. Soc., 36(2): 203-208. https://doi.org/10.1111/j.1749-7345.2005.tb00386.x
Yoshimura, H., Washio, H., Yoshida, S., Seino, T., Otaka, M., Matsubara, K. and Matsubara, M., 1995. Promoting effect of wood vinegar compounds on fruit-body formation of Pleurotus ostreatus. Mycoscience, 36: 173–177. https://doi.org/10.1007/BF02268554
Zhang, Q., Chang, J., Wang, T. and Xu, Y., 2007. Review of biomass pyrolysis il properties and upgrading research. Energy Conv. Manage., 48: 87-92. https://doi.org/10.1016/j.enconman.2006.05.010
To share on other social networks, click on any share button. What are these?





