Muscular Tissue Bioaccumulation and Health Risk Assessment of Heavy Metals in Two Edible Fish Species (Gudusia chapra and Eutropiichthys vacha) in Padma River, Bangladesh
Muscular Tissue Bioaccumulation and Health Risk Assessment of Heavy Metals in Two Edible Fish Species (Gudusia chapra and Eutropiichthys vacha) in Padma River, Bangladesh
Dil Afroza Khanom1, Amirun Nesa1, Muhammad Abu Sayed Jewel1, Muhammad Ayenuddin Haque1, Alok Kumar Paul1, Sonia Iqbal2, Usman Atique2,3*, Lubna Alam4
1Faculty of Agriculture, Department of Fisheries, University of Rajshahi, Rajshahi-6205, Bangladesh
2Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore, Pakistan
3Department of Bioscience and Biotechnology, Chungnam National University, South Korea
4Institute for Environment and Development (LESTARI), School of Environmental & Natural Resource Sciences, Universiti Kebangsaan Malaysia (UKM), 43600 UKM BANGI, Selangor, Malaysia
Abstract | This study investigated edible and commercially valuable fish species viz. Gudusia chapra and Eutropiichthys vacha, for the muscular tissue bioaccumulation of selected heavy metals (Pb, Cd, Cr, Zn, Cu, and Mn) and their human health risks. A total of 50 fish species (25 individuals each) were examined from the Padma River, Bangladesh, and subjected to heavy metals presence and loads by detection after wet digestion of samples. The results displayed the hierarchy of average levels of the targeted metals in G. chapra in declining order of Zn (14.37) > Cu (12.63) >Mn (1.39) > Cr (0.64) >Pb (0.38) > Cd (0.03 mg/kg) whereas, Zn (11.89) > Cu (11.18) >Mn (1.69) > Cr (0.52) >Pb (0.19) > Cd (0.01 mg/kg) in E. vacha. The t-test indicated significant differences (P<0.05) between Pb and Zn, while, the inter-species relationships were not significant for the other metals investigated. The estimated average daily dose (ADD) intake remained below the reference dose as recognized by the international guidelines of WHO, FAO, and USEPA. Furthermore, the hazard quotient (HQ) and hazard index (HI) outcomes labelled a non-carcinogenic threat to the consumers. In conclusion, this study provides useful insights into the identification of potential risks of these commercially valuable fishes to the continuous consumption of fish flesh originating from a contaminated water body may lead to heavy metals toxicity leading to mental retardation, kidney tissue damages, cancerous cell growth, and even death in case of chronic exposure.
Novelty Statement | The different metal uptakes displayed inter-species variations. The estimated daily dose remained below the reference doses as well as hazard quotient and hazard index do not pose carcinogenic threat to consumers. However, continuous consumption of fish flesh from contaminated resources may lead to metal toxicity in case of chronic exposure.
Article History
Received: January 01, 2020
Revised: March 12, 2020
Accepted: May 02, 2020
Published: June 08, 2020
Authors’ Contributions
DAK, AN, MAH and AKP collected the data and carried out the fish sampling under the supervision of MASJ. DAK, AN and UA wrote the manuscript while UA and SI revised it. LA, SI and DAK analyzed the data.
Keywords
Heavy metals, Gudusia chapra, Eutropiichthys vacha, Human health risk, Padma River, Manganese, Cadmium
Corresponding author: Usman Atique
To cite this article: Khanom, D.A., Nesa, A., Jewel, M.A.S., Haque, M.A., Paul, A.K., Iqbal, S., Atique, S. and Alam, L., 2020. Muscular tissue bioaccumulation and health risk assessment of heavy metals in two edible fish species (Gudusia chapra and Eutropiichthys vacha) in Padma River, Bangladesh. Punjab Univ. J. Zool., 35(1): 81-89. https://dx.doi.org/10.17582/journal.pujz/2020.35.1.81.89
Introduction
Heavy metals pollution has become of critical importance owing to their toxicity and bioaccumulation in the aquatic organisms (Atique et al., 2020; Saeed et al., 2020). Under with continuous environmental exposure, the heavy metals may accumulate to such high levels resulting in toxic impacts and ecological damages (Moon et al., 2019; Bae et al., 2020). The study of metal bioaccumulation levels and their patterns in edible muscular tissues is an evolving process that adds more useful information to avoid potential health damages caused by metal toxicity. The fish absorbs various chemicals and metals directly from the contaminated aquatic environment by diffusion through gills and skin, or bioaccumulate through feeding and digestion (Frank et al., 1998; Khan et al., 2018). For healthy metabolism, the vital elements are absorbed from their environment through water and food, however extreme intakes could also produce toxic effects (Iqbal et al., 2017; Haider et al., 2018; Iqbal et al., 2020). However, non-essential metals including cadmium (Cd), nickel (Ni), mercury (Hg), and lead (Pb), and are reported to show high accumulation in vital organs of fish (Canli and Atli, 2003), especially muscle tissues. Furthermore, these metals could cause disruptions in biological systems (Younus et al., 2015). Thus, the heavy metals absorbed by the aquatic organisms through food chains of polluted ecosystems are potentially threatening chemicals to the health of fish consumers (Damadharan and Reddy, 2013).
The River Padma is flowing along the Rajshahi City in Bangladesh and has become immensely important for its precious fisheries resources during the recent decades. The riverbank harbors a plethora of anthropogenic activities, which produce bulk of pollutants of the industrial, agricultural and origins (Halder et al., 2014). Due to the hazardous discharges of untreated wastes materials, the river is becoming increasingly polluted. Having established its habitat as a suitable ground for several native fish species, it has become the center of pollutant investigations. Therefore, the investigations targeting the assessment of heavy metals pollution in the river is getting significant with the advent of time (Thilsted et al., 1997; Halder et al., 2014). The Gudusia chapra and Eutropiichthys vacha are considered of enormous commercial importance due to high market value. River catfish E. vacha of Padma River is considered as one of the highly nutritious and preferred fish species owing to higher nutritional value and has been reported to decline due to over-exploitation. Further ecological degradations under the devastating impacts of human activities (Atique and An, 2019a; Atique and An, 2020) and different ecological variations in the preferred habitats are creating a disturbing situation that decidedly merits to be studied (Hossain et al., 2015; Atique and An, 2018; Atique and An, 2019b; Kim et al., 2019).
Recent reports on the socio-economic contributions have also identified G. chapra and E. vacha as essential fisheries resource in preventing malnutrition, potential deficiency of vitamins and minerals, especially in women and children of rural communities (Thilsted et al., 1997). However, the likely reason for the decline of these valuable fish species could be linked industrial and domestic pollutants, ecological degradations and change in the preferred habitat in the riverine ecosystem (Atique et al., 2019). Having established the importance of the fish species as a favorite food source, the information about the bioaccumulation patterns of significantly important toxic metals in the targetted fish species is essential with respect to the environmental and human health management. Therefore, this study was planned to evaluate the selected heavy metals viz. Pb, Cd, Cr, Zn, Cu, and Mn concentrations in G. chapra and E. vacha from Padma River, Bangladesh. We also determined the potential human health risks posed by the oral consumption of these two species that could have been exposed to the heavy metals in Padma River.
Materials and Methods
Study area and sampling stretegy
This study was carried out for five months from October 2016 to February 2017 in the Padma River stretch along the Rajshahi City Corporation, which is in the northwestern part of Bangladesh (Figure 1). Two fish species, viz. Chapila (G. chapra) and Bacha (E. vacha) were collected from Shaheb Bazar Point of Padma River. A total of fifty fish pieces (25 each species) were taken as samples. The fishes were collected by using various types of fishing gears, including cast net, square lift net, conical trap, and monofilament fixed gill net. After collection, the fish samples were stored in the icebox to prevent the decaying of muscle tissues and maintaining the moister. The fishes were transferred to the research lab immediately, often within three hours before deep freeze storage until sample processing for heavy metal assessment. These two fish species can be identified in the photographs shown in Figure 2.
Sample preparation
Muscle tissues of each fish piece were used in the present experiment to estimate the bioaccumulated heavy metal contents in edible tissues. The samples were then dried in an oven at 110 ⁰C for three days until steady weight in the laboratory. After cooling in the desiccator, the whole fish samples were ground by hand grinder and homogenized. The powdered samples were then finally stored in a pre-cleaned dry container and preserved in desiccators by sealing to avoid moisture gain until further analyses.
Digestion procedure
For the quantitative analyses of Lead (Pb), Cadmium (Cd), Chromium (Cr), Zinc (Zn), Mn (Manganese) and Copper (Cu), the fish samples were digested. The whole digestion procedure of fish samples was performed at the Bangladesh Council of Scientific and Industrial Research (BCSIR) laboratory, Rajshahi. To determine the presence levels of heavy metals, wet digestion method was used (Khalifa et al., 2010). The wet digestion of each of the fish samples was completed by the utilization of an oxy-acidic mixture of HNO3/HClO4 (4:1) (10 ml was used for the a 1.0 g sample) using 250 ml beaker. Thus, obtained mixture was boiled at 120 oC for three hours and then condensed to 25 ml volume using the de-ionized water. The blank digestion preparation was also performed by using the same method. The beaker walls were then washed down with de-ionized water and the samples was filtered (Whatman 0.42 μm) in a volumetric flask of 100 ml capacity. At the end, each of the sample was made to with the required volume by using the de-ionized water (AOAC, 2005).
Sample analysis
The sample analyses were completed at the Central Science Laboratory of University of Rajshahi, Rajshahi. Analyses of the heavy metal contents were performed with a Flame Atomic Absorption Spectrometer (Shimadzu, AA-6800) that uses the acetylene gas as its fuel and while the air is used as an oxidizer (Khalifa et al., 2010). The prepared fish samples were aspirated for the determination of final metal concentration values by using the calibration curves that were drawn according to the standard solutions prepared separately for each metal studied. For each metal assessment, the averages of three replicates were used. The following formula calculated metal content of the fish samples (dry weight basis):
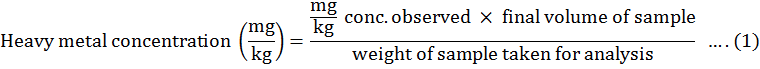
Health risk assessment from consuming fish
The dose risk evaluations were performed by employing the standard assumptions of the United States Environmental Protection Agency (USEPA) risk assessment. The details of these assumptions made for the calculation of human health risk are listed in Table 1. The potential non-cancer risk for individual heavy metals is expressed as the hazard quotient (HQ) and can be calculated as follows:
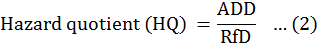
Where, ADD is the average daily dose (mg/kg/day) and RfD is oral reference dose and provides an estimate of daily exposure of a normal human being to a contaminant for a life period with a hidden risk of its harmful effects. The oral reference doses calculated for the selected heavy metals estimated in this study are given in Table 2. The risk levels are estimated if the HQ value surpasses 1.0, it is intolerable risk denoting an adverse and non-carcinogenic impact on the human health. However, the HQ < 1.0 falls within an acceptable range denoting lower level of human health risk risk (Lim et al., 2008).
The ADD is determined by employing this formula:
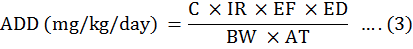
Where, C is the heavy metals level in the targeted tissue (mg/kg), IR denotes the mean ingestion rate which is equal to 0.0491 kg/day/person, EF (exposure frequency) which is 365 days per year, ED represents the duration of the exposure (30 years for non-cancer risk as designated by the USEPA, 2011), BW denotes the body weight and is taken equal to 70 kg, while AT is abbreviated from the average time (70 years × 365 days/year).
To calculate the total hazard risk of the heavy metals, all the risk assessments of the mixture of all the contaminants is calculated after summing up all the individual HQs and it provides new parameters called as hazard index (HI). The HI is calculated by the following formula:
Hazard index (HI) = HQPb+HQCd+HQCr+HQZn+HQCu+HQMn …(4)
Where the HI > 1 presents an intolerable risk of non-carcinogenic to the human health, while an HI < 1 indicates a tolerable risk level to the humans (Lim et al., 2008).
Table 1: Assumptions for average daily dose (ADD) intake calculation.
|
Assumptions |
Reference |
|
Ingested dose is equal to the absorbed pollutant dose |
|
|
Cooking has no effect on the pollutants |
|
|
The average adult body weights (BW) of the Bangladeshi assumed to be 70 kg |
|
|
Average lifetime of Bangladeshi is 70 years |
Table 2: Oral reference doses of heavy metals (USEPA, 2009; FAO/WHO, 2006).
|
Heavy metals |
Pb |
Cd |
Cr |
Zn |
Cu |
Mn |
|
RfD (mg/kg/day) |
4×10-3 |
1×10-3 |
1.5 |
3×10-1 |
4×10-2 |
0.157 |
Table 3: Summary statistics of heavy metal concentrations (mg/kg) in whole body of Gudusia chapra and Eutropiichthys vacha from Padma River with international guidelines values.
|
Fish species |
Concentration of metals (mg/kg) |
||||||
|
Pb |
Cd |
Cr |
Zn |
Cu |
Mn |
||
|
Gudusia chapra |
Min. |
0.196 |
0.001 |
0.412 |
11.095 |
10.260 |
0.085 |
|
Max. |
0.623 |
0.089 |
0.878 |
21.177 |
20.530 |
3.642 |
|
|
Average |
0.375a |
0.031a |
0.645a |
14.367a |
12.632a |
1.391a |
|
|
S.D. |
0.156 |
0.026 |
0.149 |
3.851 |
2.939 |
1.232 |
|
|
Eutropiichthys vacha |
Min. |
0.063 |
0.001 |
0.136 |
10.036 |
10.112 |
0.125 |
|
Max. |
0.294 |
0.027 |
0.824 |
13.100 |
14.820 |
3.984 |
|
|
Average |
0.193b |
0.014a |
0.525a |
11.892b |
11.180a |
1.688a |
|
|
S.D. |
0.073 |
0.008 |
0.228 |
1.039 |
1.684 |
1.266 |
|
|
t-value |
3.341 |
2.054 |
0.609 |
2.526 |
0.691 |
-0.532 |
|
|
p-value |
0.004 |
0.055 |
0.550 |
0.021 |
0.498 |
0.601 |
|
|
FAO/WHO/USFDA/EC |
2/1.5 |
0.5 |
12-13 |
30 |
30 |
1 |
|
Min.: Minimum; Max.: Maximum; S.D.: Standard deviation. Different superscripts indicate significant differences at p < 0.05 level.
Statistical analysis
The results obtained after calculations of heavy metal loads were expressed as means ± standard deviation (SD). An independent t-test was applied to decide the significant difference of the targetted metals concentration between two fishes. The significance level was set at 5% confidence level. Further, we used the Pearson’s correlation coefficient (r) to determine the relationship between inter-metal contents in fish species. All the statistical analyses were performed in the Statistical Package for the Social Sciences (SPSS v. 20).
Results and Discussion
Accumulation of heavy metals in fish species
Table 3 outlines the concentrations of targeted heavy metals in sampled fish species on the basis of their dry weight from the Padma River. The results revealed the presence of the heavy metals in the order of their mean levels as Zn (14.367) > Cu (12.632) > Mn (1.391) > Cr (0.645) > Pb (0.375) > Cd (0.031) (mean; mg/kg dry wt.) in G. chapra and Zn (11.892) > Cu (11.180) > Mn (1.688) > Cr (0.525) > Pb (0.193) > Cd (0.014) in E. vacha, respectively. This alluded to the metals content in the edible muscle tissues varied between the fish species. Therefore, it is clear that diverse fish species thriving in the same aquatic habitat may bioaccumulate dissimilar levels of heavy metals, which corroborates with the outcomes of several preceding surveys. However, the sequential order of metals observed in these examined species might also be linked with the variations in bioavailability of the metals, fundamental physiological processes in the fish, and feeding mode and preferences of each fish species. Similar findings have also been described by Eneji et al. (2011) and Lim et al. (2008) as factors for species-specific variations of edible tissues heavy metal content.
Zinc contents
Zn content in G. chapra was the highest (14.37 mg/kg) compared to other metals studied as it is an indispensable to the healthy growth as well as normal metabolic process in organisms (Meshram et al., 2014). This study recorded a significant difference (P < 0.05) between the mean concentrations of Zn content in G. chapra and E. vacha (Table 3). However, we noticed the highest mean value of Zn in G. chapra compared to E. vacha that could have been due to the omnivorous nature of this species, which promotes the accumulation of Zn from different food items (Siraj et al., 2014). Zinc is an element that tends to get stored in the body of aquatic organisms particularly their fatty tissues., However, it is established to influence the reproductive behavior and physiology in fish species (Meshram et al., 2014). This was also in accordance with the findings of Krishna et al. (2014), who testified Zn as the highest-level element found in edible muscles tissues. The possible reason for higher Zn contents in the fish muscles might be the variety of anthropogenic activities that are taking place in Rajshahi City area characterized by the disposal of raw sewage materials and household garbage. However, the Zn content of experimental fishes was well lower than the allowable limit (30 mg/kg) reported by FAO (1983).
Copper contents
Copper was reported as the second most plentiful heavy metal observed in fish muscle tissues of targeted fish species. However, we could not notice any significant difference of its mean values. The Cu contents in G. chapra and E. vacha ranged between 10.26-20.53 and 10.11-14.82 mg/kg with the mean concentration equal to 12.63 and 11.18 mg/kg, respectively (Table 3). The highest Cu content in G. chapra was also due to the omnivorous nature as reported by Siraj et al. (2014). Increased Cu content in the environment can cause the fish to undergo difficult respiration and produce stress and additional impacts by decreasing appetite, consequently compromised growth rate. The toxic impacts of Cu could damage the olfactory and sensory systems of the fish as well (McIntyre et al., 2008), resulting in a problematic response to food. If we compare the present findings with the recommended value of WHO (1995; 30 mg/kg), the level of Cu recorded in targeted fish muscle tissues remained within the acceptable limits. However, a further increase from this value might have hazardous impacts on fish physiology and its responses.
Manganese contents
The Mn contents in targetted fish species ranged between 0.09 to 3.64 mg/kg in G. chapra and 0.12 to 3.98 mg/kg in E. vacha with a mean value of 1.39 and 1.69 mg/kg in G. chapra and E. vacha, respectively (Table 3). Mn is an indispensable trace element for the human beings and animals, and it has an established and vital role in the enzymatic metabolism (Tinggi et al., 1997). Mortuza and Al-Misned (2015) reported Mn levels as 1.48 ± 0.23 mg/kg in H. ilisha from Padma River, which was higher than present mean concentrations observed in G. chapra and lower than in E. vacha. It indicates Mn potential pollution in Padma River that could have been originating from fertilizers, varnish and fungicides, industrial effluents, domestic garbage and municipal waste, and livestock feeding supplements. Mohiuddin et al. (2015) have reported similar results in Buriganga River. The variations in Mn content between these two species could be due to the diverse feeding natures and size variations (Fernandes et al., 2007). Even Though it is considered as an indispensable trace metal, it has shown toxicity at higher doses as reported by Burger and Gochfeld (1996). As the presence levels of Mn in the whole muscle samples exceeded the FAO (1983) limit of 1.0 mg/kg, thus it could pose a severe health hazard to the fishes as well as its consumers.
Chromium contents
Cr loads did not differ significantly within the two fish species during this investigation. The value of Cr in G. chapra fluctuated between 0.412 to 0.878 mg/kg and the mean concentration was 0.645 mg/kg. While in E. vacha, the Cr content ranged from 0.136 to 0.824 mg/kg and the mean level was recorded as 0.525 mg/kg, respectively (Table 3). Cr can induce serious health hazards in fish, ranging from swimming and feeding disorders, erosions of fin rays, ulcerations, and even mortality (Abbasi and Soni, 1984). Cr is reported for its serious threats to the aquatic environment and human health owing to its cancer-causing and endocrine-disrupting impacts to the human beings (Atique et al., 2020). The Cr content in these fish species was fairly lower than the permissible values (12-13 mg/kg) of USFDA (1993) for human consumption. It was also lower than the Cr level stated by Ahmad et al. (2010) for G. chapra (6.27, 5.28 and 7.38 mg/kg during seasons based on monsoon ranfall, and Paudel et al. (2016) for E. vacha (10.00 ± 3.92 mg/kg). The possible reasons might also be the higher inflow of pollutants in the river as compared to the present study area.
Lead contents
The Pb levels diverged significantly (P < 0.05) among the targetted fish species. Pb is a known as a neurotoxin that induces continuous behavioural discrepancies in the fish species (Weber and Dingel, 1997) as well as affects growth and survival rates, embryo development, learning and metabolic process, and increases the mucus production in various fish species (Eisler, 1988). The level of Pb was detected ranging between 0.196 to 0.623 in G. chapra and 0.063 to 0.294 mg/kg in E. vacha with a mean value of 0.375 and 0.193 mg/kg in G. chapra and E. vacha, respectively (Table 3). The Pb content of G. chapra from the Buriganga River was observed by Ahmad et al. (2010) in as 9.12, 13.52 and 10.12 mg/kg during the pre-monsoon, monsoon and post-monsoon months, respectively, which was higher than our findings. It is perhaps associated with the sampling area of our study being a less contaminated place as compared to Buriganga River owing to rapid industrialization, urbanization, and various anthropological activities adding higher Pb contents. However, the Pb contents in G. chapra were lower than that of Hilsa ilisha. This fish belongs to the same family of G. chapra from Padma River reported by Mortuza and Al-Misned (2015) might be due to the smaller body size of the targeted fish species. The lower contents of Pb in E. vacha (0.193 mg/kg) could be linked with the specific feeding behavior (carnivorous feeding habit) of this fish living in the similar habitat, a fish species having the omnivorous feeding nature would bioaccumulate more metal contents in comparison with the species having carnivorous feeding preferences (Siraj et al., 2014). Being omnivorous, G. chapra was exceedingly exposed to metal bioaccumulation having several live foods options. However, the Pb content of these fish species were well below the allowable limits (2 mg/kg) of WHO (1995) as well as by that of EC (2006) (1.5 mg/kg).
Cadmium contents
The Cd concentrations ranged between 0.001 to 0.089 mg/kg in G. chapra and 0.001 to 0.027 mg/kg in E. vacha with the mean levels of 0.031 and 0.014 mg/kg, respectively (Table 3). However, no significant difference (P < 0.05) among the mean values of the Cd contents in targeted fishes. However, the Cd content was higher in G. chapra compared to E. vacha, could be for comparable reasons reported above for Pb content. The Cd content of G. chapra was below the published values (0.83, 1.05 and 1.07 mg/kg, during the seasons including pre-monsoon, monsoon and post-monsoon, respectively) from the Buriganga River (Ahmad et al., 2010). On the other hand, the value of Cd in E. vacha was below the results (0.61 ± 0.18 mg/kg) reported by Paudel et al. (2016) in Kathmandu Valley, Nepal. However, the Cd content observed during this study period remained lower than the allowable value (0.5 mg/kg) reported by FAO (1983).
Inter-metal relationship in fishes
Majority of the targeted heavy metal contents indicated strong positive correlations in both fish species in Padma River (Table 4). Significantly positive correlations among the metals in G. chapra were observed among the Pb-Cd (r=0.806), Pb-Cu (r=0.769), Cd-Mn (r=0.647), and Cr-Mn (r=0.851). On the other hand, in E. vacha, significantly positive correlations were found among the metal combinations of Pb-Cd (r=0.682), Pb-Cr (r=0.757), Pb-Mn (r=0.799), Cd-Cr (r=0.657), Cd-Cu (r=0.733), Cr-Cu (r=0.767), and Cr-Mn (r=0.638). Such stronger and noticeably significant relationships among the metal contents could be indicating to a similar source of these metals, similar level occurrences and as well as hinting at the identical biogeochemical pathways leading to the bioaccumulation in these fish species and their edible tissues (Paudel et al., 2016; Atique et al., 2020). These relationships among the heavy metals could have resulted from the analogous tendencies of bioaccumulation in the fish species as well as their ecological and biological connections (Rejomon et al., 2010). However, bioaccumulation of these metal elements in the studied fish species might have been influenced by the duration of exposure, route of these metals and their uptake, environmental parameters including temperature, salinity level, pH conditions and, hardness). There could also be several other factors including the metal properties as well as the intrinsic factors such as age of the fish and feeding preferences (Jezierska and Witeska, 2006).
Table 4: Pearson’s correlation indicating the inter-metal relationship in G. chapra and E. vacha.
|
Pb |
Cd |
Cr |
Zn |
Cu |
Mn |
|
|
Gudusia chapra |
||||||
|
Pb |
1 |
|||||
|
Cd |
0.806** |
1 |
||||
|
Cr |
0.388 |
0.473 |
1 |
|||
|
Zn |
0.163 |
-0.128 |
0.332 |
1 |
||
|
Cu |
0.769** |
0.473 |
0.076 |
0.087 |
1 |
|
|
Mn |
0.569 |
0.647* |
0.851** |
0.344 |
0.236 |
1 |
|
Eutropiichthys vacha |
||||||
|
Pb |
1 |
|||||
|
Cd |
0.682* |
1 |
||||
|
Cr |
0.757* |
0.657* |
1 |
|||
|
Zn |
0.439 |
0.287 |
0.629 |
1 |
||
|
Cu |
0.471 |
0.733* |
0.767** |
0.612 |
1 |
|
|
Mn |
0.799** |
0.619 |
0.638* |
0.418 |
0.557 |
1 |
*. Correlation is significant at the 0.05 level (2-tailed). **. Correlation is significant at the 0.01 level (2-tailed).
Health risk assessment from consuming contaminated fish
The bioaccumulation patterns of targeted heavy metals in the edible muscles could be used as an efficient tool for measuring the presence burden of those elements that are detected at an elevated level along with an assessment into their impacts of public health (Bastami et al., 2015). The muscular tissues and not the active sites of elemental biotransformation and bioaccumulation (Elnabris et al., 2013). Nevertheless, in the contaminated aquatic ecosystems, the presence levels of hazardous elements in the fish muscles could exceed their allowable ranges for the human ingestion and imply critical health threats. Heavy metals tend to bioaccumulate in different organs of body in aquatic organisms, particularly fishes. This is how they could enter the human metabolic pathways via regular intake of contaminated fish flesh, concomitantly leading to serious human health threats (Rahman et al., 2012). The mean whole-body metal levels were summed up to conduct the health hazard evaluations to the humans after the consumption of contaiminated fish. The estimated average daily dose (ADD) intake values were 1.127, 9.319, 1.939, 4.282, 3.797 and 4.182 mg/kg/day for G. chapra whereas 5.802, 4.209, 1.578, 3.361, 3.575 and 5.074 mg/kg/day in E. vacha for Pb, Cd, Cr, Zn, Cu and Mn, respectively (Table 5). The ADD outcomes calculated for each of the heavy metals in targeted fish species in this study were below the recommended levels (FAO/WHO, 2011; USEPA, 2012). The ADD values calculated for each element estimated in the targetted fish species were less than the previously reported studies by Mortuza and Al-Misned (2015) in Padma River near Rajshahi City of Bangladesh. The ADD values indicated low concentration of all target heavy metals, indicating lower contamination of the targeted fish species in Padma River.
Table 5: Hazard quotient (HQ) and hazard index (HI) of heavy metals calculated from consumption of two fish species collected from the Padma River.
|
Metals |
Gudusia chapra |
Eutropichthys vacha |
||
|
ADD (mg/kg/day) |
HQ |
ADD (mg/kg/day) |
HQ |
|
|
Pb |
1.127 ×10-4 |
0.0282 |
5.802 ×10-5 |
0.0145 |
|
Cd |
9.319 ×10-6 |
0.0931 |
4.209 ×10-6 |
0.0042 |
|
Cr |
1.939 ×10-4 |
0.0001 |
1.578 ×10-4 |
0.0001 |
|
Zn |
4.282 ×10-3 |
0.0143 |
3.361 ×10-3 |
0.0112 |
|
Cu |
3.797 ×10-3 |
0.0949 |
3.575 ×10-3 |
0.0894 |
|
Mn |
4.182 ×10-4 |
0.0027 |
5.074 ×10-4 |
0.0032 |
|
Hazard index (HI) |
0.2333 |
0.1226 |
||
ADD: Average daily dose; HQ: Hazard quotient.
This study also quantified potential risks to the human health as HQ for the heavy metals including Pb, Cd, Cr, Zn, Cu and Mn in the two targeted fishes in Padma River. The estimated HQ from Pb, Cd, Cr, Zn, Cu and Mn for G. chapra found in the present study were 0.0282, 0.0931, 0.0001, 0.0143, 0.0949 and 0.0027, while it was 0.0145, 0.0042, 0.0001, 0.0112, 0.0894 and 0.0032 for E. vacha, respectively. The HI considering the human populations for consuming G. chapra and E.vacha were 0.2333 and 0.1226 (Table 5). The observed HQ and HI were calculated as lower than the safe limits (HI ≤ 1). Consequently, the outcomes of these findings confirmed the intake of these fishes (if continued) at this rate may not be harmful to the human beings for the observed values of studied meals (Pb, Cd, Cr, Zn, Cu and Mn), be it alone or in combinations with each of these metals.
Conclusion
In conclusion, a significant difference was discovered in the metal contents in the edible muscle tissues of these targeted fish species. Zn was observed at a higher quantity, while Cd at a lower amount in both fish species in Padma River. However, studied metals were found significantly correlated to each other that indicated a similar source of these metals to fish. Most of the metals did not exceed the permissible limits given by different regulatory organizations. According to the daily dietary allowance, the estimated average daily dose intake for the examined fish species were far below the risk level except for manganese. Therefore, the human health risks posed by contamination of targeted metals bioaccumulation in the edible muscle tissues of the fish species with respect to the targeted heavy metals were low. However, constant monitoring of the Padma River environment should be recommended to reduce future risks of heavy metal toxicity posed by the bioaccumulation in riverine fish species.
Acknowledgement
The authors are grateful to the fishermen along the Padma River for supplying fish samples and Bangladesh Council of Scientific and Industrial Research (BCSIR), Rajshahi for their cordial assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
Abbasi, S.A., and Soni, R., 1984. Toxicity of lower than permissible levels of chromium (VI) in the freshwater teleost Nuria Denricus. Environ. Pollut., 36: 75-82. https://doi.org/10.1016/0143-1471(84)90199-5
Ahmad, M.K., Islam, S., Rahman, S., Haque, M.R., and Islam, M.M., 2010. Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int. J. Environ. Res., 4: 321-332.
AOAC, 2005. International official methods of analysis of AOAC international, 18th edition. Gaithersburg, Maryland, USA.
Atique, U., and An, K.-G., 2018. Stream health evaluation using a combined approach of multi-metric chemical pollution and biological integrity models. Water, 10: 661. https://doi.org/10.3390/w10050661
Atique, U., and An, K.-G., 2019. Reservoir water quality assessment based on chemical parameters and the chlorophyll dynamics in relation to nutrient regime. Polish J. Environ. Stud., 28: 1043–1061. https://doi.org/10.15244/pjoes/85675
Atique, U., and An, K.-G., 2020. Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecol. Indic., 110: 105813. https://doi.org/10.1016/j.ecolind.2019.105813
Atique, U. and An, K.-G., 2019. Water quality and trophic state index analysis in relation to land use patterns in agricultural reservoirs. In: Korea Soc. Environ. Ecol. Proc., pp. 110.
Atique, U., Iqbal, S., Khan, N., Qazi, B., Javeed, A., Anjum, K.M., Haider, M.S., Khan, T.A., Mahmood, S. and Sherzada, S., 2020. Multivariate Assessment of Water Chemistry and Metals in a River Impacted by Tanning Industry. Fresenius Environ. Bull., 29: 3013-3025.
Atique, U., Byungjin, L., Johee, Y., and An, K.-G., 2019. Biological Health Assessments of Lotic Waters by Biotic Integrity Indices and their Relations to Water Chemistry. Water, 11: 436. https://doi.org/10.3390/w11030436
Bae, D.-Y., Atique, U., Yoon, J., Lim, B., and An, K.-G., 2020. Ecological Risk Assessment of Urban Streams Using Fish Biomarkers of DNA Damages and Physiological Responses. Polish J. Environ. Stud. 29: 1–10. https://doi.org/10.15244/pjoes/104660
Bastami, K.D., Afkhami, M., Mohammadizadeh, M., Ehsanpour, M., Chambari, S., Aghaei, S., Esmaeilzadeh, M., Neyestani, M.R., Lagzaee, F., and Baniamam, M., 2015. Bioaccumulation and ecological risk assessment of heavy metals in the sediments and mullet Liza klunzingeri in the northern part of the Persian Gulf. Mar. Pollut. Bull., 94: 329–334. https://doi.org/10.1016/j.marpolbul.2015.01.019
Burger, J., and Gochfeld, M., 1996. Heavy metal and selenium levels in Franklin’s Gull (Larus pipixcan) parents and their eggs. Arch. Environ. Contam. Toxicol., 30: 487–491. https://doi.org/10.1007/BF00213400
Canli, M., and Atli, G., 2003. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb and Zn) levels and the size of six Mediterranean fish species. Environ. Pollut., 121: 129–136. https://doi.org/10.1016/S0269-7491(02)00194-X
Cooper, C.B., Doyle, M.E., and Kipp, K., 1991. Risk of consumption of contaminated seafood, the Quincy Bay Case Study. Environ. Hlth. Perspect., 90: 133-140. https://doi.org/10.1289/ehp.90-1519503
Damadharan, U., and Reddy, M.V., 2013. Heavy metal bioaccumulation in edible fish species from an industrially polluted river and human health risk assessment. Arch. Pol. Fish., 21: 19-27. https://doi.org/10.2478/aopf-2013-0003
EC, 2006. European Commission Regulation no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union, L634/5-24.
Eisler, R., 1988. Lead hazards to fish, wildlife, and invertebrates: A synoptic review. US Fish Wildl. Ser. Rep., Washington DC. 85(1.14): 1-94.
Elnabris, K.J., Muzyed, S.K., and El-Ashgar, N.M., 2013. Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J. Assoc. Arab Univ. Basic Appl. Sci., 13: 44-51. https://doi.org/10.1016/j.jaubas.2012.06.001
Eneji, I.S., Rufus, S., and Annune, P.A., 2011. Bioaccumulation of heavy metals in fish (Tilapia zilli and Claria garienpinus) Organs from River Benue. Pak. J. Anal. Environ. Chem., 12: 25-31.
FAO, 1983. Compilation of legal limits for hazardous substances in fish and fishery products. Fd. Agric. Organ., FAO Fish. Circ., 464: 5-100.
FAO/WHO, 2006. A model for establishing upper levels of intake for nutrients and related substances. Food and Agriculture Organization of the United Nations/World Health Organization,
FAO/WHO, 2005. Nutrient risk assessment workshop, 2–6 May 2005, WHO Headquarters, Geneva.
FAO/WHO, 2011. Evaluations of the Joint FAO/WHO Expert committee on food additives (JECFA). http://apps.who.int/ipsc/database/evaluations/search.aspx?fcc=2.
Fernandes, C., Fontainhas-Fernandes, A., Peixoto, F., and Salgado, M.A., 2007. Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon. Portugal. Ecotoxicol. Environ. Saf., 66: 426-431. https://doi.org/10.1016/j.ecoenv.2006.02.007
Frank, A.P.C.G., John, B.W., Ronald, W.R., and Haffner, G.D., 1998. Mechanism of biomagnification in fish under laboratory and field conditions. Environ. Sci. Technol., 33: 133-141. https://doi.org/10.1021/es980681m
Haider, M.S., Javid, A., Azmat, H., Abbas, S., Ashraf, S., Altaf, M., Atique, U., Iqbal, S., Iqbal, K.J. and Baool, M., 2018. Effect of processed fish waste on growth rate and digestive enzymes activities in Cyprinus carpio. Pak. J. Zool. Suppl. Ser: 191–198.
Halder, P.K., Paul, N., Hoque, M.E., Parvez, M.S., Rahman, M.H., and Ali, M., 2014. Municipal solid waste and its management in Rajshahi City, Bangladeh: A source of energy. Int. J. Renew. Energy Res., 4: 1.
Hossain, M.Y., Hossain, M.A., Ahmed, Z.F., Islam, R., Hossen, M.A., Rahman, M.M., and Ohtomi, J., 2015. Threatened fishes of the world: Eutropiichthys vacha (Hamilton, 1822) (Siluriformes: Schilbeidae). Croatian J. Fish., 73: 80–82. https://doi.org/10.14798/73.2.798.
Iqbal, S., Atique, U., Mughal, M.S., Khan, N., Haider, M.S., Iqbal, K.J., and Akmal, M., 2017. Effect of Selenium Incorporated in Feed on the Hematological Profile of Tilapia (Oreochromis niloticus). J. Aquact. Res. Dev., 8: 1000513. https://doi.org/10.4172/2155-9546.1000513
Iqbal, S., Atique, U., Mughal, M.S., Younus, M., Rafique, M.K., Haider, M.S., Iqbal, H.S., Sherzada, S., and Khan, T.A., 2020. Selenium-Supplemented Diet Influences Histological Features of Liver and Kidney in Tilapia (Oreochromis niloticus). Jordan J. Biol. Sci. 13: [In Press].
Jezierska, B., and Witeska, M. 2006. The metal uptake and accumulation in fish living in polluted waters. Soil Water Pollut. Monit., Prot. Rem. pp. 107-114. https://doi.org/10.1007/978-1-4020-4728-2_6
Khalifa, K.M.A., Hamil, M.A., Al-Houni, Q.A., and Ackacha, M.A., 2010. Determination of Heavy Metals in Fish Species of the Mediterranean Sea (Libyan coastline) Using Atomic Absorption Spectrometry. Int. J. Pharm. Tech. Res., 2: 1350-135.
Khan, N., Atique, U., Ashraf, M., Mustafa, A., Mughal, M.S., Rasool, F., Azmat, H., Tayyab, M. and Iqbal, K.J., 2018. Effect of various protein feeds on the growth, body composition, hematology and endogenous enzymes of catfish (Pangasius hypophthalmus). Pak. J. Zool., Suppl. Ser: 112–119.
Kim, J.-J., Atique, U., and An, K.-G., 2019. Long-Term Ecological Health Assessment of a Restored Urban Stream Based on Chemical Water Quality, Physical Habitat Conditions and Biological Integrity. Water, 11: 114. https://doi.org/10.3390/w11010114
Krishna, P.V., Jyothirmayi, V., and Rao, K.M., 2014. Human health risk assessment of heavy metal accumulation through fish consumption, from Machilipatnam Coast, Andhra Pradesh, India. Int. Res. J. Publ. Environ. Hlth., 1: 121.
Lim, H.S., Lee, J.S., Chon, H.T., and Sager, M., 2008. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor., 96: 223–230. https://doi.org/10.1016/j.gexplo.2007.04.008
McIntyre, J.K., Baldwin, D.H., Meador, J.P., and Scholz, N.L., 2008. Chemosensory deprivation in juvenile Coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ. Sci. Technol., 42: 1352–1358. https://doi.org/10.1021/es071603e
Meshram, L.N., Udawant, S.M., Pawar, S., and Mishra, P.S., 2014. Bioaccumulation of heavy metals (Zn, Pb, Cd, and Ni) in tissues of Penaeus monodon (Fabricius, 1798) from India. Int. J. Adv. Res., 2: 548-555.
Mohiuddin, K.M., Alam, M.M., Ahmed, I., and Chowdhury, A.K., 2015. Heavy metal pollution load in sediment samples of the Buriganga river in Bangladesh. J. Bangladesh Agric. Univ., 13: 229–238. https://doi.org/10.3329/jbau.v13i2.28784
Moon, W.-K., Atique, U., and An, K.-G., 2019. Ecological risk assessments and eco-toxicity analyses using chemical, biological, physiological responses, DNA damages and gene-level biomarkers in Zebrafish (Danio rerio) in an urban stream. Chemosphere, 239: 124754. https://doi.org/10.1016/j.chemosphere.2019.124754
Mortuza, M.G., and Al-Misned, F.A., 2015. Trace elements and heavy metals in five cultured and captured fishes from Rajshahi City, Bangladesh. BMST, 1: e2. http://www.mdtcanada.ca/bmst.html
Paudel, P.N., Pokhrel, B., Kafle, B.K., and Gyawali, R., 2016. Analysis of heavy metals in some commercially important fishes of Kathmandu Valley, Nepal. Int. Fd. Res. J., 23: 1005-1011. http://www.ifrj.upm.edu.my
Rahman, M.S., Molla, A.H., Saha, N., and Rahman, A., 2012. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Dhaka, Bangladesh. Fd. Chem., 134: 1847–1854. https://doi.org/10.1016/j.foodchem.2012.03.099
Rejomon, G., Nair, M., and Joseph, T., 2010. Trace metal dynamics in fishes from the southwest coast of India. Environ. Monit. Assess., 167: 243-255. https://doi.org/10.1007/s10661-009-1046-y
Saeed, F., Iqbal, K.J., Atique, U., Javid, A., Khan, N., Iqbal, S., Majeed, H., Azmat, H., Khan, B.Y.A., Baboo, I., Shahid, M.T. and Afzal, G., 2020. Toxic trace metals assessment in selected organs of edible fish species, sediment and water in Head Punjnad, Punjab, Pakistan. Punjab Univ. J. Zool., 35: 43-50. https://dx.doi.org/10.17582/journal.pujz/2020.35.1.43.50
Siraj, M., Shaheen, M., Sthanadar, A.A., Khan, A., Chivers, D.P., and Yousafzai, A.M., 2014. A comparative study of bioaccumulation of heavy metals in two freshwater species, Aorichthys seenghala and Ompok bimaculatous at River Kabul, Khyber Pakhtunkhwa, Pakistan. J. Biodiver. Environ. Sci., 4: 40-54.
Thilsted, S.H., Roos, N., and Hasan, N., 1997. The role of small indigenous fish species in food and nutrition security in Bangladesh. Naga, ICLARM Q., 20: 82-84, 102.
Tinggi, U., Reilly, C., and Patterson, C., 1997. Determination of manganese and chromium in foods by atomic absorption spectrometry after wet digestion. Fd. Chem., 60: 123–128. https://doi.org/10.1016/S0308-8146(96)00328-7
USEPA, (United States Environmental Protection Agency), 2011. USEPA Regional Screening Level (RSL) Summary Table: November 2011.
USEPA, (United States Environmental Protection Agency), 2012. EPA Region III Risk-Based Concentration (RBC) Table 2008 Region III, 1650 Arch Street, Philadelphia, Pennsylvania 19103.
USEPA, 1989. Risk assessment guidance for super fund. Human health evaluation manual (part A) Interim final, Washington (DC): United States Environmental Protection Agency.
USEPA, 2009. Risk-based Concentration Table. Philadelphia PA: United States Environmental Protection Agency, Washington, DC.
USFDA, 1993. United States Food and drug administration, Guidance document for chromium in shellfish. DHHS/PHS/FDA/CFSAN/office of seafood, Washington, DC.
Weber, D.N., and Dingel, W.M., 1997. Alterations in Neurobehavioral Responses in Fishes Exposed to Lead and Lead-Chelating Agents. Am. Zool., 37: 354-362. https://doi.org/10.1093/icb/37.4.354
WHO, 1995. Environmental Health Criteria No 165: Inorganic Lead. Geneva (Switzerland): World Health Organization (WHO). http://www.inchem.org/documents/ehc/ehc/ehc165.htm
Younus, M., Iqbal, S., Mughal, M.S., Javid, A., Rafique, M.K., Khan, A.U., Khan, N., and Atique, U., 2015. Effect of Selenium Incorporated In Feed on the Hematological Profile of Oreochromis niloticus. Abstr. Appl. Sci. Eng., pp. 1–22.
To share on other social networks, click on any share button. What are these?








