Length-Weight Relationship and Reproductive Biology of the Indian River Shad, Gudusia chapra (Hamilton, 1822) from Central Brahmaputra Valley of Assam, North-East India
Length-Weight Relationship and Reproductive Biology of the Indian River Shad, Gudusia chapra (Hamilton, 1822) from Central Brahmaputra Valley of Assam, North-East India
Abdul Malik Ahmed, Bhargav Bhushan Nath, Ayub Ali, Jiten Sarma, Bipul Phukan, Imtiaz Ahmed* and Jyotirmoyee Das
Department of Fisheries Resource Management, College of Fisheries, Assam Agricultural University, Raha, Nagaon, Assam 782103, India
ABSTRACT
The Indian River shad, Gudusia chapra (Hamilton, 1822), is a very popular food fish with high market price in the Central Brahmaputra Valley of Assam. A total of 458 specimens comprising of 161 males and 297 females with total length (TL) 7.8 to 15.6 cm and total weight (TW) 5.01 to 38.19 gm were collected monthly basis from August 2017 to July, 2018 from Thekera beel (wetland) of Morigaon District of Assam. The length-weight relationship of Gudusia chapra was found to be W=0.01L2.897, W=0.01L2.9165 and W=0.01L2.9214 for male, female and pooled samples, respectively. The ‘b’ value was recorded to be deviating (P<0.01) from the value of ‘3’ indicating a negative allometric growth of the species. The overall sex ratio (M:F) was 1:1.84 (Chi-square 40.38, P <0.01), indicating the predominance of females over males. Monthly analysis data of Gonado Somatic Index (GSI) showed two peaks with one in April and another in August suggesting Gudusia chapra breeds twice a year. The species was recorded to be highly fecund with fecundity ranging from 7,095-48,238. The relationship between fecundity and variables viz total length, body weight and ovary weight were observed to be linear and highly significant (r =0.979).
Article Information
Received 22 January 2021
Revised 13 March 2021
Accepted 25 March 2021
Available online 10 September 2021
(early access)
Published 23 April 2022
Authors’ Contribution
AA and JS provided overall guidance for research. BP and BBN helped during laboratory analysis. IA
guided in statistical analysis and paper writing. JD helped in sampling.
Key words
Length-Weight relationship, Sex ratio, Gonadosomatic index, Fecundity, Thekera beel, Central Brahmaputra Valley.
DOI: https://dx.doi.org/10.17582/journal.pjz/20210122140157
* Corresponding author: imtiaz.ahmed@aau.ac.in
0030-9923/2022/0004-1869 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Indian River shad, Gudusia chapra is one of the commercially important small indigenous freshwater clupeid fish belonging to order Clupeiformes and family Clupeidae , widely distributed in the rivers of Indian sub-continent mainly in the Ganga, Brahmaputra and Mahanadi river (Whitehead, 1985); also, in ponds, lakes, ditches and inundated fields (Rahman, 1989). The Indian River Shad is a prolific breeder (Reide, 2004) and is an important source of nutrition for rural people, supporting the livelihoods of many of the subsistence and artisanal fishermen Indian subcontinent (Talwar and Jhingran, 1991; Jayaram, 1999; Daniels, 2002). The species has high potential in the market because of its nutritional quality, wide abundance and round the year availability and thus can be identified as a candidate species for rearing in inundated fields, paddy fields, wetlands etc.
A length-weight relationship is an important tool in fishery management. It can be used as a tool for differentiation of small taxonomic units, as the exponent b may be different for fish of different localities, of different sexes and different stages of the developmental process but constant for fishes similar in these aspects (Le Cren, 1951). The establishment of Length-Weight relationship (LWR) is necessary for calculation of production and biomass of a fish population (Anderson et al., 1983). The estimation of length-weight relationship and relative condition factors enables estimation of the population of the same species from different localities. Besides that, knowledge of fecundity i.e., the total number of eggs produced by a female fish during a year is essential for assessing the spawning potential of the stock. Gonadosomatic index (GSI) is used as an indicator of gonadal development as marked by maturity and indicates the phase of the reproductive cycle; and also to assess the ripeness of the ovary (Nandikeswari et al., 2014; Islam et al., 2008; Gafferi et al., 2011; Jan et al., 2014). Sex ratio gives statistics on representation of male and female individuals in a population and the proportion of male to female required in a population (Amtyaz et al., 2013). In nature, the proportion is assumed to be 1:1. A good deal of works has been carried out concerning reproductive biology, length-weight relationships of Gudusia chapra in India and Bangladesh. However, such works related to this species in the North-Eastern region of India is scanty. So, the present study aimed to provide basic information in the aspects of biology for future research to enable them make the comparison between regions and year.
Materials and methods
The study of length-weight relationship and different aspects of the reproductive biology of Gudusia chapra was carried out from August, 2017 to July, 2018. The fish samples were collected from Thekera Beel (26°12/N, 92°25/E) of Central Brahmaputra Valley, Morigaon district of Assam (Supllementary Fig. S1) on a monthly interval. Experimental fishing was done using gill net of different mesh size ( 10-15 mm and 15-20 mm). The collected fishes were stored in iceboxes and brought to the laboratory for further examination. The morphometric measurements were taken from the left-hand side of the fish body and photographs were taken in fresh condition. Total length (TL) was measured to nearest 1 mm by using a digital caliper and body weight (BW) was measured with a digital weighing machine with the precision of 0.1 mg using Sartorius BSA224S-CW electronic balance. After dissection, the fish ovaries were taken out and measured followed by preservation in 10% neutral formalin for further study. The present study was based on a total of 458 individuals ranging in the size from 7.8 cm to 15.6 cm in total length (TL) and 5.01 g to 38.19 g in total weight (TW) comprising of 161 males and 297 females.
The length- weight relationship of Gudusia chapra was established using the formula (Le Cren, 1951):
W= aLb
Where, W is the total weight (g) of the fish, L is the total length (cm) or standard length (cm) of fish and ‘a’ and ‘b’ are the constants or this equation can be linearly represented as Log W= Log a + b Log L. Analysis of covariance was employed to test whether the ‘b’ value of two equations differed at 5% level of significance (Snedecor and Cochran, 1967). The‘t’ test was done to determine whether the regression co-efficient significantly deviated from the expected cubic value (Snedecor and Cochran, 1980).
For calculating the gonadosomatic index, the weight of the individual fish was taken. The gonads were removed carefully and weighed on an electronic balance after removing the excess moisture using a blotting paper. GSI was estimated as per the following equation (Parmeshwaran et al., 1974):
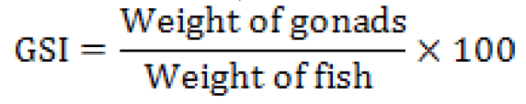
The sex of individual fish was ascertained by dissecting the fish and examining the gonads for every sample collected. Data on sex ratio were analyzed by χ2 (chi-square) test assuming that the ratio of male and female in the population may be 1:1 (p<0.05) (Snedecor and Cochran, 1967).
The weight of the ovaries of each fish was recorded with the help of an electronic balance. Then 0.2 g of the mature ovary from the anterior, middle and posterior region was weighed to 0.10 mg accuracy. The subsamples were then mounted in a glass slide and numbers of mature ova were counted manually. Fecundity was calculated by using the formula of Le Cren (1951):
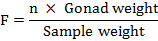
Where, F is Fecundity, and n is number of eggs in sample.
Results
The relationship between length and weight indicated allometric growth for both males, females and pooled as the value of coefficient b were different from the isometric value of 3 (t- test; P<0.01) (Table I, Fig. 1). GSI ranged from 0.11-8.97 in males and 0.60-13.58 in females. Month wise changes in mean GSI values are presented in Supplementary Tables S1, SII and Figure 2. The values of mean GSI of male ranged from 1.07±0.15 to 6.03±0.61 and that of female ranged from 1.64±0.16 to 8.14±0.61, both showing two peaks one in April (5.06 for male and 5.98 for female) and another in August (6.03 for male 8.14 for female). Minimum GSI for both males and females was recorded in January (1.07 for males and 1.64 for females).
Table I.- Descriptive statistics and estimated length-weight relationship parameters for Gudusia chapra during the experimental period.
|
Species |
n |
Length range (cm) |
Weight range (g) |
Regression parameters |
||||
|
a |
b |
95% confidence level of b |
r2 |
|||||
|
Gudusia chapra |
Male |
161 |
8.1-14.3 |
6.02-31.1 |
0.1465 |
2.897 |
2.79-3.00 |
0.947 |
|
Female |
297 |
7.8-15.6 |
5.01-38.19 |
0.0116 |
2.916 |
2.84 -2.98 |
0.959 |
|
|
Pooled |
458 |
7.8-15.6 |
5.01-38.19 |
0.1439 |
2.921 |
2.86 -2.98 |
0.954 |
|
The monthly variation of the sex ratio of Gudusia chapra is shown in Table II. The observed ratio was tested against 1:1 using Chi-square test for (n-1) degrees of freedom at 5% level of significance. The ratio between the male and female ranged from 1:1.26 (April) to 1:2.53 (December). The ratio significantly departed from the expected 1:1 ratio in January, March, May, June, July (P <0.05) and December (<0.01). During these months the females significantly outnumbered the males. The overall sex ratio for the whole specimens sampled over twelve months also varied significantly from the expected ratio of 1:1 (male: female =1:1.84, χ2= 40.38, P <0.01).
Table II.- Monthly variation in sex ratio (male:female) of Gudusia chapra during the experimental period.
|
Month |
Male (%) |
Female (%) |
M: F |
Chi square |
P- value |
S/SN |
|
Aug-2017 |
8 (34.78) |
15 (65.22) |
1: 1.8 |
2.1304 |
0.144 |
NS |
|
Sept |
9 (32.14) |
19 (67.86) |
1: 2.1 |
3.5714 |
0.058 |
NS |
|
Oct |
15 (41.67) |
21 (58.33) |
1: 1.4 |
1.0000 |
0.317 |
NS |
|
Nov |
10 (37.04) |
17 (62.96) |
1: 1.7 |
1.8148 |
0.178 |
NS |
|
Dec |
13 (28.26) |
33 (71.74) |
1: 2.5 |
8.6957 |
0.003 |
S** |
|
Jan-2018 |
12 (32.43) |
25 (67.57) |
1: 2.0 |
4.5676 |
0.033 |
S* |
|
Feb |
13 (37.14) |
22 (62.86) |
1: 1.6 |
2.3143 |
0.128 |
NS |
|
Mar |
13 (33.33) |
26 (66.67) |
1: 2.0 |
4.3333 |
0.037 |
S* |
|
Apr |
27 (44.26) |
34 (55.74) |
1: 1.2 |
0.8033 |
0.370 |
NS |
|
May |
17 (33.33) |
34 (34.00) |
1: 2.0 |
5.6667 |
0.017 |
S* |
|
Jun |
12 (33.33) |
24 (66.67) |
1: 2.0 |
4.0000 |
0.045 |
S* |
|
Jul |
12 (30.77) |
27 (69.23) |
1: 2.2 |
5.7692 |
0.016 |
S* |
|
Overall |
161 (35.15) |
297 (64.85) |
1: 1.8 |
40.384 |
0.000 |
S** |
A total number of 35 mature female specimens were randomly selected for the estimation of fecundity as this specimens were observed to be at oocyte stage IV and V. The fecundity of mature Gudusia chapra ranged from 7095.6 to 48,238 with an average of 21,150.60 was presented in Table III. The number of ova present per g of body weight ranged from 539.42 (March) to 2509.21 (April) with an average of 1182.82 ova/gm of body weight, which showed that the fish was highly fecund. The highest ova were seen in August and lowest in March. The relationship between the fecundity and three variables i.e., total length (TL), body weight (BW) and gonad weight were analysed separately (Fig. 3).
Table III.- Absolute and relative fecundity of different length of Gudusia chapra during the experimental period.
|
S. No |
Total length (cm) |
Total weight (g) |
Ovary weight (g) |
Absolute fecundity |
Relative fecundity |
|
1 |
9.8 |
8.80 |
0.77 |
10995.60 |
1249.50 |
|
2 |
11.5 |
16.91 |
1.27 |
21755.10 |
1287.28 |
|
3 |
11.5 |
16.90 |
1.28 |
20614.40 |
1219.78 |
|
4 |
13 |
23.26 |
2.13 |
44037.75 |
1893.28 |
|
5 |
11.4 |
15.03 |
1.79 |
31146.00 |
2072.25 |
|
6 |
14.5 |
29.70 |
2.01 |
40883.40 |
1376.55 |
|
7 |
11.5 |
13.39 |
1.04 |
16510.00 |
1233.01 |
|
8 |
12.3 |
17.81 |
1.02 |
15386.70 |
863.94 |
|
9 |
8.6 |
07.11 |
0.54 |
7095.60 |
997.97 |
|
10 |
11.5 |
14.75 |
0.98 |
15655.50 |
1061.39 |
|
11 |
13.8 |
26.22 |
2.29 |
48238.85 |
1839.77 |
|
12 |
12.6 |
18.25 |
1.56 |
28399.80 |
1556.15 |
|
13 |
12.1 |
15.78 |
1.01 |
16402.40 |
1039.44 |
|
14 |
13.6 |
25.28 |
1.51 |
26357.05 |
1042.60 |
|
15 |
13.3 |
21.23 |
1.54 |
26318.60 |
1239.69 |
|
16 |
12.8 |
16.06 |
1.31 |
22387.90 |
1394.02 |
|
17 |
10.9 |
08.09 |
0.52 |
6858.80 |
847.81 |
|
18 |
12.9 |
18.23 |
1.74 |
31659.30 |
1736.66 |
|
19 |
12.8 |
18.84 |
0.65 |
10162.75 |
539.42 |
|
20 |
10.4 |
08.76 |
0.62 |
8373.10 |
955.83 |
|
21 |
12.0 |
15.11 |
1.28 |
23072.00 |
1526.94 |
|
22 |
13.9 |
24.82 |
1.43 |
26583.70 |
1071.06 |
|
23 |
12.9 |
22.10 |
1.26 |
16827.30 |
761.42 |
|
24 |
10.7 |
13.50 |
1.84 |
33874.40 |
2509.21 |
|
25 |
12.3 |
16.78 |
0.87 |
14067.90 |
838.37 |
|
26 |
12.1 |
18.55 |
1.32 |
19912.20 |
1073.43 |
|
27 |
10.6 |
12.55 |
0.87 |
12193.05 |
971.56 |
|
28 |
12.0 |
19.6 |
2.10 |
27121.40 |
1383.74 |
|
29 |
13.5 |
22.8 |
1.05 |
14248.50 |
624.93 |
|
30 |
15.2 |
32.4 |
1.62 |
29046.60 |
896.50 |
|
31 |
10.6 |
13.37 |
0.67 |
7514.05 |
562.01 |
|
32 |
11.3 |
13.33 |
0.92 |
13726.40 |
1029.74 |
|
33 |
12.5 |
16.6 |
0.88 |
12042.80 |
725.47 |
|
34 |
13.6 |
24.12 |
1.36 |
20794.40 |
862.12 |
|
35 |
10.4 |
17.93 |
1.29 |
20007.90 |
1115.89 |
Discussion
The present findings conform with earlier reports of Vinci et al. (2005) who have reported negative allometric growth (2.857) pattern in Gudusia chapra from a floodplain wetland in West Bengal. The value of ‘b’ less, equal to or greater than 3 shows negative allometric, isometric, positive allometric growth, respectively (Morey et al., 2003). In the present study, the value of ‘b’ for males, females and pooled samples showed less than 3 suggesting a negative allometric growth of fish. Hile (1936) and Martin (1949) observed that the value of exponent b (regression coefficient) lies in the range of 2.5-4.0 and for ideal fish, it assumes the value close to 3.0 which supports that the present study was valid. Hile (1936) interpreted that change in the value exponent ‘b’ from the 3.0 is associated with the change in direction or change in form or condition of fish. In simple words, b ˂ 3 indicates elongation or a decrease in condition with an increase in length, b>3 indicates an increase in condition or height or width with an increase in length and suggested that the greater the deviation from the 3.0 the greater the change in form. If the b assumes the value close to 3.0 it means, the fish did not change form during ontogenetic growth (Costa and Araujo, 2003). Length-weight parameters (a, b) of fishes are affected by myriad of factors such as seasons, habitat, gonad maturity, sex, diet, stomach fullness, the health of individuals in their natural habitats, preservation techniques and annual differences in environmental conditions (Bagenal, 1978; Forese, 2006; Hossain et al., 2006).
Recently various attempts have been made to study LWRs of indigenous freshwater fish species from Brahmaputra basin (Borah et al., 2017, 2018, 2020; Koushlesh et al., 2018; Nath et al., 2019), Barak basin (Nath et al., 2017), Ganga basin (Baitha et al., 2018) and from rivers of peninsular India (Borah et al., 2019). The ‘b’ value of the present study also concurs with the value of earlier researchers observed from the wetlands and reservoirs (Kumari et al., 2019) indicating negative allometric growth. Contrary to the above results, Hossain et al. (2009) recorded the positive allometric growth pattern of G. chapra from their study in North-Western Bangladesh.
The GSI is a good indicator of the state of gonadal development of fish. The GSI value of the present study revealed that spawning season prolonged from March to October with two distinct peaks for both sexes, one in April (5.06 for male and 5.98 for female) and another in August (6.03 for male 8.13 for female). Minimum GSI value for both male and female G. chapra was recorded in January (1.07 for male and 1.64 for female). The decreasing GSI value from highest in August to lowest in January implies the decreasing development of gonad which indicates peak season is in August. The GSI value increases with the advancement of maturation and reaches the highest in the peak maturity period and then changes dramatically (Parween et al., 1993). The findings of the study are similar to the result of Kabir et al. (1998) who recorded G. chapra spawn for several months with two spawning peaks, one in April and another in August. Vinci et al. (2005) reported that the spawning season of G. chapra extended from March to October sampled from floodplain wetland in West Bengal. Conversely, Ahamed et al. (2014) reported higher GSI value from March to September with a single peak in April indicating this as the main spawning season. Similarly, in contrast to the present findings, Rahman and Haque (2008) observed two spawning peaks of Gudusia chapra one in March and the other in July as indicated by the peaks of the gonadosomatic index. The change in the gonadal condition of fishes may be subjected to size, age, food availability, prevailing environmental conditions and that is why the spawning peaks may vary from region to region.
The sex-ratio studies provide information for the assessment of reproduction potential of fish and stock size in a population (Vicentini and Arajau, 2003). The overall sex ratio for the whole specimens sampled for twelve months also varied significantly from the theoretical ratio of 1:1 (male : female = 1:1.84, Chi-square = 40.38, P <0.01). The finding of the present study is similar to Mondal and Kaviraj (2010) who noticed a weightage of females over males. The overall male-female ratio (1:1.56) differed significantly from the predicted sex ratio of (1:1). In addition to that, the monthly sex ratio also had shown weightage of females over males as well as the divergence of the sex ratio from the predicted 1:1 ratio. In Amblypharyngodon mola, whose breeding phenomenon is similar to Gudusia chapra, Gupta and Banerjee (2013) have also recorded the deviation of the sex ratio from the predicted value and female superiority over the male population.
Knowledge of fish fecundity has much relevance to study the population dynamics, successful management and exploitation of fish stocks (Alam et al., 1997). The findings of the present study are similar to the findings of Mondal and Kaviraj (2010) recorded the fecundity of Gudusia chapra in the range of 5,795 to 26,240. According to Hossain et al. (2009), the mean fecundity of Gudusia chapra was 20,200±6,500 and ranged from 10,800 (fish with body length 11.1 cm and weight 15 gram) to 36,200 (fish with body length 15.4 cm and weight 43.60 gram). Mostafa and Ansari (1983) reported the fecundity of Gudusia chapra from Baigal and Nanaksagar reservoir of India to vary from 11,393 to 82,719 and 11,544 to 56,824, respectively. Kabir et al. (1998) observed that the fecundity ranged from 25,220 to 1, 54,528 in the earthen ponds from Bangladesh, which was very high compare to the results obtained in the present study.
An individual female facundity varies according to several factors, including age, gender, species and environmental conditions, such as the availability of food, water temperature and salinity (Lagler et al., 1967; Doha and Hye, 1970; Bagenal, 1978; Masoud et al., 2011). Fertility (F) regression ratio of total length (TL), total body weight (BW) and gonadal weight (GW) showed a strongly positive correlation by Chapra. The statistical analysis showed that the relationship between fecundity and TL, TW, GW was found to be significant at the level of 1 percent (p<0.01). In the current research, relative to the smaller ones, the larger fish were found to be more fecund. Length and weight are accurate measures that assess an individual’s reproductive potential as fertility increases with a rise in size and weight, according to Bagenal (1978). The fecundity of G. chapra within the context of the present analysis may be best represented by the weight of the ovary than the fish’s total length and body weight. In the present work, the linear relationship between gonadal weight and fecundity is consistent with those of previous studies e.g. Kabir et al. (1998), Akter et al. (2007), Narejo et al. (2006) and Das et al (1989).
Acknowledgement
The authors are thankful to the authority of Assam Agricultural University, Dean College of Fisheries, Dean, College of Fisheries, Assam Agricultural University, Raha, for their kind support and providing all the necessary facilities to carry out the research work. The authors are also thankful to the local fishermen of Thekera beel for their kind help in the collection of the specimens.
Supplementary material There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20210122140157
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Ahamed, F., Ahmed, Z.F., Hossain, M.Y. and Ohtomi, J., 2014. Population biology of Indian River Shad, Gudusia chapra (Clupeidae) in the old Brahmaputra River, North Eastern Bangladesh. Sains Malay., 43: 1645-1655.
Akter, M.A., Hossain, M.D., Hossain, M.K., Afza, R. and Bhuiyan, A.S., 2007. The fecundity of Hilsa ilisha from the river Padma near Godagari of Rajshahi district. J. Zool. Rajshahi Univ., 26: 41-44. https://doi.org/10.3329/ujzru.v26i0.696
Akther, S. and Akther, S., 2011. Some aspects of the reproductive biology and sex-ratio of Cirrhinus reba (Hamilton). Univ. J. Zool., 30: 21-24. https://doi.org/10.3329/ujzru.v30i0.10739
Alam, M.J., Kamal, D. and Kader, M.A., 1997. Fecundity of the Gangetic anchovy, Setipinna phasa (Hamilton-Buchanon) from the Bay of Bengal. J. Asiat. Soc. Bangladesh Sci., 23: 1-8.
Amtyaz, M.A., Khan, M.Z. and Hashmi, M.U.A., 2013. Studies on gonadosomatic index and stages of gonadal development of striped piggy fish, Pomadasys stridens (Forsskal, 1775) (Family; Pomadasyidae) of Karachi coast, Pakistan. J. Ent.Zool. Stud., 1: 28-31.
Anderson, D.M., Chishlom, S.W. and Watraj, C.J., 1983. Importance of life cycle events in population dynamics of Gonyaulax tamarensis. Mar. Biol., 76: 179-189. https://doi.org/10.1007/BF00392734
Azadi, M.A. and Rahman, A.S.M.S., 2008. Reproductive biology of the clupeid, Gonialosa manmina (Hamilton-1822) from the Kaptai Lake, Bangladesh. Chittagong Univ. J. biol. Sci., 3: 139-148. https://doi.org/10.3329/cujbs.v3i1.13414
Bagenal, T.B., 1978. Aspects of fish fecundity. In: Ecology of freshwater fish production (ed. S.D. Gerking). Blackwell Scientific Publications, Oxford, pp. 75-101.
Biswas, S.P., 1993. Manual of methods in fish biology. South Asian publishers Pvt. Ltd., New Delhi, pp. 157.
Baitha, R, Sinha, A., Koushlesh, S.K., Chanu, T.N., Kumari, K., Gogoi, P., Ramteke, M.H., Borah, S. and Das, B.K., 2018. Length-weight relationship of ten indigenous freshwater fish species from Gandak River, Bihar, India. J. appl. Ichthyol., 34: 233-236. https://doi.org/10.1111/jai.13555
Borah, S., Bhattacharjya, B.K., Saud, B.J., Yadav, A.K., Debnath, D., Yengkokpam, S., Das, P., Sharma, N., Singh, N.S. and Sarma, K.K., 2017. Length- weight relationship of six indigenous fish species from Deepor beel, a Ramsar site in Assam, India. J. appl. Ichthyol., 33: 655-657. https://doi.org/10.1111/jai.13348
Borah, S., Gogoi, P., Bhattacharjya, B.K., Suresh, V.R., Yadav, A.K., Baitha, R., Koushlesh, S.K., Kakati, A., Ray, B.C. and Das, B.K., 2018. Length–weight and length–length relationship of two endemic snakehead fish species from Brahmaputra river basin, Assam, India. J. appl. Ichthyol., 34: 788–790. https://doi.org/10.1111/jai.13685
Borah, S., Vaisakh, G., Jaiswar, A.K., Bhattacharjya, B.K., Sahoo, A.K., Deshmukhe, G., Raman, R.K. and Das, B.K., 2019. Length-weight relationship and condition factor of three geographically isolated populations of Hilsa Shad, Tenualosa ilisha (Hamilton, 1822). J. Inland Fish. Soc. India, 51: 49-54.
Borah, S., Das, P., Bhattacharjya, B.K., Yadav, A.K., Saud, B.J. and Das, B.K., 2020. A report on the occurrence of Bangana dero (Hamilton, 1822) from Deepor beel (Ramsar site No. 1207), Brahmaputra Valley, Assam. J. appl. Nat. Sci., 12: 202-206. https://doi.org/10.31018/jans.vi.2288
Costa, M.R. and Araújo, F.G., 2003. Length-weight relationship and condition factor of Micropogonias furnieri (Desmarest)(Perciformes, Sciaenidae) in the Sepetiba Bay, Rio de Janeiro State, Brazil. Rev. Brasil. Zool., 20: 685-690.
Daniels, R.J.R., 2002. Fresh water fishes of Peninsula India. Universities Press, Hyderabad.
Das, M., Dewan, S. and Debnath, S.C., 1989. Studies on fecundity of Heteropneustes fossilis (Bloch) in a mini pond of Bangladesh Agricultural University, Mymensingh. Bangladesh J. agric. Sci., 16: 1-6.
Doha, S. and Hye, M.A., 1970. Fecundity of Padma river hilsa (Hilsa ilisha). Pak. J. Sci., 22: 176-184.
Froese, R., 2006. Cube law, condition factor and weight-length relationships: History, meta- analysis and recommendations. J. appl. Ichthyol., 22: 241-253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Ghaffari, H., Ardalan, A.A., Sahafi, H.H. and Babaei, M.M., 2011. Annual changes in gonadosomatic index (GSI), hepatosomatic index (HIS) and condition factor (K) of large scale tonguesole Cynoglossus arel (Bloch & Schneider, 1801). The Coastal Waters of Bandar Abbas, Persian Gulf. J. appl. Sci. Res., 5: 1640-1646.
Gupta, S. and Banerjee, S., 2013. Studies on some aspects of reproductive biology of Amblypharyngodon mola (Hamilton-Buchanan, 1822). Int. Res. J. biol. Sci., 2: 69-77. https://doi.org/10.15373/22778179/MAY2013/98
Hamilton, F., 1822. An account of fishes found in the river Ganges and its branches. Edinburgh and London, pp. 1-405. https://doi.org/10.5962/bhl.title.59540
Hile, R., 1936. Age and growth of the cisco, Leucichthys artedi (Le Sueur), in the lakes of the northeastern highlands, Wisconsin. Bull. Bur. Fish., 48: 211-317.
Hossain, M.Y., Ahmed, Z.F., Leunda, P.M., Roksanul, I.A.K.M. and Jasmine, S., 2006. Length-weight and length-length relationships of some small indigenous fish species from the Mathabhanga river, Southwestern Bangladesh. J. appl. Ichthyol., 22: 301-303. https://doi.org/10.1111/j.1439-0426.2006.00801.x
Hossain, M.Y., Jasmine, S., Ibrahim, A.H.M., Ahmed, Z. F., Rahman, M.M. and Ohtomi, J., 2009. Length- weight and length- length relationships of 10 small fish species from the Ganges, Bangladesh. J. appl. Ichthyol., 25: 117-119. https://doi.org/10.1111/j.1439-0426.2008.01168.x
Islam, M.A., Begum, M., Alam, M.J., Pal, H.K. and Shah, M.M.R., 2007. Growth and survival of esturine cat fish, Mystus gulio (Ham.) larvae fed on live and prepared feeds. Bangladesh J. Zool., 35: 325-330.
Jan, M., Jan, U. and Shah, G., 2014. Studies on fecundity and gonadosomatic index of Schizothorax plagiostomus (Cypriniformes: Cyprinidae). J. Threatened Taxa, 6: 5375-5379. https://doi.org/10.11609/JoTT.o3269.5375-9
Jayaram, K.C., 1999. The freshwater fishes of Indian region. Narendra Publishing House, New Delhi.
Kabir, A.K.M.A., Hossain, M.A., Rahmatullah, S.M., Dewan, S. and Islam, M.S., 1998. Studies on the gonadosomatic index and fecundity of chapila (Gudusia chapra Ham.). Bangladesh J. Fish Res., 2: 195-200.
Koutrakis, E.T. and Tsikliras, A.C., 2003. Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). J. appl. Ichthyol., 19: 258–260. https://doi.org/10.1046/j.1439-0426.2003.00456.x
Koushlesh, S.K., Sinha, A., Kumari, K., Borah, S., Chanu, T.N., Baitha, R., Das, S.K., Gogoi, P., Sharma, S.K., Ramteke, M.H. and Das, B.K., 2018. Length-weight relationship and relative condition factor of five indigenous fish species from Torsa River, West Bengal, India. J. appl. Ichthyol., 34: 169-171. https://doi.org/10.1111/jai.13518
Kumari, S., Sandhya, K.M., Karnatak, G., Lianthuamluaia, L., Sarkar, U.K., Panda, D. and Mishal, P., 2019. Length-weight relationship and condition factor of Gudusia chapra (Hamilton, 1822) from Panchet Reservoir, Jharkhand, India. Indian J. Fish., 66: 136–139. https://doi.org/10.21077/ijf.2019.66.3.81017-18
Lagler, K.F., Bardach, J.F.Z. and Miller, R.R., 1967. Ichthyology. John Wiley and Sons Inc., New York, London, Sydney, pp. 59-301.
Lakra, W.S., Sarkar, U.K., Kumar, R.S., Pandey, A., Dubey, V.K. and Gusain, O.P., 2010. Fish diversity, habitat ecology and their conservation and management issues of a tropical River in Ganga basin, India. Environmentalist, 30: 306–319. https://doi.org/10.1007/s10669-010-9277-6
Le Cren, E.D., 1951. The length-weight relationship and seasonal cycles in gonad weight and condition in perch (Perca fluviatilis). J. Anim. Ecol., 20: 201-219. https://doi.org/10.2307/1540
Martin, W.R., 1949. The machanics of environmental control of body form in fishes. Univ. Toronto Stud. Biol., 58: 1-91.
Masoud, A., Amir, H.S., Hamid, M. and Rokhsarch, M., 2011. Reproductive biology and age determination of Garra rufa (Heckel, 1843) (Actinopterygii: Cyprinidae) in Central Iran. Turkish J. Zool., 35: 317-323.
Mondal, D.K. and Kaviraj, A., 2010. Feeding and reproductive biology of Indian Shad Gudusia chapra in two floodplain lakes of India. Electronic J. Biol., 6: 98-102.
Mondal, G., 2014. Impact of anthropogenic and natural drivers on Ganges, Brahmaputra, Meghna River fish diversity in Bangladesh. In: River for life: Proceedings of the International Symposium on river biodiversity Ganges- Bramhaputra- Meghna system, pp. 176-183.
Morey, G., Moranta, J., Massuti, E., Grau, A., Linde, M., Riera, F. and Morales-Nin, B., 2003. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fish. Res., 62: 89–96. https://doi.org/10.1016/S0165-7836(02)00250-3
Mostafa, S. and Ansari, R., 1983. Fecundity of Gudusia chapra from Baigai and Nanaksagar reservoirs (Nainital: India). Z. Angew. Zool., 70: 139-144.
Nandikeswari, R., Sambasivam, M. and Anandan, V., 2014. Length weight relationship of Terapon jarbua (Forsskal, 1775) from Pondicherry Waters. Int. J. Biol. Vet. Agric. Fd. Engin., 3: 278-282.
Narejo, N.T., Lashari, P.K., Laghari, Y.M., Mastoi, A.M. and Khoso, H.B., 2006. Fecundity of Palri, Gudusia chapra (Hamilton) from fish ponds of Chilya Hatchery (District, Thatta), Sindh, Pakistan. Pakistan J. Zool., 38: 69-272.
Nath, K.D., Borah, S., Yadav, A.K., Bhattacharjya, B.K., Das, P., Deka, P.M., Darngawn, O. and Devnath, D.J., 2017. Length-weight and length-length relationship of four Native fish species from Barak river, Assam, India. J. exp. Zool. India., 20: 977-979.
Nath, B.B., Das, J., Ahmed, A.M., Sarma, J., Phukan, B., Ali, A., Sharma, A.K. and Borah, S., 2019. Length-weight and length-length relationship of two tropical freshwater fish species from Central Brahmaputra Valley, Assam. J. exp. Zool. India, 22: 321-324.
Parameshwaran, S., Selvaraj, C. and Krishan, S.R., 1974. The study of the biology of Labeo gonius in confined waters. Indian J. Fish., 21: 54-75.
Parween, S., Begum, N., Rahman, M.H. and Hossain, M.A., 1993. On the breeding periodicity of Esomus dandricus (Hamilton). Univ. J. Zool. Rajshahi Univ., 12: 31-34.
Qasim, S.Z., 1973. An appraisal of the studies on maturation and spawning In marine teleosts from the Indian waters. Indian J. Fish., 20: 166-181.
Rahman, A.K.A., 1989. Fresh water fishes of Bangladesh. Zoological Society of Bangladesh. Department of Zoology, University of Dhaka, pp. 364.
Rahman, M.A. and Haque, M.M., 2005. Population dynamics and stock assessment of Gudusia chapra (Hamilton-Buchanan) in the Rajdhala reservoir, Netrakona, Bangladesh. Asian Fish Sci., 19: 281-292.
Rahman, M.A. and Haque, M.M., 2008. Beel fishery and livelihood of the local community in Rajdhala, Netrakona, Bangladesh. J. Fish. Res., 12: 95108.
Reide, K., 2004. Global register of migratory species- from global to regional scales. Final Report of the R&D- Project 80805081. Federal agency for Nature Conservation, Bonn, Germany, pp. 329.
Shafi, S., 2012. Study on fecundity and GSI of Carassius carassius (Linneaus, 17580 introduced from Dal Lake Kashmir. J. Biol. Agric. Healthc., 2: 68-75.
Snedecor, G.W. and Cochran, W.G., 1967. Statistical methods, 6th edn. Iowa State University Press, Ames, Iowa.
Snedecor, G.W. and Cochran, W.G., 1980. Statistical methods, 7th edn. Iowa State University Press, Ames, Iowa.
Talwar, P.K. and Jhingran A.G., 1991. Inland fishes of India and adjacent countries. Oxford and IBH Publishing, Calcutta.
Vicentini, R.N. and Araujo, F.G., 2003. Sex ratio and size structure of Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) in Sepetiba bay, Rio de Janeiro, Brazil. Braz. J. Biol., 3: 559-566. https://doi.org/10.1590/S1519-69842003000400003
Vinci, G.K., Suresh, V.R. and Bandyopadhyaya, M.K., 2005. Biology of Gudusia chapra (Hamilton-Buchanan) from a floodplain wetland in West Bengal. Indian J. Fish., 52: 73-79.
Whitehead, P.J.P., 1985. FAO species catalogue, Vol. 7. Clupeoid fishes of the world (suborder Clupeioidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolfherrings. Part 1 – Chirocentridae, Clupeidae and Pristigasteridae. FAO Fish Synop., 125: 1–30.
To share on other social networks, click on any share button. What are these?










