Investigation of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity in Some Inflammatory Conditions in Barki Sheep
Investigation of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity in Some Inflammatory Conditions in Barki Sheep
Asmaa A. Darwish
Researcher at Department of Animal and Poultry Health, Animal and Poultry Production Division, Desert Research Center, Mataria, Egypt, Postal code: 11753.
Abstract | Inflammation is a double-edged weapon. Although it starts as a protective mechanism, it ends with serious cliniopathological alterations, which disappoint our therapeutic programs and frustrate our expectation for curing. This work aimed to assess the most important clinicopathological alterations related to sheep pneumonia, arthritis and enteritis and spot the light on MMP-2 and MMP-9 diagnostic and prognostic accuracy in these conditions. 80 barki lambs were divided into four groups control group (CG), pneumonic group (PG), arthritic group (AG) and enteric group (EG). Blood samples were collected from the four groups then haematologically, biochemically and statistically analyzed. A significant (P< 0.05) microcytic hypochromic anemia was observed in the PG and AG while, EG suffered from a macrocytic hyperchromic anemia. On contrast, a neutrophilic leukocytosis and lymphocytopenia was described in the three diseased groups. Biochemically, a significant (P< 0.05) increase in total protein, globulin, liver enzymatic activities, kidney function tests, total lipids, triglycerides, MMP-2 and MMP-9 concentrations was depicted in the three diseased groups. On the contrary, the total cholesterol, HDL-cholesterol, LDL-cholesterol, minerals, electrolytes, trace elements and total antioxidants capacity concentrations significantly (P< 0.05) decreased in the three diseased groups. Both of MMP-2 and MMP-9 yielded a sensitivity and NPV as 100% in the sheep pneumonia, arthritis and enteritis and correlated with some parameters in PG and EG. Ovine inflammatory conditions usually result in different clinicopathological changes. Monitoring of them may be useful in improving our therapeutic intervention and enhancing the animal response to treatment. Both of MMP-2 and MMP-9 is a good marker for sheep inflammatory conditions.
Editor | Muhammad Abubakar, National Veterinary Laboratories, Park Road, Islamabad, Pakistan.
Received | November 06, 2019; Accepted | December 15, 2019; Published | February 17, 2020
*Correspondence | Asmaa A. Darwish, Researcher at Department of Animal and Poultry Health, Animal and Poultry Production Division, Desert Research Center, Mataria, Egypt, Postal code: 11753; Email: asmaa_vet25@yahoo.com
Citation | Darwish, A. A. 2020. Investigation of matrix metalloproteinase-2 and matrix metalloproteinase-9 activity in some inflammatory conditions in Barki sheep. Veterinary Sciences: Research and Reviews, 6(1): 25-32.
DOI | http://dx.doi.org/10.17582/journal.vsrr/2020/6.1.25.32
Key words | Sheep, Pneumonia, Arthritis, Enteritis, Matrix metalloproteinases (2-9), clinicopathological changes.
Introduction
Barki sheep breeding is an integral part of the Bedouin life in the Egyptian North West coast. Beside its economic importance as a source of meat and wool, it has a socio-religious value (Othmana et al., 2016; Donia et al., 2018). Unfortunately, different inflammatory conditions attack sheep flocks in large scales leading to high mortalities, poor growth rates, low carcass yield and high condemnated organs percentage. The high cost of prophylactic and therapeutic programs are another serious economic losses added to the previous losses (Zein-Eldin et al., 2013; Jesse et al., 2017; Donia et al., 2018). Surely, absence of good knowledge about the hematological and biochemical changes associated with different ovine inflammatory conditions as well as late detection of them partially participate in augmentation of the prior economic losses.
MMP-2 and MMP-9 are two proteolytic enzymes, classified under a super family of Zn containing endopeptidases, called matrix metalloproteinases (MMPs). Generally, MMPs are responsible for extracellular matrix turnover and cleavage while, MMP-2 and MMP-9 are mainly specialized in gelatin degradation so called gelatinseaes. In addition to gelatin, they are capable of collagens, elastin and vitronectin degeneration (Garg et al., 2006; Meszaros and Malemud, 2012; Li et al., 2016). Physiologically, they are embedded in various processes such as angiogenesis, endothelial cells apoptosis, vascular basement membrane degradation thus facilitating cellular infiltration and pro-inflammatory mediators stimulation and suppression. They are strictly controlled by tissue inhibitor of metalloproteinases (TIMPs). The imbalance between MMPs between TIMPs is tightly connected to a large section of diseases pathogenesis particularly malignancy and inflammation (Strup-Perrot et al., 2004; Gao et al., 2005). In spite of their curial importance in human medicine either as sensitive markers or therapeutic targets for different diseases, they are still little unknown in veterinary medicine (Meszaros and Malemud, 2012; Li et al., 2016).
Hence, this research aimed to monitor the most important clinicopathological changes associated with three major inflammatory conditions (pneumonia, arthritis, enteritis) in barki sheep and focus on MMP-2 and MMP-9 diagnostic and prognostic accuracy and their relation with other clinicopathological changes in these conditions.
Material and Methods
According to the ethical guidelines of animal treatment and after the owners’ agreements, blood samples were collected sporadically from 80 female barki lambs (6 months age) from Matrouh governorate different cities.
They were divided into 4 groups:
Control group (CG): 20 apparently healthy lambs (normal appetite, posture, body weight, plus (80-100 beat/min), temp (39-40oC) and respiration (36-40 breath/min), no nasal or ocular discharge (The Merck Veterinary Manual, 2006).
Pneumonic group (PG): 20 lambs suffered from respiratory manifestation (emaciation, loss of appetite, dyspnea, stretched head and neck, hyperthermia (41-42oC), nasal discharge, wheezes and crackles on auscultation, rapid abdominal respiration (41-46 breath/min)).
Arthritic group (AG): 20 lambs suffered from lameness (in most cases the animal carried the affected leg), swollen knee some times, hyperthermia (41-42oC), pain and anorexia. Enteric group (EG): 20 lambs suffered from diarrhea, pain, off food, low weight, dullness, depression and fever (41-42oC).
Blood samples were collected from all lambs by jugular vein puncture, and then divided in two parts: Potassium EDTA salt solution was added to the first part to stop the coagulation cascade and this part was used immediately for hematological parameters assessment according to Feldman et al. (2000) method. While, the second part was let to coagulate and was centrifugated at 3000 RPM. for 20 minutes at 37 ºC, to get serum which used for estimation of different biochemical parameters spectrophotometrically by using Biodiagnostic company® commercial kits, Cairo, Egypt and serum MMPs concentrations by using Cloud- Clone Corp company® ELISA kits, Huston, USA (all manual instructions were taken in consideration).
Statistical analysis
SPSS® program version 23 was used to evaluate the differences between the estimated parameters means (one-way ANOVA test), the post hoc differences between means (a multiple comparison Tukey`s HSD test) and the correlations between the selected parameters (Pearson’s simple correlation method). A difference was considerable significant at P< 0.05.
Graphed prism version 5 program was used to evaluate the cut off points, sensitivity, specificity and likelihood ratio (LR) for the measured MMPs between PG, AG, EG and CG.
The positive predictive value (PPV), negative predictive value (NPV) and accuracy rate for them were calculated according the next equations:
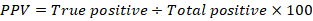
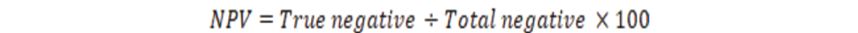
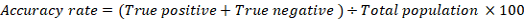

Results and Discussion
Table 1 revealed that a significant (P< 0.05) decrease in the red cell parameters (RBCs, Hb, PCV, MCV, MCH, MCHC) between PG, AG and CG, but these parameters significantly (P< 0.05) increased in EG in relation to CG, PG and AG. On contrast, a significant (P< 0.05) neutrophilic leukocytosis and lymphocytopenia were detected between PG, AG, EG and CG.
Table 2 showed a significant (P<0.05) hyperproteinemia, hyperglobulinemia, decreased A/G in PG, AG, EG when compared to CG. While, the albumin and glucose concentrations significantly (P< 0.05) decreased in PG, AG in relation to CG and significantly (P< 0.05) increased in EG in relation to CG.
A significant (P< 0.05) decrease was observed between PG, AG, EG and CG in the total cholesterol, HDL-cholesterol, LDL-cholesterol, minerals (Ca, Mg, P), electrolytes (Cl, Na), trace elements (Cu, Zn) and total antioxidants capacity (TAC) concentrations. While K significantly (P< 0.05) increased in PG in relation to CG, AG, EG and significantly (P< 0.05) decreased in AG, EG in relation to CG. On the other hands, total lipids, triglycerides, the liver enzymatic activities (AST, ALT, ALP), kidney function tests (blood urea, Creatinine (Cr)), MMP-2 and MMP-9 significantly (P< 0.05) increased between PG, AG, EG and CG.
The comparison between the three diseased groups exhibited a significant (P< 0.05) raise in TLC, lymphocytes, total protein, albumin, glucose, total lipids, triglycerides, total cholesterol, HDL-cholesterol, minerals (Mg, P), Na, trace elements (Cu, Zn), total antioxidants capacity (TAC), liver enzymatic activities, kidney function tests, MMP-2 and MMP-9 in EG when compared to PG and AG. While, a significant (P< 0.05) hyperglobulinemia was detected between EG and AG only.
In connection with the assessment of MMP-2 and MMP-9 importance as markers for the ovine pneumonia, arthritis and enteritis, Table 3 cleared that both of them yielded a sensitivity and NPV as 100% in sheep pneumonia, arthritis and enteritis. While, MMP-9 had higher values of specificity, LR, PPV and accuracy rate than MMP-2 but the percentage of increase was in the sake of MMP-2. The difference between the three groups showed that the PPV and accuracy rate for MMP-2 and MMP-9 were higher in PG than the other two groups. On the other hand, the highest percentage of increase was reported in EG followed by AG then PG. Meanwhile, Table 4 illustrated a significant (P< 0.05) positive correlation between MMP-2 and TP in PG. Meanwhile, there was a significant (P< 0.05) positive correlation between MMP-9 and TLC, glucose, AST as well as a significant (P< 0.05) negative correlation between MMP-9 and Zn, Mg, Na and Cu, but MMP-2 significantly (P< 0.05) positively correlated with neutrophils and glucose and significantly (P< 0.05) negatively correlated with Na levels in the EG. There was no significant correlations between MMP-2 and MMP-9 and other clinicopathological alterations in AG.
Inflammation is a double-faced process, arises primarily during different diseases courses as a defensive mechanism (Nahed et al., 2016; Jesse et al., 2017). Regrettably, it usually leaves behind a mess of serious hematological as well as biochemical alterations. Concerning the hematological changes, the present study revealed that both of ovine pneumonia and arthritis accompanied with a typical microcytic hypochromic anemia. Basically, this anemia originated as a part of the protective inflammatory process. As the invigorated pro-inflammatory cytokines stimulate the hepcidin secretion which minimizes the dietary iron absorption from intestine and maximizes iron storage as ferritin and hemosiderin. Thus, prevent the invading microorganisms from getting the iron necessary for their growth and multiplication. By the time, this mechanism results in the outstanding anemia (Nahed et al., 2016; Jesse et al., 2017; Donia et al., 2018). In addition, these pro-inflammatory cytokines hinder the erythropoiesis through inhibition of erythropoietin and stem cell factor expression and decrease erythrocytes age (Nahed et al., 2016; Jesse et al., 2017). Furthermore, they stimulate the massive production of different free radicals causing the oxidative stress increment and the subsequent red blood cell destruction and dysfunction (Garcia-Gonzalez et al., 2015; Allam et al., 2017). On contrast, the detected increase in the red blood cell parameters in
Table 1: Comparison between the hematological parameters of the studied groups. Values are means ± SD.
| Parameter | CG | PG | AG | EG |
|
RBCs (×106/μl) |
12.59±0.11d |
8.59±0.29a |
8.64±0.29a |
14.11±0.07a,b,c |
| Hb (g/dl) |
14.18±0.23d |
8.03±0.06a |
8.03±0.07a |
16.94±0.03a,b,c |
| PCV (%) |
33.15±0.81d |
20.17±0.11a |
20.15±0.11a |
38.27±0.24 a,b,c |
| MCV (fl) |
26.32±0.62d |
23.49±0.80a |
23.35±0.81a |
27.12±0.22 a,b,c |
| MCH (pg) |
11.25±0.23d |
9.36±0.34a |
9.30±0.29 a |
12.01±0.07 a,b,c |
| MCHC (%) |
42.78±1.07d |
39.84±0.38a |
39.85±0.41a |
44.27±0.26 a,b,c |
|
TLC (×103/μl) |
7.21±0.13d |
10.73±0.18a |
10.75±0.35a |
11.18±0.20a,b,c |
|
Neutrophils (×103/μl) |
2.15±0.02d |
7.12±0.17a |
7.07±0.30a |
7.14±0.03a |
|
Lymphocytes(×103/μl) |
4.06±0.07d |
2.64±0.22 a |
2.71±0.28 a |
3.09±0.06a,b,c |
|
Monocytes (×103/μl) |
0.44±0.07 | 0.46±0.05 | 0.46±0.05 | 0.42±0.07 |
|
Eosinophils (×103/μl) |
0.53±0.07 | 0.46±0.05 | 0.46±0.05 | 0.50±0.09 |
|
Basophils (×103/μl) |
0.04±0.05 | 0.05±0.05 | 0.05±0.05 | 0.03±0.04 |
a(significant with CG); b(significant with PG ); c(significant with AG ); d(significant between the four groups); considered statistically significant at P<0.05
Table 2: Comparison between the biochemical parameters of the studied groups. Values are means ± SD.
| Parameter | CG | PG | AG | EG |
| Total protein (g/dl) |
6.52±0.24d |
7.42±0.22a |
7.39±0.24a |
8.89±0.06a,b,c |
| Albumin (g/dl) |
3.99±0.20d |
3.13±0.58a |
3.40±0.51a |
4.29±0.51a,b,c |
| Globulin (g/dl) |
2.53±0.30d |
4.29±0.59a |
3.99±0.49a |
4.60±0.09a,c |
| A\G |
1.61±0.27d |
0.76±0.24a |
0.87±0.21a |
0.93±0.05a |
| Glucose (mg/dl) |
92.90±2.07d |
75.23±2.16a |
74.85±1.98a |
122.30±1.78a,b,c |
| Total lipids (mg/dl) |
355.40±9.53d |
356.40±10.72 | 359.47±13.85 |
373.28±5.38a,b,c |
| Triglycerides (mg/dl) |
73.17±2.12d |
126.50±2.56a |
127.06±2.95a |
135.82±3.77a,b,c |
| Phospholipids (mg/dl) | 161.04±8.88 | 159.47±9.42 | 161.79±10.66 | 162.88±2.02 |
| Cholesterol (mg/dl) |
121.19±1.98d |
70.43±3.34a |
70.62±3.49a |
74.58±3.63a,b,c |
| HDL-cholesterol(mg/dl) |
86.90±1.39d |
46.21±1.72a |
46.31±1.74a |
49.50±2.25a,b,c |
| LDL-cholesterol (mg/dl) |
34.29±1.40d |
24.21±1.72a |
24.31±1.74a |
25.08±2.70a |
| Ca (mg/dl) |
11.01±0.27d |
8.76±0.28a |
8.77±0.31a |
8.94±0.05a |
| P (mg/dl) |
6.35±0.27d |
3.77±0.53a |
3.79±0.51a |
4.87±0.05 a,b,c |
| Cl (mmol/L) |
105.23±2.64d |
89.79±4.31a |
90.06±4.91a |
92.94±1.71a,b |
| Na (mmol/L) |
142.40±2.80d |
108.93±0.66a |
108.97±0.70a |
126.61±3.56 a,b,c |
| K (mmol/L) |
3.47±0.18d |
6.05±0.13a |
2.82±0.39a,b |
2.90±0.04a,b |
| Mg (mg/dl) |
3.71±0.50d |
2.66±0.04a |
2.66±0.04a |
2.97±0.04a,b,c |
| Cu (μmol/L) |
23.55±1.31d |
16.72±0.26a |
16.72±0.28a |
20.59±0.72a,b,c |
| Zn (μg/dl) |
155.72±7.65d |
127.82±3.34a |
127.57±3.51a |
133.93±6.73a,b,c |
| TAC (Mm/L) |
1.23±0.11d |
0.89±0.05a |
0.90±0.05a |
0.70±0.04a,b,c |
| AST (U/L) |
26.74±1.61d |
33.41±1.41a |
33.13±1.32a |
37.01±1.38a,b,c |
| ALT (U/L) |
36.74±1.61d |
43.42±1.72a |
43.36±1.68a |
46.86±1.40a,b,c |
| ALP (U/L) |
28.54±0.30d |
40.19±1.57a |
40.33±1.52a |
43.54±1.44a,b,c |
| Blood urea (mg/dl) |
24.74±0.73d |
38.14±4.94a |
38.27±5.38a |
44.68±0.76a,b,c |
| Cr (mg/dl) |
0.75±0.11d |
1.20±0.21a |
1.19±0.18a |
1.75±0.11a,b,c |
| MMP-2 (ng/ml) |
15.39±0.75d |
61.32±1.14a |
61.18±1.11a |
65.46±0.89a,b,c |
| MMP-9 (ng/ml) |
22.75±1.07d |
66.69±2.96a |
67.63±3.17a |
71.84±3.18a,b,c |
a(significant with CG); b(significant with PG ); c(significant with AG ); d(significant between the four groups); considered statistically significant at P<0.05.
Table 3: Cut off points (ng/ml), sensitivity, specificity, LR, PPV, NPV, accuracy rate and percentage of increase for MMP-2 and MMP-9 in PG, AG, EG compared to CG.
| Statistical parameter | PG | AG | EG | |||
| MMP-2 | MMP-9 | MMP-2 | MMP-9 | MMP-2 | MMP-9 | |
| Cut off | 15.9 | 23.5 | 15.9 | 23.5 | 15.9 | 23.5 |
| Sensitivity | 100% | 100% | 100% | 100% | 100% | 100% |
| Specificity | 70% | 75% | 70% | 75% | 70% | 75% |
| LR | 3.33 | 4 | 3.33 | 4 | 3.33 | 4 |
| PPV | 83.33% | 85.71% | 76.92% | 80% | 76.72% | 80% |
| NPV | 100% | 100% | 100% | 100% | 100% | 100% |
| Accuracy rate | 88% | 90% | 85% | 87.5% | 85% | 87.5% |
| % of increase | 298.44% | 193.14% | 297.53% | 197.27% | 325.34% | 215.78% |
EG is fundamentally attributed to the excess fluid loss and related haemocncentration (Zein-Eldin et al., 2013). While, the remarkable neutrophilic leukocytosis noticed in the three diseased groups, is another outcome for the pro-inflammatory cytokines activation and dependant stimulation of the bone marrow production of the leukocytes especially neutrophils which are the first line of defense against pathogenic microorganisms (Zein-Eldin et al., 2013; Nahed et al., 2016; Jesse et al., 2017). On the opposite side, the lymphocytopenia registered in PG, AG, EG in the present work may be assigned to the lymphocytes migration to the infected organs (lung, joint, intestine) in order to prevent the infection expansion until the developing of specific immunity (Kumar et al., 2015; Nahed et al., 2016; Hassanein et al., 2017).
Regarding the biochemical changes, the protein profile of the pneumonic, arthritic, enteric lambs here presented a hyperglobulinemia followed by a hyperproteinemia and decreased A/G ratio. This hyperglobulinemia referred to immunoglobulins formation (humeral immunity) as well as release of innate immunity components (acute phase proteins, cytokines, MMPs) to limit the infective microorganisms propagation and spread as well as destroy them (Zein-Eldin et al., 2013; Nahed et al., 2016; Jesse et al., 2017). On the other hand, the recorded hypoalbuminemia in PG and AG was because of the acute phase response and the oxidative stress related to the inflammatory process. Whereas, albumin is a potent negative acute phase reactant and antioxidant in the same time (Zein-Eldin et al., 2013; Nahed et al., 2016; Jesse et al., 2017). Rationally, the hyperthermia and dependent anorexia (which are basic parts from the inflammatory disease pathogenesis) are extremely involved in the outstanding hypoglycemia and hypoalbuminemia in PG and AG as well as hypocholesterlemia, HDL-hypocholestelemia, LDL-hypocholesterlemia, hypocalcemia, hypophosphatemia, hypomagenesemia, hyponateremia, hypocholeredemia, hypozincemia and hypocupremia in PG, AG and EG and hypokalemia in both of AG and EG (Zein-Eldin et al., 2013; Joshi et al., 2015; Nahed et al., 2016; Bozukluhan et al., 2017; Jesse et al., 2017; Rodríguez-Carrio et al., 2017; Donia et al., 2018). Unlike AG and EG, PG showed a marked hyperkalemia whereas the blood buffering system responds to the respiratory acidosis (mostly accompanied the pneumonia cases) via pumping the excess H+ ions intracelluarly and K+ extracelluarly in what known as potassium efflux (Nahed et al., 2016; Donia et al., 2018). No doubt, intestinal villi damage and subordinate decreased intestinal absorptive surface are extra causes for the decreased cholesterol (total, HDL, LDL), minerals, electrolytes and trace elements levels in EG (Kumar et al., 2015; Hassanein et al., 2017).
It worth to mention that, the hyperalbuminemia and hyperglycemia stated in EG in relation to CG, PG, AG in this study as well as the increased TLC, lymphocytes, total protein, albumin, globulin, A/G ratio, total lipids, triglycerides, cholesterol (total, HDL), Mg, P, Cl, Na, Cu, Zn, TAC, kidney function tests, liver enzymatic activity, MMP-2 and MMP-9 in EG compared to PG and/or AG are not true and were mostly connected to the hypovolemia and fluid lose due to diarrhea (Zein-Eldin et al., 2013). Logically, the prior anorexia and hypoglycemia resulted in enhancing of the fat stores lipolysis to get energy leading to a massive triglycerides liberation in the diseased animals’ circulation. Therefore, a prominent hypertriglycerdemia and consequent hyperlipidemia were obtained in PG, AG and EG in this work as well as previous studies (Joshi et al., 2015; Bozukluhan et al., 2017; Rodríguez-Carrio et al., 2017).
Table 4: The correlation between MMP-2, MMP-9 and the other clinicopathological parameters in PG, AG and EG (Pearson’s correlation test, values = r).
|
MMP-2 |
MMP-9 |
|||||
| PG | AG | EG | PG | AG | EG | |
| RBCs | -0.120 | -0.002 | -0.077 | -0.017 | -0.179 | -0.052 |
| Hb | -0.182 | -0.269 | -0.137 | -0.287 | -0.106 | -0.042 |
| PCV | -0.070 | -0.038 | -0.229 | -0.097 | -0.018 | -0.055 |
| MCV | -0.131 | -0.007 | -0.222 | -0.003 | -0.178 | -0.077 |
| MCH | -0.074 | -0.073 | -0.030 | -0.046 | -0.168 | -0.033 |
| MCHC | -0.189 | -0.191 | -0.282 | -0.176 | -0.073 | -0.077 |
| TLC | 0.130 | 0.377 | 0.324 | 0.113 | 0.190 | 0.545* |
| Neutrophils | 0.034 | 0.175 | 0.704* | 0.098 | 0.142 | 0.030 |
| Lymphocytes | -0.106 | -0.278 | -0.249 | -0.027 | -0.060 | -0.215 |
| TP | 0.421* | 0.364 | 0.043 | 0.273 | 0.322 | 0.073 |
| Albumin | -0.126 | -0.101 | -0.039 | -0.073 | -0.124 | -0.433 |
| Globulin | 0.279 | 0.073 | 0.003 | 0.173 | 0.028 | 0.328 |
| A\G | -0.190 | -0.042 | -0.012 | -0.115 | -0.084 | -0.397 |
| Glucose | -0.078 | -0.184 | 0.460* | -0.066 | -0.164 | 0.513* |
| Triglycerides | 0.66 | 0.034 | 0.072 | 0.249 | 0.189 | 0.400 |
| Cholesterol | -0.090 | -0.127 | -0.040 | -0.124 | -0.081 | -0.023 |
| HDL-cholesterol | -0.090 | -0.127 | -0.182 | -0.124 | -0.081 | -0.159 |
| LDL-cholesterol | -0.090 | -0.127 | -0.099 | -0.124 | -0.081 | -0.023 |
| Ca | -0.163 | -0.324 | -0.008 | -0.015 | -0.019 | -0.271 |
| P | -0.129 | -0.032 | -0.078 | -0.030 | -0.001 | -0.061 |
| Cl | -0.003 | -0.124 | -0.312 | -0.091 | -0.154 | -0.076 |
| Na | -0.082 | -0.084 | -0.460* | -0.095 | -0.186 | -0.513* |
| K | -0.071 | -0.189 | -0.068 | -0.071 | -0.241 | -0.082 |
| Mg | -0.212 | -0.213 | -0.357 | -0.036 | -0.033 | -0.513* |
| Cu | -0.036 | -0.189 | -0.103 | -0.028 | -0.044 | -0.983* |
| Zn | -0.103 | -0.087 | -0.114 | -0.028 | -0.074 | -0.975* |
| TAC | -0.021 | -0.123 | -0.108 | -0.154 | -0.195 | -0.001 |
| AST | 0.127 | 0.047 | 0.280 | 0.127 | 0.203 | 0.597* |
| ALT | 0.031 | 0.118 | 0.135 | 0.062 | 0.180 | 0.359 |
| ALP | 0.259 | 0.063 | 0.145 | 0.002 | 0.045 | 0.063 |
| BUN | 0.126 | 0.227 | 0.263 | 0.177 | 0.117 | 0.186 |
| Cr | 0.234 | 0.365 | 0.325 | 0.117 | 0.063 | 0.045 |
Statistical significance of correlations * was recorded at (P < 0.05).
The distinguished decline in the TAC in the three diseased groups was attributed to the afore-mentioned pro-inflammatory cytokines activity which promotes different immune cells to produce free radicals in large amounts. Fundamentally, these free radicals damage the invading pathogens through reacting with their proteins, DNA and carbohydrates. They are physiologically neutralized by different antioxidants but in the current research they exceed the neutralizing capacity of the antioxidants causing the noticed decreased TAC in PG, AG and EG (Zein-Eldin et al., 2013; Donia et al., 2014; Rodríguez-Carrio et al., 2017). Additionally, the above-mentioned hypozincemia and hypocupremia in the diseased groups hinder the enzymatic antioxidants synthesis, leading to oxidative stress augmentation as well as lowering of TAC in the diseased animals. Moreover, these free radicals attack different body cells particularly liver and kidney cells result in the observed increase in hepatic enzymatic activities and renal function tests in the diseased groups (Zein-Eldin et al., 2013; Donia et al., 2014; Jesse et al., 2017). The infection metastasis as well as the invading pathogens migration also may take a part in this elevation of the organs function tests (Zein-Eldin et al., 2013; Donia et al., 2014; Jesse et al., 2017).
MMPs are a group of 28 proteolytic enzymes, among them MMP-2 and MMP-9 are the most important (Bircan et al., 2015). Both of them displayed a pronounced activity in sheep inflammatory diseases in this research. Similar results were reported before in human respiratory diseases (Bircan et al., 2015; Li et al., 2016), arthritis (Meszaros and Malemud, 2012) and enteritis (Strup-Perrot et al., 2004; Gao et al., 2005; Garg et al., 2006). Whereas, the pro-inflammatory cytokines (TNF-α, IL-1β) induce their secretion from various immune cells in order to maintain the immune response by activation of specific proteins, neutrophil migration, reactive oxygen species generation and bacterial phagocytosis (Garg et al., 2006; Meszaros and Malemud, 2012; Li et al., 2016). Additionally, their sensitivity, specificity, LR, PPV, NPV, accuracy rate, percentage of increase and their correlation with some parameters in the diseased groups (PG, EG) in the current work as well as their correlation with the inflammation severity in previous researches confirmed their value as good indicators for sheep pneumonia, arthritis and enteritis and suggested them as new therapeutic target in these conditions (Meszaros and Malemud, 2012; Li et al., 2016).
Conclusion and Recommendations
Overall then, this research cleared that the ovine pneumonia, arthritis and enteritis are associated with dramatic haemato-boichemical alterations. Following theses alterations during the diseases course and counteracting them by supportive treatment may be valuable in reducing the diseases side effects, shortening their duration and enhancing their recovery rates. Both of MMP-2 and MMP-9 is a good inflammatory marker for ovine pneumonia, arthritis and enteritis.
Acknowledgements
The authors acknowledged the staff members of Animal Health and Poultry Department, Desert Research Center, Egypt.
Authors Contribution
All authors equally contributed to the work.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
Allam. T.S., Saleh, N.S., Abo-Elnaga T.R. and Darwish, A.A. 2017. Cytokine response and immunological studies in camels (Camelus dromedarius) with respiratory diseases at Matrouh province. Alexandria J. Vet. Sci., 53: 116-124. https://doi.org/10.5455/ajvs.259788
Bircan, H.A., Akir, M., Kapulu, İ.Y., Sutcu, R., Kaya, S. and Ozturk, O., 2015. Elevated serum matrix metalloproteinase-2 and -9 and their correlations with severity of disease in patients with community-acquired pneumonia. Turk. J. Med. Sci., 45: 593-599. https://doi.org/10.3906/sag-1402-51
Bozukluhan, K., Merhan, O., Gokce, H.I., Deveci, H.A., Gokce, G., Ogun, M. and Marasli, S., 2017. Alterations in lipid profile in neonatal calves affected by diarrhea. Vet. World. 10(7): 786-789. https://doi.org/10.14202/vetworld.2017.786-789
Donia, G.R., El Ebissy, I.A. and Wassif, I.W., 2018. Biochemical and immunological studies on the respiratory diseases in north western coast. European J. Biomed. Pharmaceut. Sci., 5(12): 34-41.
Feldman, B.F., Zinkl, J.C. and Jain, N.C. 2000.Schalm’s veterinary hematolog. 5th edn. Lippincott Williams and Wilkins, Philadelphia, London.
Gao, Q., Meijer, M., Kubben, F., Sier, C., Kruidenier, L., Duijn, W., van den Berg, M., Hogezand, R., Lamers, C. and Verspaget, H., 2005. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with Inflammatory Bowel Disease (IBD). Short title: gelatinases in IBD. Dig. Liver Dis., 37: 584-592. https://doi.org/10.1016/j.dld.2005.02.011
Garg, P., Rojas, M., Ravi, A., Bockbrader, K.., Epstein, S., Vijay-Kumar, M., Gewirtz, A., Merlin, D. and Sitaraman, S., 2006. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: Contrasting role of gelatinases in the pathogenesis of colitis. J. Immunol. 177: 4103-4112. https://doi.org/10.4049/jimmunol.177.6.4103
Hassanein, K., Sayed, M. and Hassan, A., 2017. Pathological and biochemical studies on enterotoxemia in sheep. Comp. Clin. Pathol., 26: 513–518. https://doi.org/10.1007/s00580-017-2407-5
Joshi, V., Gupta, V.K., Dimri, U., Mandal, R.S.K. and Sharma, D.K., 2015. Evaluating Serum Lipid Profile in Bacterial Bovine Respiratory Disease (BRD) Affected Calves. Intas Poli Vet., 16(2): 187-188.
Kumar, S., Jakhar, K.K., Nehra, V. and Pal, M., 2015. Pathomorphological and microbiological studies in sheep with special emphasis on gastrointestinal tract disorders. Vet. World, 8(8): 1015-1020. https://doi.org/10.14202/vetworld.2015.1015-1020
Li, Y., Wang, Y., Lee, H., Lu, M. and Yang, S., 2016. Elevated Plasma Matrix Metalloproteinase-9 and Its Correlations with Severity of Disease in Patients withVentilator-Associated Pneumonia. Int. J. Med. Sci., 13(8): 638-645. https://doi.org/10.7150/ijms.16187
Meszaros, E. and Malemud, C.J., 2012. Prospects for treating osteoarthritis: enzyme–protein interactions regulating matrix metalloproteinase activity. Ther. Adv. Chronic. Dis., 3(5): 219-229. https://doi.org/10.1177/2040622312454157
Nahed, S.S., Tarek, R.A.E., Amani, A.H., Iman, A.E.E. and Asmaa, A.D., 2016. Clinicopathological and Bacteriological Studies on Pneumonia in Camel (Camelus dromedarius). J. Vet. Adv., 6(4): 1228-1236. https://doi.org/10.5455/jva.20160409123446
Othmana, O.E., Payet-Duprat, N., Harkat, S., Laoune, A., Maftahb, A., Lafri, M. and Silva, A.D., 2016. Sheep diversity of five Egyptian breeds: Genetic proximity revealed between desert breeds Local sheep breeds diversity in Egypt. Small Ruminant Res., 144: 346-352. https://doi.org/10.1016/j.smallrumres.2016.10.020
Rodríguez-Carrio, J., Alperi-López, M., López, P., López-Mejías, R., Alonso-Castro, S., Abal, F., Ballina-García, F.J., González-Gay, M.Á. and Suárez, A. 2017. High triglycerides and low high-density lipoprotein cholesterol lipid profile in rheumatoid arthritis: A potential link among inflammation, oxidative status, and dysfunctional high-density lipoprotein. J. Clin. Lipidol., 11(4): 1043-1054. https://doi.org/10.1016/j.jacl.2017.05.009
Strup-Perrot, C., Mathe, D., Linard, C., Violot, D., Milliat, F., Francois, A., Bourhis, J. and Vozenin-Brotons, M., 2004. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am. J. Physiol. Gastrointest. Liver Physiol., 287: 875-885. https://doi.org/10.1152/ajpgi.00088.2004
The Merck Veterinary Manual. 2006. Merck and Co., Inc. web site station NJ. USA Marial Ltd.
Zein-Eldin, M.M., Ghanem, M.M., Abd El-Raof, Y.M. and El-Attar, H.M., 2013. Clinical, haematobiochemical and electrocardigraphic changes of diarrheic sheep following treatment by nutmeg and oxytetracycline. Benha Vet. Med. J. 24(1): 329-334.






