Investigation of Azadirachta indica A Juss as a Source of Potential Antimicrobial and Cytotoxic Compounds
Research Article
Investigation of Azadirachta indica A Juss as a Source of Potential Antimicrobial and Cytotoxic Compounds
Nuzhat Munawar1, Abrar Hussain1, Imran Sajid2, Zahra Noreen1, Muhammad Aslam3*, Zeeshan Rehman1 and Aamir Ali3,4
1Department of Botany, Division of Science and Technology Division, University of Education, Lahore, Pakistan; 2Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan; 3Department of Chemistry, Division of Science and Technology Division, University of Education, Lahore, Pakistan; 4Geological Survey of Pakistan, Lahore.
Abstract | Azadirachta indica A Juss. (Neem) is a very valuable therapeutic plant being used as herbal medicine to cure various diseases due to the variety of biologically active compounds present in it. Neem leaves of the plant were collected, dried, powdered, and extracted by using ethyl acetate, chloroform, and acetone as solvents. Qualitative phytochemical analysis showed the presence of active compounds. The antibacterial potential of various plant extracts was evaluated using well diffusion and disc diffusion assay against various disease-causing microorganisms. In the case of well diffusion assay, the plant exhibited a maximum 16mm inhibition zone against B. subtilis a minimum 10 mm inhibition zone against P. aeruginosa, with acetone extract. The same extract seems inactive against K. pneumoniae as compared to control. When tested on S. aureus, chloroform showed a 10 mm inhibition zone while it was inactive against all other experienced bacteria. Ethyl acetate extract gave an inhibition zone of 10 mm against S. aureus, B. subtilis, and P. aeruginosa while it was inactive against the rest of the bacteria. Using disc diffusion assay, ethyl acetate extract gave 12 mm, 8mm, and 7 mm zone of inhibition against B. subtilis, S. aureus, K. pneumoniae respectively, while it was inactive against P. aeruginosa and E. coli. Chloroform extract did not show any pronounced activity as compared to the negative control against E. coli and K. pneumoniae while it was inactive against other tested bacteria. Cytotoxic effect of all the extracts was also evaluated using micro well cytotoxicity assay against brine shrimp larvae where chloroform extract showed minimal cytotoxic level (23.37%) while acetone extract showed maximum cytotoxicity (65%).
Received | January 25, 2021; Accepted | June 16, 2022; Published | June 20, 2022
*Correspondence | Muhammad Aslam, Department of Chemistry, Division of Science and Technology Division, University of Education, Lahore, Pakistan; Email: maslamchemist@hotmail.com
Citation | Munawar, N., A. Hussain, I. Sajid, Z. Noreen, M. Aslam, Z. Rehman and A. Ali. 2022. Investigation of Azadirachta indica A Juss as a source of potential antimicrobial and cytotoxic compounds. Biologia Lahore), 68(1): 11-17.
DOI | https://dx.doi.org/10.17582/journal.Biologia/2022/68.1.11.17
Keywords | Azadirachta indica, Phytochemical analysis, Well diffusion assay, Disk diffusion assay, Microwell cytotoxicity assay
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
It is a fact that the various contagious diseases are treated with plants, and these are the key medicinal sources in some emerging countries (Mothana and Lindequist, 2005). Many pharmaceutical plants are considered to have several antimicrobial drugs. Selection of proper medicinal plants for antimicrobial activity will improve economic formulations, having better safety and worth (Parashar et al., 2018). This expectancy established that some of the naturally found herbal compounds could slay antibiotic-resistant strains of bacteria, for instance, E. coli, S. aureus, B. cereus, and M. luteus (Friedman, 2006). Plants having such phytochemicals revealed great promise for the cure of inflexible infectious human diseases, including viral contagions (Cowan, 1999). The combined action of these phytochemicals is likely to be responsible for the various anti-bactericidal and fungal properties that are shown by oils (Babatunde et al., 2019). Further efficient screening of plants could have resulted in the invention of new valued compounds (Nitta et al., 2002).
Neem (Azadirachta indica), family Meliaceae is the most adaptable tree species grown in the tropics, having massive potential. Various compounds are synthesized during secondary metabolism, which have antimicrobial properties due to which this plant has been used in the past. These properties are due to active substances, like phenolic, in essential oils (Janssen et al., 1987). The natural activities are attributed to various biologically active compounds in their various parts (Sonia and Srinivasan, 1999). The extract of leaves is used as a fillip to boost up hunger and remove intestinal worms and is used for its hypoglycaemic, hypolipidemic, hepatoprotective, and hypotensive activities along with fever control. Medicinally, leaf juice is also used for its antimicrobial potential against dental diseases.
Moreover, in the traditional medicine system, the respective herb is also used to treat malarial fever. Its products even are used to cure cancer, skin infections, digestive diseases, and AIDS. The present work aims to study the antimicrobial potential of leaf extracts of various solvents against certain pathogenic bacteria and to find out the cytotoxic potential against Brine shrimp larvae.
Materials and Methods
Extract preparation
The Azadirachta indica leaves were collected, dried under shade, and powdered using an electrical grinder. After grinding, the powder was taken in a beaker with the subsequent addition of 500 ml methanol (mother extractant), with two to three times off and on shaking in a day. Then the filtrate was obtained using Whatman’s filter paper no. 1. following condensation of filtrate using hot plate magnetic stirrer, until the filtrate turned into gummy solid. The gummy solid (methanolic extract) was further processed by adding 100 ml of distilled water and shaking to dissolve crude extract. The obtained solution was poured in a separating funnel and fractionated by 150 ml of ethyl acetate, chloroform, and acetone (Al-Hashemi and Hossain, 2016).
Phytochemical analysis
Phytochemical analysis was performed on all the extracts of plant to check the presence of different phytochemicals using respective standard protocols such as for alkaloids (Evans, 1997), carbohydrates (Herborne, 1973), reducing sugars (Sofowora, 1993), tannins, phenols (Mace, 1963), flavonoids (Trease and Evans, 1989), terpenoids (Robinson, 1980), saponins, cardiac glycosides (Krisgnaveni and Sailaja, 2014) and fixed oils and fats (Krishna et al., 2013).
Test microorganisms
S. aureus, B. subtilis, K. pneumoniae, E. coli, and P. aeruginosa were used to check the antimicrobial activity of plant extract. These all bacterial strains were obtained from the Department of Microbiology, and Molecular Genetics, University of the Punjab, Lahore, and the antimicrobial assays were performed in the BSL2 lab facility of the department.
Preparation of bacterial inoculum
0.5 M Farland inoculum was incubated on to LB (Luria Bertani) medium having 10g Bacto-tryptone, 5g yeast extract, 5g NaCl, 15g agar; pH 7, and the inoculum was autoclaved at 121oC, 15 lbs pressure for 15 minutes.
Determination of antibacterial activity
Well diffusion assay and disc diffusion assay were performed to check the antimicrobial activity of the plant. Moreover, all pure solvents (ethyl acetate, chloroform, and acetone) were also used as a negative control to assess the plant’s potential.
Well diffusion assay
10 ml of 0.5 McFarland solutions were prepared separately in a test tube for all five to be tested organisms. The trial plates were prepared by adding 20 ml of LB (Luria bertani) and inoculated with 50µl of 0.5 McFarland inoculum of each test organism. Five wells (1 in center and four on sides) were made using a sterile cork borer in each plate. The 50 µl of each extract was loaded inside wells and 50 µl of pure solvent, i.e., acetone, ethyl acetate, and chloroform, in the central well. The test plates were incubated at 37oC for 16-24 hours. The inhibitory zone appeared around each well after an incubation period; the diameter of each zone was measured in mm (Lertcanawanichakul and Sawangnop, 2008).
Disk diffusion assay
The test plates were prepared for all to be tested organisms by adding16 ml of LB medium. After hardening, the layer was overlapped with 4 ml of molten LB agar media injected with 100 µl of 0.5 McFarland inoculum of the test organism as a seed layer. The sterilized discs of 6mm diameter were prepared using Whatman filter paper no. 1. sterilized filter paper disks were then impregnated with 40 µl of crude extract solution, dried under hygienic conditions, and put on the surface of test plates using sterile forceps. All test plates were incubated (37ºC) for 16–24 hours. After this, the diameter of inhibition zones was measured in mm (Garhardt et al., 1994).
Brine shrimp (Artemia salina) microwell cytotoxicity assay
Brine shrimp dried eggs were hatched in separating funnel (500ml) using artificial seawater (400ml). Bubbling air was used to oxygenate the suspension using an aquarium pump in the funnel. The aeration was stopped after 24-48 h incubation at room temperature, and the larvae were kept undisturbed for 1 hour. One side was covered with aluminium foil, while the other side of the funnel was illuminated using a lamp, and the larvae were separated using a pipette. Then the larvae were shifted to a deep well microtiter plate of 1.8cm diameter and 2cm depth, filling it with 0.2 ml of synthetic seawater. At this stage, the dead larvae counted (valued as N). Then 20 µg of crude extract was dissolved in 5-10 µl of dimethyl sulfoxide and poured in the plate kept in the dark at room temperature. The A value was again counted after 24 h, using a microscope. To count the total number of the larvae, 0.5 ml methanol was poured into killing the surviving larvae. Each test row was supplemented with a blind sample of pure DMSO. For 100% mortality, actinomycin D (10 µg/ml) was used as a positive control. The following formula was used to calculate the death rate (Solts et al., 1993).
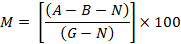
Where; M = % of the dead larvae after 24 h; A = No. of the dead larvae after 24 h; B = Average number of the dead larvae in blind sample; N = No. of the dead larvae before starting the test; G = Total number of larvae.
Results and Discussion
Phytochemical screening
Preliminary phytochemical screening was done using evaporated extracts of the plant. The qualitative screening of ethyl acetate, chloroform, and acetone extracts showed the presence of most of the secondary metabolites, except phenols not found in the ethyl acetate and chloroform layer and tannins, which were found to be absent in the chloroform and acetone layer (Table 1).
Table 1: Phytochemical constituents of plant extracts prepared in different solvents.
|
Content |
Azadirachta indica |
||
|
E.A |
Chl |
Ace |
|
|
Carbohydrates |
+ |
+ |
+ |
|
Tannins |
+ |
- |
- |
|
Flavonoids |
+ |
+ |
+ |
|
Phenols |
- |
- |
+ |
|
Saponins |
+ |
+ |
+ |
|
Terpenoids |
+ |
+ |
+ |
|
Glycosides |
+ |
+ |
+ |
|
Reducing sugar |
+ |
+ |
+ |
|
Oils |
+ |
+ |
+ |
|
Alkaloids |
+ |
- |
+ |
+, indicating presence; -, indicating the absence.
Antibacterial activity of extracts
Well-diffusion activity: Ethyl acetate extract gave a maximum inhibition zone (10mm) against S. aureus, B. subtilis, and P. aeruginosa while it was inactive against the rest of the strains. The pure solvent was also tested as a control which was not active against any strains of bacteria (Figure 1). The chloroform extract was found only active against S. aureus, giving a 10 mm inhibition zone around well but was inactive against the other tested strains. Pure chloroform, tested as control, didn’t show any activity against understudy bacterial strains (Figure 2).
The acetone extract gave 16 mm, i.e., maximum inhibition zone against B. subtilis following 12 mm against S. aureus, 11 mm against E. coli, 10mm against P. aeruginosa, and 9 mm against K. pneumoniae. Acetone, as control, was inactive against S. aureus, B. subtilis, and P. aeruginosa, but it showed a 13 mm and 12 mm inhibition zone against E. coli and K. pneumoniae, respectively (Figure 3).
Disc diffusion activity
Ethyl acetate extract of Azadirachta indica was found to be active against S. aureus (8mm) and B. subtilis (12 mm), and K. pneumonia (7 mm). The negative control (pure ethyl acetate) was found to be active only against K. pneumonia (7 mm) (Figure 4).
The chloroform extract of Azadirachta indica gave 8mm and 9mm zones of inhibition against B. subtilis and E. coli, respectively, inactive against rest bacterial strains. In contrast, negative control showed more pronounced activity against E. coli (12mm) and somehow equal activity against B. subtilis (9 mm) (Figure 5). Acetone extracts gave 11mm, 10mm, and 7mm inhibition zones against B. subtilis, E. coli and K. pneumonia, respectively. Pure acetone was found to be active only against K. pneumonia (7 mm) (Figure 6).
The cytotoxicity potential of the plant was checked against brine shrimp larvae. Among all extracts, chloroform extract exhibited the least cytotoxicity with a 23.37% mortality rate, while ethyl acetate extract showed a bit higher cytotoxicity level with a 26.64% mortality rate. On the other hand, acetone extract proved to be the most toxic, with a 65% mortality rate (Figure 7).
Although a lot of work has been done on medicinal plants, this could not be discontinued due to an increase in antibiotic-resistant strains of bacteria. So, there is a need to work on antimicrobial and cytotoxic compounds of medicinal plants, which can form the basis for the formulation of new antibiotic agents. The present research follows such an effort that preliminary phytochemical screening showed the presence of most biologically active compounds similar to the earlier worker (Al-Hashemi and Hossain, 2016). However, Alkaloids were found absent in the chloroform layer (Table 1).
Well diffusion assay was performed to check the antibacterial potential of the under-study plant against a panel of test bacteria. The previously reported antimicrobial potential of the plant leaves showed a zone of inhibition of 20 mm against E. faecalis and 7.1 mm against C. albicans (Bohora et al., 2010). Zone of inhibition ranging from 0.8cm to 2cm was given by results of aqueous and alcoholic leaf extract against E. coli, S. aureus, S. Bacillus bacteria, and Rhizopus fungi through cup-plate agar diffusion method (Gupta et al., 2013). The difference between the result of antibacterial activity of P. aeruginosa and E. coli was probably due to the difference in the solvent extraction method (using soxhlet) and variations in solvent being used. For chloroform extract, the results were in accordance with the previous report about Pseudomonas sp., but not in agreement with E. coli and Klebsiella sp. (Shinde and Mulay, 2015; Zwetlana et al., 2014). A similar result was also reported against S. aureus, E. coli, P. aeruginosa, and K. pneumoniae using acetone extract of the plant (Bassey et al., 2016; Irshad et al., 2011; Shinde and Mulay, 2015).
In a previously reported disc diffusion assay result, the respective test extracts of Azadirachta indica possess significant activity against test microorganisms (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, and Klebsiella pneumonia) (Latha et al., 2015). The difference in results against E. coli and P. aeruginosa could be due to different method used to prepare plant extracts. Performing disc-diffusion assay for chloroform extract of the plant, the same results were reported in the previous study against P. aeruginosa, E. coli, and S. aureus (Al-Jadidi and Hossain, 2015; Victor and Igeleke, 2012). The understudy acetone extract results were also in agreement with earlier reported results against E. coli., K. pneumonia, and P. aeruginosa, but results differed against S. aureus and B. subtilis, which is probably due to differences in experimental conditions (Irshad et al., 2011; Victor and Igeleke, 2012).
Cytotoxicity of plant extracts
In reported results of water and methanol extracted samples of Azadirachta and Myrica for brine shrimp, the toxicity level was 101.26 ± 3.7, 61.43 ± 2.9 (μg/ml) and 328.22 ± 10.9, 320.17 ± 1.6 (μg/ml), respectively (Kirira et al., 2006). Comparing reported results with current cytotoxicity results revealed that using ethyl acetate and chloroform as an extracting agent will be much less toxic.
Conclusions and Recommendations
Most of the biologically active compounds were found in ethyl acetate and acetone extracts of the plant. Using agar well diffusion assay, chloroform and acetone samples were most active, giving 16mm inhibition zone against B. subtilis. The disc diffusion assay was also used to determine the antimicrobial potential of the respective plant in which ethyl acetate extracted sample indicated a 12 mm maximum zone of inhibition against B. subtilis. With brine shrimps, microwell cytotoxicity assay chloroform extract indicated least toxicity (23.37%). Overall, ethyl acetate and chloroform extracts of Azadirachta indica could be promising antibacterial and anti-cytotoxic agents. Still, more precise and intensive research work is needed before it can be used in the pharmaceutical industry.
Novelty Statement
This paper could be used as the basics for further use of Azadiracta indica extract in medicinal field.
Author’s Contribution
MA: Manuscript submission.
NM, ZR: Performed experimental work.
IS, AH: Study, experimental design and laboratory experiments.
ZN, AA: Manuscript writing and data analysis.
Conflict of interest
The authors have declared no conflict of interest.
References
Al-Hashemi, Z.S.S., and M.A. Hossain. 2016. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac. Sci. Rev. A Nat. Sci. Eng., 18: 128-131. https://doi.org/10.1016/j.psra.2016.09.013
Al-Jadidi, H.S.K. and M.A. Hossain. 2015. Studies on total phenolics, total flavonoids and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni-Suef Univ. J. Basic Appl. Sci., 4: 93-98. https://doi.org/10.1016/j.bjbas.2015.05.001
Babatunde, D.E., G.O. Otusemade, M.E. Ojewumi, O. Agboola, E. Oyeniyi and K.D. Akinlabu. 2019. Antimicrobial activity and phytochemical screening of neem leaves and lemon grass essential oil extracts. Int. J. Mech. Eng. Technol., 10(3): 882-889.
Bassey, E.E., G.A. Mohammed, H.M. Bala, U.S. Ogonna, B.B. Yawuri and O.C. Maduchi. 2016. Phytochemical analysis and antimicrobial activity of methanolic, ethanolic and acetonic extracts of stem bark and leaf of neem plant (Azadirachtaindica). J. Dis. Med. Plants, 2: 14-23. https://doi.org/10.11648/j.jdmp.20160203.11
Bohora, A., V. Hegde and S. Kokate. 2010. Comparison of the antibacterial efficiency of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. albicans and mixed culture-An in vitro study. Endodontology, 22: 8-12. https://doi.org/10.4103/0970-7212.351972
Cowan, M.M., 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev., 12: 564-582. https://doi.org/10.1128/CMR.12.4.564
Evans, W.C., 1997. Trease and evans pharmacology. Harcourt Brace Company. Asia. Pvt. Ltd., Singapore.
Friedman, M., 2006. Antibiotic activities of plant compounds against non-resistant and antibiotic-resistant foodborne human pathogens. ACS Symp. Ser., 931: 167-183. https://doi.org/10.1021/bk-2006-0931.ch012
Garhardt, P., R.G.E. Murray, W.A. Wood and N.R. Krieg. 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington DC.
Gupta, A.K., N.K. Ahirwar, N. Shinde, M. Choudhary, Y.S. Rajput and A. Singh. 2013. Phytochemical screening and antimicrobial assessment of leaves of Adhatoda vasica, Azadirachta indica and Datura stramonium. J. Pharm. Biosci., 1: 42-47. https://doi.org/10.20510/ukjpb/1/i1/91118
Herborne, J.B., 1973. Phytochemical methods. A guide to modern techniques of plant analysis. https://doi.org/10.1007/978-94-009-5921-7_1
Irshad, S., M. Butt and H. Younus. 2011. In-vitro antibacterial activity of two medicinal plants Neem (Azadirachta indica) and Peppermint. Int. Res. J. Pharma., 1: 9-14.
Janssen, A.M., J.J.C. Scheffer and A.B. Svendsen. 1987. Antimicrobial activity of essential oils: a 1976-1986 literature review. Aspects of the test methods. Planta Med., 53: 395-398. https://doi.org/10.1055/s-2006-962755
Kirira, P.G., G.M. Rukunga, A.W. Wanyonyi, F.M. Muregi, J.W. Gathirwa, C.N. Muthaura, S.A. Omar, F. Tolo, G.M. Mungai and I.O. Ndiege. 2006. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts of Kenya. J. Ethnopharmacol., 106: 403-407. https://doi.org/10.1016/j.jep.2006.01.017
Krisgnaveni, G. and O. Sailaja. 2014. Identification and estimation of phytochemicals from the plant Pedicularis Bicornuta leaf extract by UV-spectrophotometry. Res. Desk., 3: 410-418.
Krishna, T.P.A., V.N.S. Raj, S. Juliet, S.N. Nair, R. Ravindran and S. Sujith. 2013. Evaluation of phytochemical constituents and proximate contents of the ethanolic leaf extract of Tetrastigmaleucostaphylum (Dennst.) Alstone (Vitaceae) found in Western Ghats of Kerala, India. Res. J. Pharm. Sci., 2(10): 1-6.
Latha, R.C.R., S. Kavitha and S. Sathya. 2015. Antimicrobial efficacy of Azadirachta Indica leaf. Int. J. Sci. Nat., 6(3): 432-440.
Lertcanawanichakul, M. and S. Sawangnop. 2008. A comparison of two methods used for measuring the antagonistic activity of Bacillus species. J. Sci. Technol., 5: 161-171.
Mace, M.E., 1963. Histochemical localization of phenols in healthy and diseased banana roots. Physiol. Plant, 16: 915-925. https://doi.org/10.1111/j.1399-3054.1963.tb08367.x
Mothana, R.A.A., and U. Lindequist. 2005. Antimicrobial activity of some medicinal plants of the island Soqotra. J. Ethnopharmacol., 96: 177-181. https://doi.org/10.1016/j.jep.2004.09.006
Nitta, T., T. Arai, H. Takamatsu, Y. Inatomi, H. Murata, M. Iinuma, T. Tanaka, T. Ito, F. Asai and I. Ibrahim. 2002. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J. Health Sci., 48: 273-276. https://doi.org/10.1248/jhs.48.273
Parashar, G., N. Sutar and S. Sanap. 2018. Antibacterial activity of mixture of leaf extracts of Neem (Azadirachta indica Linn.) and Tantani (Lantana camara). Int. J. Pharm. Sci. Res., 9: 2545-2549.
Robinson, T., 1980. The organic constituents of higher plants: Their chemistry and interrelationships.
Shinde, A.B., and Y.R. Mulay. 2015. Phytochemical analysis and antibacterial properties of some selected Indian medicinal plants. Int. J. Curr. Microbiol. Appl. Sci., 4: 228-235.
Sofowora, A., 1993. Medicinal plant and traditional medicine in Africa 2nd Ed., Sunshine House, Ibadan.
Solts, P.N., C.W. Wright, M.M. Anderson, M.P. Gupta and J.D. Phillipson. 1993. A microwell cytotoxicity assay using Artemia salina. Plant Med., 59(3): 250-252. https://doi.org/10.1055/s-2006-959661
Sonia, B. and B.P. Srinivasan. 1999. Investigations into the anti-diabetic activity of Azadirachta indica. Indian J. Pharmacol., 31(2): 138-141.
Trease, G.E., and W.C. Evans. 1989. Pharmacognosy, 11th Ed., Bailliere Tindall, London.
Victor, I.U. and C.L. Igeleke. 2012. Antimicrobial properties of the extracts of locally sold garlic and neem leaf in Benin City, Nigeria. Int. J. Biosci., 2: 21-27.
Zwetlana, A., M. Nandini and K. Dorcas. 2014. Antimicrobial activity of medicinal plant extracts on gram negative bacteria. J. Med. Plants Stud., 2: 51-54.
To share on other social networks, click on any share button. What are these?








