Influence of Harvesting Stages and Packaging on Storage Life of Apricot (Prunus armeniaca L.) Cv. Trevett
Research Article
Influence of Harvesting Stages and Packaging on Storage Life of Apricot (Prunus armeniaca L.) Cv. Trevett
Rashid Khan1, Muhammad Sajid2, Saud Khan3*, Jehanzeb4 and Aslam Noor5
1Research Officer, Agriculture Research Station, Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan; 2Department of Horticulture, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Agriculture Officer, Agriculture Extension Department South Waziristan, Tribal District, Khyber Pakhtunkhwa, Pakistan; 4Agriculture Officer, Agriculture Extension Department D.I. Khan, Khyber Pakhtunkhwa, Pakistan; 5Research Officer Agriculture Research Station, South Waziristan, Tribal District, Khyber Pakhtunkhwa, Pakistan.
Abstract | Supply of quality fruits to consumer is a major challenge due to storage and packing issues in developing countries. The current study was designed to evaluate postharvest quality of apricot at different harvesting stages i.e., M1 (greenish), M2 (light yellow) and M3 (orange-yellow) while, storage conditions i.e., packed in plastic bags with F0 (closed), F5 (5 pores), F10 (10 pores), F15 (15 pores), F20 (20 pores) and F25 (25 pores) at 5+2oC. The fruits were then analyzed for different quality parameters at 0 and 25 days storage. The harvesting stages (HS), perforated plastics (PP), storage duration (SD) as well as HS×SD and PP×SD significantly influenced various quality attributes of apricot fruits. The fruits harvested at the greenish stage had the lowest physiological weight loss and decay index that shows an increase in fruits harvested at orange yellow stage respectively after 25 days of storage. Similarly, after 25 days of storage, fruits stored in plastic bags having 10 pores retained fruit weight and attained minimum physiological weight loss with low decay index. Similarly, the fruits harvested at the greenish stage and stored for 25 days attained the highest titratable acidity, fruit firmness, and ascorbic acid in comparison with other harvesting stages. The highest titratable acidity, fruit firmness, and ascorbic acid were recorded in 10 pores plastic bags, while the lowest titratable acidity, fruit firmness and ascorbic acid were recorded in closed plastic bags after 25 days of storage. The lowest value of TSS and TSS: acid was recorded at a greenish stage, which increased at later stages of harvesting. In case of Pp ×SD, the range of TSS and TSS: Acid at day 0 increased after 25 days of storage with the lowest TSS and TSS: Acid was observed in plastic bags having 10 perforations. Apricot fruits harvested at the greenish stage (10.31 0 Brix) packed in plastic bags having 10 perforations shows the best in retaining most of the quality parameters at storage.
Received | May 12, 2022; Accepted | February 17, 2023; Published | March 28, 2023
*Correspondence | Saud Khan, Agriculture Officer, Agriculture Extension Department South Waziristan, Tribal District, Khyber Pakhtunkhwa, Pakistan; Email: saudwazir@aup.edu.pk
Citation | Khan, R., M. Sajid, S. Khan, Jehanzeb and A. Noor. 2023. Influence of harvesting stages and packaging on storage life of apricot (Prunus armeniaca L.) Cv. trevett. Sarhad Journal of Agriculture, 39(1): 278-286.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.1.278.286
Keywords | Apricot shelf life, Fruit quality parameter, Harvesting stages, Perforated plastics, Stages and Packaging
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Apricot (Prunus armeniaca L.) is a deciduous fruit belongs to the family Rosaceae and is mostly grown in temperate climate of all continents of the world. Apricot is referred to stone fruits, with outer fleshy exocarp and mesocarp (skin and flesh) surrounds a stone of hardened endocarp having seed inside. Stone fruits also include peach, plum, nectarine, almond and cherry, which are closely related to each other, because of large and hard stone inside. All these belong to the same genus: Prunus, family Rosaceae (Penn state Extension, 2015). Among all stone fruits apricot has unique importance because of its great market value as of fresh as well as dried food commodity and has good market share of agriculture income. It has enough amount sugar, protein crude fibre, crude fate, Vitamin A, C, K, B complex and organic acid (Fatima et al., 2018). Turkey is the world largest producer of apricot with total production of 750,000 tonnes, followed by Uzbekistan and Iran with production of 493842 and 342479 tonnes respectively. Pakistan ranked seventh globally with total production of 128382 tonnes (FAO, 2018). This production is much lower as compared to advance countries and there is still a huge potential to boost the apricot production in the country. Post apricot is a climacteric fruit, which makes it susceptible to spoilage and post-harvest decay after being picked and became susceptible to more shrinkage and dehydration due to non-waxy nature of the skin (Ali et al., 2013). To keep these commodities fresh for as long as possible, their respiration rate should be decreasing without harming the quality of the fruit commodities. Generally, the respiration process is reduced by low temperature and modified atmosphere (Hussain, 1986). For this purpose, polymeric films have been used for packaging of fresh fruits and vegetables for a long time to control water loss, protect it from skin abrasion and reduce several other metabolic activities. It also has the ability to minimize the spread of disease from one fruit to another (Kader, 1980). The reduced metabolic activities depend on the relative permeability of the films, which allow the regulation of respiratory gases. This makes a modified atmospheric condition, which allows low level of oxygen and high level of carbon dioxide within the package and ultimately reduces the respiration rate of the fruits (Scetar et al., 2010). A single film in modified atmosphere packaging cannot provide all the properties needed for a modified atmosphere package (Alique et al., 2003). Modification in these films alters the gas permeability and rate of respiration at specific temperature (Scetar et al., 2010). Similarly, optimization of pre harvest management practices like application of GA3 and harvesting the fruit at proper physiological maturity stage increases the storage life of stone fruits (Vangdal et al., 2012). The critical factor that influences the fruit quality and storage performance of fruit is to harvest them at proper maturity stage (Crisosto et al., 2004).
Pakistan has massive potential for apricot production and is among the main producers in the world. However, limited literature is available on the storage life of apricot fruit from this region. There is increasing cultivation of apricots year by year in Pakistan and the fruit produced here has good quality and high market value but unfortunately the local producer is totally unaware about its storage under the prevailing environmental conditions which results in huge post-harvest losses. Keeping in view the above facts, this study was planned to determine the post-harvest life of apricot fruits in cold storage enclosed in perforated plastics harvested at different harvesting stages.
Materials and Methods
This experiment was planned to retain postharvest quality attributes of fruit for a longer period during cold storage. For this purpose, the fruits of uniform size (20-22gm) were harvested at three different stages of maturity according to the skin color, representing M1 (greenish stage with total soluble solids 10.31 oBrix), M2 (light yellow stage with total soluble solids 12.32 oBrix) and M3 (orange-yellow stage with total soluble solids 13.62 oBrix) from the healthy trees located at Horticulture Research farm, The University of Agriculture Peshawar, Pakistan. Fruits were harvested at each stage and divided into two lots. Lot no 1 fresh fruits of different stages were analyzed for various qualities attributes i.e. (Physiological weight loss, decay index, Fruit firmness, Fruit juice pH, Total soluble solid, Titratable acidity, TSS: acid ratio and Ascorbic acid), while the fruits of lot no 2 (54 no of fruits of each harvesting stage with 09 number of fruits in each packaging) were enclosed in plastic bags (20 x 30 cm, 30 microns) with different perforation (diameter of each pore was 2mm and these perforations were made through 2mm hole puncher) representing F0 (0 pores), F5 (5 pores), F10 (10 pores), F15 (15 pores), F20 (20 pores), F25 (25 pores) and shifted to cold storage and stored for 25 days at 5+2 oC. These perforations were equally distributed in each plastic bag and the plastic bags were placed in such a position in the cold storage so that all the perforations remained open.
The experiment was laid out in Completely Randomized Design (CRD) with three factors replication. The analysis of variance (ANOVA) for different parameters was determined using Statistix 8.1. The data of different parameters were analyzed through ANOVA techniques to observe the differences among different parameters as well as their interactions. In cases where the differences were significant at P≤0.05 the means were separated by Least Significant Difference (LSD) test (Steel and Torrie, 1997). Data on the following parameters were recorded during the experiments.
Physiological weight loss
Physiological weight loss was determined using an electrical balance by the difference between the initial and final weight of each treatment. Each fruit was weighed individually and then averaged. The following equation was used to determine the physiological weight loss, which is expressed in percent (%) (Amayogi and Alloli, 2007).
Decay index (%)
It was determined by dividing the percentage of fruits showing disease or decay incidence visually by total fruits stored in each treatment of all replication.
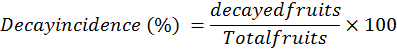
Fruit firmness
Fruit firmness was determined using hand held penetrometer of the randomly selected fruits for each treatment in each replication (Effigi, FT-11) (Pocharski et al., 2000).
Fruit juice pH
pH meter (Model No. INOLAB. pH 720) was used for measuring fruit juice pH of the randomly selected fruit.
Total soluble solids (ºBrix)
Total soluble solids (TSS) was recorded with the help of hand refractometer (Ranganna, 1986). A small quantity of crushed fruit pulp of randomly selected fruit was used for extracting few drops of juice that were placed on clean dry prism of the hand refractometer (Kernco Instruments Co Texas) and the reading was recorded. Distilled water and tissue paper were used to clean the prism between consecutive readings.
Titratable acidity (%)
Randomly selected fruits in each replication for all treatments were analyzed for titratable acidity as determined by the recommended method of (AOAC, 2000). 10 ml juice sample of randomly selected apricot fruit was taken in a beaker and their volume was increased up to 100 ml by addition of distilled water. Then the 10 ml diluted sample was titrated against 0.1N NaOH solution using 2-3 drops of phenolphthalein as an indicator. Data was recorded after the appearance of pink color, which persisted for 15 seconds. The data was recorded three times in each treatment and the following formula was used to calculate percent acidity. Average was calculated later.
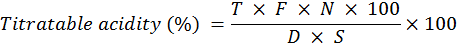
Where; T = Burette solution (ml of NAOH); N= Normality of sodium hydroxide (NAOH); S = Quantity of diluted sample (ml) taken for titration; D = Quantity of sample (gm) taken for dilution; F = Constant acid factor (primary acid in the fruit) = 0.0067 (malic acid in apricot).
TSS: Acid ratio
The TSS: Acid was calculated by using the formula given below:
Ascorbic acid (mg. 100g-1)
Dye method was used for determining ascorbic acid contents in randomly selected fruits in each replication for all the treatments according to the method of (AOAC, 2000). 0.4% oxalic acid solution was used for the dilution of 10 gm of apricot fruit pulp and then its volume was raised to 100 ml. 10 ml of this diluted sample was then titrated against the dye solution till the appearance of light pink color. Ascorbic acid was then calculated using the following formula.
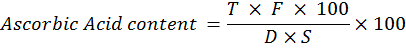
Where; F= dye factor; T= dye solution (ml) used for titration; D= diluted sample (ml) taken for titration; S= Apricot fruit pulp (gm) taken for titration.
Results and Discussion
Physiological weight loss (%)
Perforated plastic (Pp), harvesting stages (HS) and storage duration (SD) had as significant influence on physiological weight loss of apricot fruits (Table 1). The highest physiological weight loss (4.18%) occurred in fruits harvested at orange yellow stage (M3), followed by fruit harvested at light yellow stage (M2) with (3.11%) weight loss after 25 days of storage. However, fruits harvested at greenish stage (M2) retained their weight with minimum (2.87%) weight loss after 25 days of storage in comparison with fresh fruits. Similarly, maximum weight loss (4.04%) occurred after 25 days of cold storage duration in closed plastic bags (F0), while the minimum weight loss (2.82%) occurred in plastic bags having 10 pores (F10), which again increased to (3.69 %) in plastic bags with 25 perforations F25). Respiration and water loss in fruits is a continuous process, which causes fruits deterioration and post-harvest losses. Maximum fruit weight loss in closed plastic bags could be because of O2 injury and low O2 levels (anaerobic condition) (Tariq et al., 2001). To avoid such conditions, there must be control flow of gases at optimum rate depending on the nature of the film and type of fruits (Pretel et al., 2000). Similarly, more physiological weight loss occurred in fruits harvested at later stages of maturity could be attributed to higher respiration and transpiration rates (Ngcobo et al., 2012). In addition, the Hardenburg et al. (1990) also stated that water loss through transpiration and loss of carbon in respiration process is responsible for weight loss in different fruits, which generally increases with delaying harvesting. The results are also in line with Rashidi et al. (2014) who reported that moisture content of nectarine could be conserved with wrapping and packaging material. Similarly, lower moisture loss was recorded in grapes during storage. Further, Sabir et al. (2011) also reported that water loss in minimally processed table grapes can be prevent through MAP, as a most common wrapping material. Rab et al. (2016) also reported less weight loss in plum fruits harvested at pale green stage and packed in plastic bags having 15 pores after 25 days of cold storage duration.
Decay index (%)
Fruits harvested at various stages were observed with varying decaying indexes More fruits were decayed (18.05%) at orange-yellow stage (M3), followed by fruits harvested at light yellow stage (M2) with (4.86%) decay while minimum increase (4.17%) occurred in fruits harvested at greenish stage (M1) at the end of 25th day of storage in comparison with fresh fruits. Similarly, after 25 days of storage in cold storage conditions maximum decay index (26.39%) was observed in fruits stored in closed plastic bags (F0), followed by F5 (plastic bags with 5 pores), while minimum decay index (2.77%) occurred in plastic bags with 10 pores (F10), which again increased 6.94%) in plastic bags with 25 perforations (F25). Fruits in closed plastic bags were found more susceptible to deterioration as compared to the perforated ones. Closed plastic bags do not allow the moisture contents to go out, released during continuous transpiration and hence accumulate more humidity. Besides this, high CO2 injury and humid conditions in closed plastic bags encourage microbial activities and as a result decaying percentage increase (Jawandha et al., 2012). Navjot and Sukhjit (2010) further reported that increase in respiration rate, enzymatic activities and disassociation of cells in the cell wall results in higher spoilage in later harvested fruits, which leads to the softening and ripening of fruits. The Juan et al. (1999) observed similar results in apple.
Rab et al. (2016) reported least disease incidence in plum fruit harvested at pale green stage, and packed in plastic packaging having 15 perforations after 25 days of cold storage duration. Our results are also in line with Khan et al. (2013), who reported that fruit firmness of plum fruit can be retained with different packaging material. Similarly, decrease in plum fruit firmness was recorded by with increasing durations (Rashidi and Bahri, 2014).
Fruit firmness (kg.cm-2)
Fruit firmness is one of the important quality attributes. It was observed that fruits showed significant variations in firmness at different stages stored for different durations in cold and normal conditions (Table 1). Initial fruit firmness of greenish stage (M1), light yellow stage (M2) and orange yellow stage (M3) was recorded 3.55 kg.cm-2, 2.01 kg.cm-2 and 1.54 kg.cm-2, respectively which, finally decreased to 0.64 kg.cm-2, 0.57 kg.cm-2, and 0.38 kg.cm-2, respectively. Greenish (M1) stage showed maximum fruit firmness (0.64 kg.cm-2), while minimum fruit firmness was recorded in orange yellow (M3) after 25 days of cold storage. Similarly, initial firmness of the fruits for F0, F5, F10, F15, F20 and F25was 2.37 kg.cm-2, 2.36 kg.cm-2, 2.38 kg.cm-2, 2.35 kg.cm-2, 2.36 kg. cm-2 and 2.36 kg. cm-2, respectively, which decreased to 0. 42kg.cm-2, 0.54 kg.cm-2, 0.65 kg.cm-2, 0.61 kg.cm-2, 0.5 kg.cm-2 and 0.47 kg.cm-2, respectively. The maximum fruit firmness (0.64 kg. cm-2) was recorded in F10, while minimum fruit firmness (0.42 kg. cm-2) was recorded in F0 after 25 days of storage. Our results are in agreement with (Tariq et al., 2001); they stated that the fruit firmness decreases as result of fruit damages due to the decreased concentration of O2 and increase CO2 in modified atmosphere. Such condition leads to an anaerobic condition, which further accelerates the catabolic process fruits became softer and reduced firmness. Our finding also confirms the results of Jan et al. (2012) who concluded that the fruits harvested at early stage of maturity have maximum fruit firmness, while least firmness was recorded in later harvesting stages. The texture of the flesh and changes in primary cell wall during ripening is responsible for the firmness of the fruit. Enzymatic activities, pectin solubilization and disassociation of the primary cell wall and middle lamella structures might be involved in it, which results in the decrease of mechanical strength of cell walls and thus the fruit firmness decreases (Kov and Felf, 2003; Kov et al., 2005).
Table 1: Effect of harvesting stages × storage duration and perforated plastics × storage duration on physiological weight loss (%), decay index (%), and fruit firmness (kg.cm-2 of apricot fruit Cv. Trevatt after 25 days of storage (5+2 oC).
|
Harvesting stages |
Physiological weight loss (%) |
Decay index (%) |
Fruit firmness (Kg. cm-2) |
|||
|
0 day |
25th day |
0 day |
25th day |
0 day |
25th day |
|
|
Greenish stage (M1) |
-- |
2.87c |
-- |
4.17b |
3.55a |
0.64d |
|
Light yellow stage (M2) |
-- |
3.11b |
-- |
4.86b |
2.01b |
0.57d |
|
Orange yellow stage (M3) |
-- |
4.18a |
-- |
18.06a |
1.54c |
0.38e |
|
LSD at 0.05 |
-- |
0.21 |
-- |
2.48 |
0.04 |
|
|
Perforated plastics |
||||||
|
F0 |
-- |
4.04a |
-- |
26.39a |
0.42e |
|
|
F5 |
-- |
3.28cd |
-- |
8.33b |
0.54cd |
|
|
F10 |
-- |
2.82e |
-- |
2.78e |
0.65b |
|
|
F15 |
-- |
3.05de |
-- |
4.17cd |
0.61bc |
|
|
F20 |
-- |
3.44bc |
-- |
5.55bcd |
0.50de |
|
|
F25 |
-- |
3.69b |
-- |
6.94bc |
0.47de |
|
|
LSD at 0.5 |
0.30 |
3.52 |
ns |
0.07 |
||
Means followed by similar letter(s) in column do not differ significantly from one nother. Ns: Non significant.
Table 2: Effect of HS × SD and perforated plastics × storage duration on ascorbic acid (mg. 100g-1), total soluble solid (0 Brix), titratable acidity (%) and total soluble solids: Acid of apricot fruit Cv. Trevatt after 25 days of storage (5+2 oC).
|
Harvesting stages |
Ascorbic acid |
Total soluble solid |
Titratable acidity |
TSS:Acid |
||||
|
0 day |
25th day |
0 day |
25th day |
0 day |
25th day |
0 day |
25th day |
|
|
Greenish stage (M1) |
1.00a |
0.33d |
10.31f |
12.92d |
0.94a |
0.41d |
10.95e |
31.71c |
|
Light yellow stage (M2) |
0.75b |
0.24e |
12.33e |
14.09b |
0.84c |
0.40d |
14.73d |
35.90b |
|
Orange yellow stage (M3) |
0.63c |
0.15f |
13.63c |
16.23a |
0.88b |
0.6e |
15.47d |
49.37a |
|
LSD at 0.05 |
0.09 |
0.01 |
1.21 |
0.04 |
||||
|
Perforated plastics |
||||||||
|
F0 |
0.80 |
0.13f |
12.09 |
14.71a |
0.88 |
0.37e |
13.76 |
42.02a |
|
F5 |
0.79 |
0.26cd |
12.12 |
14.33bc |
0.89 |
0.40cd |
13.70 |
37.74c |
|
F10 |
0.81 |
0.36b |
12.07 |
14.17d |
0.89 |
0.42b |
13.64 |
36.22c |
|
F15 |
0.79 |
0.30c |
12.09 |
14.26cd |
0.89 |
0.41bc |
13.64 |
37.26c |
|
F20 |
0.79 |
0.22de |
12.10 |
14.40b |
0.88 |
0.38de |
13.79 |
39.90b |
|
F25 |
0.77 |
0.17ef |
12.07 |
14.60a |
0.88 |
0.38de |
13.75 |
40.80ab |
|
LSD at 0.5 |
ns |
0.13 |
ns |
0.02 |
ns |
1.71 |
ns |
0.06 |
Means followed by similar letter(s) in column do not differ significantly from one another. Ns: non significant.
Ascorbic acid (mg. 100g-1)
Apricot fruits harvested at various stages were observed with different concentrations of ascorbic acid. Similarly, perforated plastic and storage duration also significantly influenced the ascorbic acid of apricot fruits (Table 2). The initial data recorded for different maturity stages was 1.0, 0.75 and 0.63 mg. 100g-1 for M1, M2 and M3 respectively, which declined to 0.33, 0.24 and 0.15 mg. 100g-1, respectively. The study also showed maximum ascorbic acid (0.33 mg. 100g-1) in fruits harvested at greenish stage (M1), while the minimum ascorbic acid (0.15 mg. 100g-1) was seen in fruits harvested at orange-yellow stage (M3). Similarly, he initials ascorbic acid contents of the fruits stored in various perforated plastic bags was 0.80, 0.79, 0.81, 0.79, 0.79 and 0.77 mg. 100g-1 for F0, F5, F10, F15, F20 and F25, respectively which, finally decreased to 0.13, 0.26, 0.36, 0.30, 0.22 and 0.17 mg. 100g-1 respectively after 25 days in cold storage. However, maximum ascorbic acid (0.36 mg. 100g-1) was reported in plastic bags with 10 perforations (F10), while the minimum ascorbic acid (0.13 mg. 100g-1) was recorded in plastic bags having no pores (F0) after 25 days of storage. Our result was confirmed by Chambroy et al. (2013), stated that the level of CO2 and O2 concentration is dependent on film permeability at a defined temperature. Findings are in line with Shafique et al. (2006) and reported similar decease in ascorbic contents. Nasrin et al. (2008) also recorded similar results and reported slight decline in vitamin content in tomato by packing it in perforated polythene bags during refrigeration storage. Lee and Kader (2000) stated that the regulation of oxidative process might be responsible for the reduction of ascorbic acid in fruit. They also concluded that many other factors like temperature, gaseous composition of storage and post-harvest storage might also induce heavy losses to ascorbic acid.
Total soluble solids (oBrix)
Total soluble solids determine the proper maturity time of the fruits and have close relation with harvesting stages, packaging materials and storage duration. Initially, data showed that there were 10.31, 12.33, and 13.63 (oBrix) for greenish stage M1), light yellow stage M2) and orange yellow stage (M3), which were increased to 12.92, 14.09, and 16.23oBrix), respectively. On the other hand, total soluble solids of the fruits, stored in perforated plastic bags for 25 days were12.09, 12.12, 12.07, 12.09, 12.10 and 12.07 oBrix, for F0, F5, F10, F15, F20 and F25, respectively for fresh fruits, which increased to 14.71, 14.33, 14.17, 14.26, 14.40, 14.60 oBrix respectively after 25 days of storage. The conversion of starches and pectins into simple sugar during ripening leads to increase in total soluble solids of the fruits. The results of the current study were in line with (Chambroy et al., 2013; Nasrin et al., 2008; Rajkumar and Mitali, 2009). Juan et al. (1999) who stated that more starch and pectin content is present at early mature stage as compared to mid and late mature stage due to which total soluble solid increases with delaying harvesting.
Titratable acidity (%)
Titratable acidity of apricot fruits is significantly influenced by perforated plastic, harvesting stages and storage duration (Table 2). Freshly harvested fruits at various stages such as M1, M2, and M3 were recorded with 0.94%, 0.84% and 0.88% respectively, which finally decreased to 0.41%, 0.40% and 0.36%, respectively after 25 days. However, fruits stored in plastic bags having 10 holes (F10). retained the titratable acidity (0.42%) after 25 days in cold storage conditions However, fruit in closed plastic bags (F0) were observed with minimum (0.37%) titratable acidity after 25 days of storage. In the current study, acidity was decreased in closed plastic bags that might be because of anaerobic conditions Tariq et al. (2001) also stated that acidity of citrus fruits decreased in both conditions i.e., 0% oxygen:10% CO2 and 21% oxygen: 0% CO2. They also stated that, it is possible that anaerobic condition which is created by lower level of oxygen might be responsible for decreasing fruit acidity, during which the organic acid might be used as a reserve source for energy production. On the other hand, high rate of respiration at higher level of oxygen might decrease acidity.
Further, Beaudry et al. (1992) stated that during respiratory metabolism organic acid are consumed. Package perforations generated modified atmosphere, which helps to retain high level of organic acid and thus delaying ripening process. However, Tariq et al. (2001) reported that loss of organic acid may induce due to high rate of respiration in sealed packages due to low oxygen. It was reported that acidity of the fruit decline during ripening and storage. Findings are also in line with Rab et al. (2016), who recorded highest titratable acidity after 25 days of cold storage in plum fruits harvested at pale green stage and packed in plastic bags having 15 perforations. Khan et al. (2007) and Pretel et al. (2000) also reported less titratable acidity in apricot and persimmon with storage durations.
Total soluble solids: Acid ratio
TSS and Acid dependent on each other and TSS/acid ratio contributes toward giving many fruits their characteristic flavor. At the start of ripening process the TSS/acid ratio remain low, because of low sugar content and high acidity, which makes the fruit taste sour, during ripening process the sugar content increases and fruit acid are degraded, thus TSS/acid ratio achieves a high value. Previously, it has been observed that both TSS and acidity of the fruits were considerably varied. Similarly, the TSS: Acid was also found responsive to harvesting stages, plastic bags perforation and storage duration (Table 2). Initially, TSS: Acid for the fruits harvested stages remained at lower levels such as 10.95, 14.73, and 15.47 for M1, M2, and M3 that increased to 31.71, 35.90, and 49.37, respectively after 25 days of cold storage. Similarly, TSS: Acid was increased from F0 (13.76), F5 (13.70), F10 (13.64), F15 (13.64), F20 (13.79) and F25 (13.75) of freshly harvested fruits to F0 (42.02), F5 (37.74), F10 (36.22), F15 (37.26), F20 (39.90) and F25 (40.80), respectively after 25 days of storage. These results are in agreement with (Tariq et al., 2001). The increasing of total soluble solid and decreasing of acidity of fruits with storage and delaying harvesting is responsible for the increases of total soluble solids: Acid. The results of the present study were also in agreement with the earlier conclusion of (Juan et al., 1999; Dhillon and Cheema, 1991) in apple.
Conclusions and Recommendations
This study concluded that harvesting of apricot fruits at greenish stage (10.31 oBrix) attained minimum physiological weight loss and decay index as compared to other harvesting stages after 25 days of storage. Similarly, fruits packed in plastic bags contained 10 pores retained fruit weight and had maximum titratable acidity, fruit firmness and ascorbic acid with minimum physiological weight loss and low decay index. Similarly, the highest value of titratable acidity, fruit firmness and ascorbic acid and lower content of TSS and TSS: Acid was observed in green stage harvested fruit during 25 days of storage. In case of interaction of packaging materials and storage durations, fruits packed in plastic bags having 10 perforation showed increase in TSS and TSS: Acid value with prolonging storage duration up to 25 days. Therefore, it is concluded that apricot fruits should be harvested at green stage (10.31 oBrix) and should be packed in plastic bags having 10 perforations.
Acknowledgements
The authors are grateful to the Department of Horticulture, University of Agriculture Peshawar, Pakistan for providing laboratory facilities and guidance.
Novelty Statement
To enhance the storage life of apricot, fruits should be harvested at greenish stage (10.31 0 Brix) and should be packed in plastic bag having 10 perforations of 2mm each equally distributed.
Author’s Contribution
Rashid Khan: Conducted the research.
Muhammad Sajid: Major supervisor, provided guidelines and idea of the research.
Saud Khan: Prepared the manuscript.
Jehanzeb: Made the layout of research and determined the factors.
Aslam Noor: Finalized the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Ali, S., T. Masud, K.S. Abbasi, T. Mahmood and I. Hussain. 2013. Influence of CaCl2 on biochemical composition, antioxidant and enzymatic activity of apricot at ambient storage. Pak. J. Nutr., 12(5): 476-483. https://doi.org/10.3923/pjn.2013.476.483
Alique, R., M.A. Martinez and J. Alonso. 2003. Influence of the modified atmosphere packaging on shelf life and quality of Navalinda sweet cherry. Eur. Food. Tech., 217(5): 416-420. https://doi.org/10.1007/s00217-003-0789-x
Amayogi, R.K. and T.B. Alloli 2007. Studies on integrated nutrient and postharvest management of fig (Ficus carica L.). Ph.D. thesis. Dept. Hortic. Coll. Agric. Dharwad Univ. Agric. Sci. Dharwad. 580: 005.143.
AOAC (Association of official Analytical Chemists), 2000. Official methods of analysis, 15th edition, (Ed. Helrich, K.) Arlington, Virginia USA. 513.
Aslam, M. and Hafizullah. 2012. Agricultural statistics of Pakistan. pp. 92.
Beaudry, R.M., A.C. Cameron, A. Shirazi and Lange, D.L.D. 1992. Modified atmosphere packaging of blueberry fruit: Effect of temperature on package O2 and CO2. J. Am. Soc. Hortic. Sci., 117(3): 436- 441. https://doi.org/10.21273/JASHS.117.3.436
Chambroy, Y., M. Souty, G. Jacquemin, R.M. Gomez and J.M. Audergon. 2013. Research on the suitability of modified atmosphere packaging for shelf-life and quality improvement of apricot fruit. Acta Hortic., 384.
Crisosto, C.H., D. Garner, G.M. Crisosto and E. Bowerman. 2004. Increasing ‘Black amber’ Plum (Prunus salicina Lindell.) consumer acceptance. Postharv. Biol. Technol., 34: 237-244. https://doi.org/10.1016/j.postharvbio.2004.06.003
Dhillon, W.S. and S.S. Cheema. 1991. Physico-chemical changes during development of peach cv. Flordasun. Ind. Food. Packer. pp. 56-59.
FAO, 2018. Food and Agriculture Organization. Ranking. Countries by commodity. Apricot. http://www.fao.org/faostat/en/#ranking/countries_by_commodity.
Fatima, T., O. Bashir, G. Hani, T.A. Bhat and N. Jan. 2018. Nutritional and health benefits of apricot. Int. J. Univ. Integr. Med., 2(2): 05-09.
Hardenburg, R.E., A.E. Watada and C.Y. Wang. 1990. The commercial storage of fruits, vegetables and florist and nursery stocks. Agric. Hand book Number 66, USDA, pp. 19.
Hussain, S., 1986. Effect of different packaging material on the overall quality of fruit juices. M.Sc (Hons) thesis, Dept. Food Sci. Tech., Univ. Agric., Faisalabad. pp. 48.
Jan, I., A. Rab and M. Sajid. 2012. Storage performance of apple cultivars harvested at different stages of maturity. J. Anim. Plants Sci., 22(2): 438-447.
Jawandha, S.K., P.S. Tiwana and J.S. Randhawa. 2012. Effect of low density polyethylene packaging and chemicals on ambient storage of kinnow. J. Food Ag-Ind., 5(2): 112-118.
Juan, J.L., J. Frances, E. Montesinos, F. Camps, J. Bonany and L. Michalchuk. 1999. Effect of harvest date on quality and decay losses after cold storage of Golden Delicious apple in Grona (Spain). Acta Hortic., 485: 195-201. https://doi.org/10.17660/ActaHortic.1999.485.26
Kader, A.A., 1980. Prevention of ripening in fruits by use of controlled atmospheres. F Tech., 34(3): 45-49.
Khan, D., A.R. Khan, S. Bibi, S. Ali and I.A. Khalil. 2007. Storage stability of persimmon fruits (Diospyros kaki) stored in different packaging materials. J. Agric. Biol. Sci., 2(2): 20-23.
Khan, M.S., A. Zeb, K. Rahatullah, Ihsanullah, N. Ahmed and S. Ahmed. 2013. Storage life extension of plum fruit with different colored packaging and storage temperatures. J. Environ. Sci. Toxicol. Food Technol., 7(3): 86-93. https://doi.org/10.9790/2402-0738693
Kov E., E. Hertog and E. Vanstreels. 2005. Relationship between physical and biochemical parameters in apple softening. Acta Hortic., 68: 573-578. https://doi.org/10.17660/ActaHortic.2005.682.72
Kov, E. and E. Felf. 2003. Investigating the firmness of stored apples by non-destructive method. Acta Hortic., 59: 257-260. https://doi.org/10.17660/ActaHortic.2003.599.30
Lacey, K., N. Hancock and H. Ramsey. 2009. Measuring internal maturity of citrus. Dept. Agric. and Food Farm Note. ISSN 0726 – 934X: 1_4.
Lee, S.K. and A.A. Kader. 2000. Pre-harvest and postharvest factors influencing vitamin C content of horticultural crops. Post-Harvest Biol. Tech., 20: 207-220. https://doi.org/10.1016/S0925-5214(00)00133-2
Nasrin, T.A.A., M.M. Molla, M.A. Hossaen, M.S. Alam and L. Yasmin. 2008. Effect of postharvest treatments on shelf life and quality of tomato. Bangl. J. Agric. Res., 33(3): 579-585. https://doi.org/10.3329/bjar.v33i4.2291
Navjot, G. and K.J. Sukhjit. 2010. Influence of maturity stage on fruit quality during storage of ‘Earli Grande’ peaches. Not. Sci. Biol., 2(3): 96-99.
Ngcobo, M.E.K., U.L. Opara1 and G.D. Thiart. 2012. Effects of packaging liners on cooling rate and quality attributes of table grape (cv. Regal Seedless). Tech. Sci., 25: 73–84. https://doi.org/10.1002/pts.961
Penn State Extension, 2015. Penn state extension plants and pests. Home lawn and garden. Fruit production for the home gardener stone fruits: Peaches, Nectarines, Plums, Apricots and Cherries. Penn State college of Agriculture Sciences. Pennsylvania. USA. http://www.extension,psu.edu/plants/gardening/fphg/stone
Pocharski, W.J., D. Konopacka and J. Zwierz. 2000. Comparison of magness-taylor pressure test with mechanical, nondestructive methods of apple and pear firmness measurements. Intl. Agrophys., 14: 311-31.
Pretel, M.T., M. Souty and F. Romojaro. 2000. Use of passive and active modified atmosphere packaging to prolong the postharvest life of three varieties of apricot (Prunus armeniaca L.). Eur. Food Res. Tech., 211: 191-198. https://doi.org/10.1007/s002170050022
Rab, A., F. Ahmad, M. Ahmad and S.T. Shah. 2016. Maturity stages and packaging perforations affect the quality of Plum in cold storage. J. Anim. Plant Sci., 26(6): 1750-1757.
Rajkumar, P. and D. Mitali. 2009. Effect of different storage methods on nutritional quality of water apple fruits (Syzygium javanica L.) Bulgarian. J. Agric. Sci., 15(1): 41-46.
Ranganna, S., 1986. Hand book of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Publishing Co. Ltd., New Delhi, India. pp. 1112.
Rashidi, M. and M.H. Bahri. 2014. Effect of wrapping materials and cold storage durations on firmness of plum. Agric. Eng. Res. J., 4(3): 73-75.
Rashidi, M., S. Sayfzadeh, M.H. Bahri, S.T. Namini and S.H. Mirzaki. 2014. Effect of wrapping materials and cold storage durations on water content of nectarine. Agric. Eng. Res. J., 4(3): 51-54.
Sabir A., F.K. Sabir and Z. Kara. 2011. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. Int. J. Food Sci. Technol., 48(3): 312-318. https://doi.org/10.1007/s13197-011-0237-z
Sabir, F.K. and Sabir, A. 2013. Quality response of table grapes (Vitis vinifera L.) during cold storage to postharvest cap stem excision and hot water treatments. Int. J. Food Sci. Technol., 48: 999–1006. https://doi.org/10.1111/ijfs.12052
Scetar, M., M. Kurek and K. Galic. 2010. Trends in fruit and vegetable packaging. Croatian. J. F. Tech, Biotech. Nutr., 5(4): 69-86.
Shafique, M.Z., M. Ibrahim, M.O.H. Helali and S.K. Biswas. 2006. Studies on the physiological and biochemical composition of different mango cultivars at various maturity levels. Bangal. J. Sci. Ind. Res., 41(1-2): 101-108. https://doi.org/10.3329/bjsir.v41i1.279
Steel, R.G.D. and J.H. Torrie. 1997. Principles and procedures of statistics. MC. Graw Hill Pub. Comp. Inc. New York.
Tariq, M.A., F.M. Tahir, A.A. Asif and M.A. Pervez. 2001. Effect of controlled atmosphere storage on damaged citrus fruit quality. Int. J. Agric. Biol., 3(1): 9-12.
Vangdal, E., S. Flatland, I.L. Knutsen and H. Larsen. 2012. Factors affecting storability and shelf life in plums (Prunus domestica L.). II Eufrin plum and prune working group meeting on present constraints of plum growing in Europe. ISHS Acta Hortic. pp. 968. https://doi.org/10.17660/ActaHortic.2012.968.28
To share on other social networks, click on any share button. What are these?





 (
(


