In Vitro Plant Regeneration from Coleoptilar Node of Maize Seedling: a New Tool to Bioengineer the Maize Rapidly
In Vitro Plant Regeneration from Coleoptilar Node of Maize Seedling: a New Tool to Bioengineer the Maize Rapidly
Zaheer Abbas*, Shaukat Ali, Jalal-Ud-Din and Ghulam M. Ali*
National Institute for Genomics and Advanced Biotechnology (NIGAB), Islamabad, Pakistan.
Abstract | Efficient and reproducible regeneration system from coleoptilar node of elite maize inbred line was developed. Regeneration from coleoptilar node circumvented tedious procedures of raising the immature embryos as a starting material for genetic transformation. Unlike immature embryos, the development of coleoptilar node is seasonal free and green house independent which would accelerate the work relevant to genetic transformation of maize. MS medium fortified with 3 mgL-1 BAP and 5 mgL-1 picloram produced maize seedlings with expanded coleoptilar nodes. Longitudinal cut sections of expanded coleoptilar nodes produced maximum primary calli on callus induction medium containing both 0.5mgL-1 2, 4-D and 1 mgL-1 picloram. Eighty percent of embryogenic calli were obtained on MS medium containing only single auxin while combination of auxin and cytokine in MS and Chu’s N6 media produced 53.33 and 35.56% embryogenic calli, respectively. Inclusion of 5 mgL-1 silver nitrate in embryogenic medium enhanced the production of type II embryogenic calli by 54.90% compared to control treatment. Maximum regeneration (58.33%) was observed when embryogenic calli were placed on N6 medium supplemented with 60 gL-1 sucrose and 1 mgL-1 NAA for one week initially in dark and then three weeks on MS medium augmented with 2 gL-1 myo-inositol and 20 gL-1 sucrose under 16/8 hours light/dark photoperiod. The study confirmed that embryogenic type II calli obtained from half of coleoptilar node of maize seedling is fully capable to regenerate into whole plant under specific chemical and environmental conditions.
Received | April 27, 2017; Accepted | August 10, 2017; Published | October 29, 2017
*Correspondence | Zaheer Abbas, Ghulam M. Ali National Institute for Genomics and Advanced Biotechnology (NIGAB), Islamabad, Pakistan; Email: [email protected]; [email protected]
Citation | Z. Abbas., S. Ali., J.U. Din., G.M. Ali. 2017. In Vitro Plant Regeneration from Coleoptilar Node of Maize Seedling: a New Tool to Bioengineer the Maize Rapidly. Sarhad Journal of Agriculture, 33(4): 606-614.
DOI | http://dx.doi.org/10.17582/journal.sja/2017/33.4.606.614
Keywords | Coleoptilar node, Embryogenic calli, Maize regeneration, Maize tissue culture, Seedling derived callus.
Introduction
The efficiency of regeneration protocol is one of the most important components for the successful development of transgenic crops (Sahoo et al., 2011). However, regeneration capacity varies with the genotype and even among cells within the same plant (Wang et al., 2009). When we compare maize with other cereals, it was remarkably noticed that for callus induction and plant regeneration, maize is particularly a challenging crop (Vega et al., 2008; Wang et al., 2009).
Green and Phillips (1975) were the first to explore regeneration in maize using immature embryos as an explant. Since then, regeneration from immature embryos of different maize genotypes has been reported by many authors around the globe (Ishida et al., 1996; Frame et al., 2002; Guruprasad et al., 2016).Wan et al. (1995) utilized type I calli derived from immature embryos to regenerate transformed lines while Walters et al. (1992) used type II calli. Armstrong and Green (1985) established and maintained friable embryogenic calli from immature embryos of A188 inbred line for more than one year and obtained regeneration from it. However availability of immature embryos or explants derived from immature embryos is seasonal limited or requires enough green house facility and strictly limited to specific days after pollination (Odour et al., 2006; Pathi et al., 2013). This imposes tedious tissue culture procedures within specified time period (Abebe et al., 2008) and continuous planting is required in same season after regular interval to ensure availability of immature embryos for extended time period. Sidorov et al. (2006) extended the regeneration in maize to auxiliary bud in coleoptilar node obtained from germinating maize seeds in vitro. Since then, maize regeneration was achieved by embryogenic and organogenic calli obtained from coleoptilar node (Pathi et al., 2013).
Presently very limited numbers of regeneration studies have been conducted globally on elite maize inbred lines. All the published protocols relevant to regeneration of maize are based on model inbred lines or model hybrids that are still required to be improved as the elite inbred lines are difficult to regenerate with these protocols. The present project was designed to extend regeneration system to our indigenously developed maize inbred line using longitudinal cut sections of coleoptilar node.
Material and methods
Effect of germination media on morphological parameters of maize inbred line
Maize Inbred line-5 (MIBL-5) was obtained from the maize hybrid program of Crop Science Institute, National Agricultural Research Centre, Islamabad. The sterilized seeds were germinated on two different media to identify better media for optimal coleoptilar node development and to observe the variations in different morphological parameters in response to different germination media. Germination Medium-I (GM-I) was consisted of MS salts (Phytotechnology Laboratories, USA), 10 ml MS vitamins (100X), 30 gL-1 sucrose and 2.8 gL-1 gellan gum agar. The Germination Medium-II (GM-II) was the same as GM-I except the additions of 3 mgL-1 BAP and 5 mgL-1 picloram. The data was recorded after eight days on various parameters such as shoot length, number of roots, root length, number of adventitious roots, biomass and analyzed statistically using T-Statistic. The physical appearance of coleoptilar node was also observed.
Callus induction response from coleoptilar node
Fifty explants (half coleoptilar node) raised on Germination Medium-I (GM-I) and fifty explants raised on Germination Medium-II (GM-II) were subjected to callus induction medium. The callus induction medium was composed of 4.33gL-1 MS basal medium with vitamins (Phytotechnology Laboratories, USA), 3% sucrose, 1.38 gL-1 proline, 0.5 gL-1 MES, 0.2 gL-1 casin hydrolysate, 0.1 gL-1 myoinositol, 0.5 mgL-1 2, 4-D, 1 mgL-1 picloram and 2.8 gL-1 gellan gum agar. The callus induction frequency was measured after 14 days using the following equation:
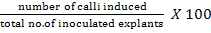
Evaluation of different media for embryogenic callusi formation
Three different media compositions were evaluated for maximum embryogenic callusi formation from primary calli. One media was based on N6 basal salts and other two media were based on MS basal salts with different combinations of growth hormones and additives. The compositions of different media are given in Table 1. The data on embryogenic callusi formation was recorded after 28 days and analyzed using Completely Randomized Design of Statistix 8.1 Software.
Table 1: Composition of different embryogenic media
| Embryogenic Media | Composition |
|
Embryogenic Medium I (EM-I) |
3.99 gL-1 Chu's N6 basal medium w/ vitamins (Phytotechnology laboratories, USA), sucrose 3%, proline 0.69 gL-1, casein hydrolysate 1 gL-1, glycine 2 mgL-1, 2, 4-D 2 mgL-1, BAP 0.2 mgL-1, gellan gum agar 2.8 gL-1. |
|
Embryogenic Medium II (EM-II) |
4.33 gL-1 MS basal medium w/ vitamins (Phytotechnology laboratories, USA), sucrose 3%, proline 0.69 gL-1, casein hydrolysate 1 gL-1, glycine 2 mgL-1, 2, 4-D 2 mgL-1, BAP 0.2 mgL-1, gellan gum agar 2.8 gL-1. |
| Embryogenic Medium III (EM-III) |
4.33 gL-1 MS basal medium w/ vitamins (Phytotechnology laboratories, USA), sucrose 3%, proline 1.38gL-1, casein hydrolysate 0.2 gL-1, myoinositol 0.1 gL-1, MES 0.5 gL-1, 2, 4-D 2 mgL-1,gellan gum agar 2.8 gL-1. |
Effect of silver nitrate on production of Type II embryogenic calli
In order to determine the effect of silver nitrate on production of type II embryogenic calli, the Embryogenic Medium-III (EM-III) was enriched with different concentrations of silver nitrate. The different concentrations of silver nitrate were selected based on previous literatures. Silver nitrate at 3.4 mgL-1 (Sidorov and Duncan, 2009), 5 mgL-1 (Zhong et al., 2011), 10 mgL-1 (Huang and Wei, 2004) and 15 mgL-1 (Carvalho et al., 1997) were evaluated for any enhancement in type II callusi production. Control treatment without silver nitrate was also included. Data was recorded after four weeks and analyzed statistically by Statistix 8.1 Software.
Effect of different methods of regeneration
Effect of different regeneration methods (REM) has been investigated. In one method (REM-I), the regeneration procedure of the Sidorov and Duncan (2009) was followed. In REM-1, embryogenic calli were cultured initially on MS medium fortified with 3.5 mgL-1 BAP, 1.36 gL-1 L-proline, 30 gL-1 sucrose and 0.05 gL-1 casamino acids for one week and then embryogenic calli were recultured on MS medium augmented with 10 gL-1 glucose, 20 gL-1 maltose, 0.15 gL-1 asparagine monohydrate and 100 mgL-1 myoinositol. In the second method (REM-II), the regeneration medium of Pathi et al. (2011) was used which was consisted of MS medium supplemented with 30 gL-1 sucrose, 100 mgL-1 myo-inositol, 0.5 mgL-1 NAA, 2 mgL-1 BAP and1 mgL-1 kinetin. In the third method (REM-III) the regeneration procedure of Rafiq et al. (2006) was followed with a little modification. In this method embryogenic calli were plated on N6 medium fortified with 60 gL-1 sucrose, 1 mgL-1 NAA for one week initially in dark and then embryogenic calli were cultured on MS medium augmented with 2 gL-1 myo-inositol and 20 gL-1 sucrose.
Results
Variations in morphological parameters in response to different germination media
Shoot length of germinated maize was not significantly affected by two different germination media while numbers of roots were severely affected by different germination media. GM-II decreased the average number of roots/plantlets by 49.3% compared to GM-I medium (Table 2 and Figure 1a, b). The plantlets having 3.19 cm average root length were produced on GM-I. Average root length produced on GM-I was 2.75 times of average root length developed on GM-II (Table 2 and Figure 1a, b). GM-II produced 0.33 average numbers of adventitious roots while GM-I supported 1.33 average numbers of adventitious roots. Increased biomass was observed on GM-I compared to GM-II. The coleoptilar node developed on GM-II was noticed as swollen, expanded and of more diameters compared to coleoptilar node produced on GM-I. (Figure 1c, d). From this experiment it was concluded that shoot length, number of roots, root length, number of adventitious roots and biomass were decreased on GM-II while the coleoptilar node was observed expanded and swollen. The length of mesocotyle was found more when the seeds were germinated on the GM-II compared to GM-I (Figure 2a, b). Moreover some portion of mesocotyle shrunk down along diameter with time in some plants when seeds were germinated on GM-II and shrinkage initiation was always observed from coleoptilar nodal side and not from scutellum nodal side (Figure 2c, d).
Table 2: Variations in morphological parameters in response to different germination media.
| Different germination media | |||||
| S. No | Parameters | GM-I ± S.E | GM-II ± S.E | T- Statistic | P- Value |
| 1 |
Shoot Length (cm) |
4.71 ± 0.31 |
3.95 ± 0.27 |
1.82ns |
0.089 |
| 2 | No. of Roots |
7.89 ± 0.48 |
4.00 ± 0.62 |
4.93** | 0.000 |
| 3 | Root length (cm) |
3.19 ± 0.13 |
1.16 ± 0.14 |
10.42** | 0.000 |
| 4 | No. of Adv.Roots | 1.33 ± 0.333 |
0.33 ± 0.17 |
2.68* | 0.021 |
| 5 | Biomass (g) | 1.10 ± 0.055 |
0.92 ± 0.06 |
2.19* | 0.045 |
| 6 | Coleoptilar node |
Less diameter |
More diameter |
||
Each value followed by standard error of mean is a mean of data taken on 9 plantlets.
*significant at 5%, ** significant at 1%, ns = non-significant
Callus induction response from coleoptilar node induced on GM-I and GM-II
Coleoptilar nodes raised on GM-II produced 90% calli while coleoptilar nodes raised on GM-I produced 44% calli on callus induction medium (Table 3). The present study shows that GM-II is better for germination of maize seeds to obtain high frequency of callus from coleoptilar node in subsequent steps of tissue culture. Callus induction response from coleoptilar nodes developed on different germination media is shown in Figure 3. The death of coleoptilar nodes without formation of callus is also shown in Figure 3a.
Table 3: Callus induction from coleoptilar node induced on GM-I and GM-II.
| Different germination media | ||
| GM-I | GM-II | |
| Explant (half coleoptilar node) | 50 | 50 |
| Calli produced | 22 | 45 |
| Percentage | 44 | 90 |
Embryogenic callus formation frequency on different media
Embryogenic Medium-III (EM-III) produced maximum number of embryogenic calli followed by EM-II and EM-I respectively. EM-II and EM-III which were MS based media performed better than N6 based EM-I medium for embryogenic calli development (Table 4). The representatives of embryogenic and non-embryogenic calli developed on EM-III are shown in Figure 4a. The study also coined that each and every primary callus is not necessary to produce somatic embryos on its surface (Figure 4a). The embryogenic calli also contained sufficient non-embryogenic portion and is depicted in Figure 4b, c.
Effect of silver nitrate on production of Type II embryogenic calli
Different concentrations of silver nitrate enhanced the production of type II embryogenic calli. 5 mgL-1 silver nitrate increased the type II embryogenic calli production by 54.9% compared to control treatment (Table 5). The results of present experiment indicate that silver nitrate has major role in enhancement of type II embryogenic calli production. Both type I and type II embryogenic calli were also produced on control treatment (0 mgL-1) of silver nitrate but the medium which contained silver nitrate significantly enhanced the production of type II embryogenic calli compared to type I. Representative of type I and type II calli are shown in Figure 5. Type I calli were found hard, slow growing and yellowish in appearance while type II calli were loose, friable, fast growing and whitish in appearance (Figure 5).
Table 4: Embryogenic callus formation frequency on different media.
| Different embryogenic media | |||
| EM-I | EM-II | EM-III | |
| Primary Calli | 45 | 45 | 45 |
| Embryogenic Calli | 16 | 24 | 36 |
| Percentage | 35.56 B | 53.33 B | 80.00 A |
|
LSD0.01 |
17.79 | ||
Effect of different methods of regeneration
REM-III supported maximum average regeneration of 58.33% followed by REM-II (43.33%) and REM-I (35%) (Table 6). Advanced stages of embryogenic calli achieved with different regeneration methods after two weeks of culturing are shown in Figure 6. Globular somatic embryos of variable sizes developed on the surface of embryogenic calli at the advanced stage prior to green spot induction are also shown in Figure 6.
Table 5: Effect of silver nitrate on production of Type II embryogenic calli.
| Different concentrations of silver nitrate | |||||
| 0 mgL-1 | 3.4 mgL-1 | 5 gL-1 | 10 mgL-1 | 15 mgL-1 | |
|
Embryogenic Calli |
28 | 46 | 49 | 45 | 44 |
| Type-II | 14 | 34 | 38 | 34 | 34 |
| Percentage | 50.00 B | 73.69 A | 77.45 A | 75.55 A | 77.27 A |
|
LSD 0.01 |
19.86 | ||||
Table 6: Effect of different methods on regeneration of maize inbred line.
| Different regeneration methods | |||
| REM-I | REM-II | REM-III | |
| Embryogenic calli | 60 | 60 | 60 |
| Regenerated Calli | 21 | 26 | 35 |
| Percentage | 35.00 B | 43.33 AB | 58.33 A |
|
LSD 0.01 |
16.73 | ||
The embryogenic calli were further allowed to grow on same regeneration media for another two weeks and data was recorded on regeneration. The calli with at least one green spot was designated as regenerated. The regenerated embryogenic calli after 4 weeks on different regeneration media are shown in Figure 7.
Various steps of regeneration of callus derived from coleoptilar node
The sterilized seeds were cultured on Germination medium (GM-II) and allowed to germinate and grow for 8 days. The 8 days old seedling is shown in Figure 8a. The coleoptilar node was cut longitudinally into two pieces (Figure 8b). The primary calli were induced from
longitudinal cut sections of coleoptilar node on callus induction medium at 28 oC under16/8 light/dark photoperiod after 14 days of culturing (Figure 8c). Embryogenic calli developed on Embryogenic Medium-III (EM-III) in dark, were shifted to Regeneration Method-III (REM-III) and green spots were induced after 4 weeks (Figure 8d). The green spots were allowed to proliferate further on REM-III and shoots were induced after another two weeks (Figure 8e, f).
Discussion
Sidorov et al. (2006) has established that embryogenic calli obtained from longitudinal cut sections of coleoptilar nodes are fully capable to regenerate whole plants. The present study extended the same regeneration system to our indigenously developed elite maize inbred line. Addition of 3 mgL-1 BAP and 5 mgL-1 picloram in MS media resulted, expanded coleoptilar node which was found suitable for further manipulation during tissue culture. The expansion in coleoptilar node may be due to induction of auxiliary bud in nodal area. Sidorov et al. (2006) also obtained expanded coleoptilar node on 10 mgL-1 picloram and 3 mgL-1 BAP. The length of mesocotyle was found more when the seeds were germinated on GM-II compared to GM-I. This enhanced length of mesocotyle may be due to increased cell division and cell elongation in response to BAP and picloram contained in GM-II. Moreover some portion of mesocotyle shrunk down along diameter with time in some plants when seeds were germinated on GM-II. The reason of shrinkage of some portion of mesocotyle is unknown.
The present study suggested that coleoptilar nodes raised on GM-II are more efficient in production of primary calli than the coleoptilar nodes raised on GM-I. Sidorov et al. (2006) optimized maximum callus induction frequency of 42.1% from L 9 genotype of maize using 2.2 mgL-1 picloram and 0.5 mgL-1 2,4-D in MS media while we obtained 90% calli from maize inbred line-5 using 1 mgL-1 picloram and 0.5 mgL-1 2, 4-D in MS media. The difference in callus induction efficiency of two maize lines seems due to difference in composition of media and different genetic makeup of these two lines.
The In vitro capacity of cells to induce embryogenic calli from primary calli may be the result of sensitivity to varying concentration of auxins (Von Arnold et al., 2002). Higher level of endogenous indole acetic acid (IAA) concentrations in maize explants is correlated with embryogenic callus development (Jimenez and Bangerth, 2001). The proper interaction of endogenous and synthetic auxin in EM-III in the present study may be the reason of development of more embryogenic calli compared to other two media. The study also coined that each and every primary callus is not necessary to produce somatic embryos on its surface. The reason of this may be due to difference in endigenous hormonal differences among explants when it was first cultured in vitro. The study also showed that embryogenic calli contained sufficient amount of non-embryogenic portion. The reason of embryogenic and non-embryogenic portion in embryogenic calli may be due to the presence of heterogeneous nature of cells in initial explant. We used both MS and N6 media to obtain the embryogenic calli and concluded that MS supported more embryogenic calli than N6 medium. Similar observation was also noticed by Malini et al. (2015) while studying different Indian maize genotypes. Azad et al. (2015) also concluded similar result that MS is better medium compared to N6 for production of somatic embryos.
Type II calli are more desirable as it maintain the capacity of regeneration for longer period of time upto three months in maize. Type II callus is preferred for in vitro manipulation and is used to establish protoplast and cell suspension cultures but type II callus usually initiates at lower frequency than type I callus (Wang et al., 2009). Songstad et al. (1991) explored the role of AgNo3 in the production of type II callus and demonstrated that addition of 10-100 µM of AgNo3 to N6 medium promoted the type II callus production from different maize genotypes. Inclusion of silver nitrate in present study also enhanced the production of type II embryogenic calli compared to control treatment.
Some embryogenic calli in the present study did not produce any green spots on its surfaces probably due to failure of expression of genes related to photosynthesis in these embryogenic calli as previously reported by (Che et al., 2006). ElItriby et al. (2003), also reported same observation that embryogenic calli not always necessarily possess regenerability. In the present study the regeneration method of Rafiq et al. (2006) with little modification was found best and 58.33% embryogenic calli were regenerated while Rafiq et al. (2006) obtained higher regeneration response of 86.4% in BR-6, 77% in EX-285 and 63% in EX-295 using nearly same regeneration method, suggesting that different genotypes respond differently on same regeneration media. Similar to present study, Gonzalez et al. (2012) also obtained regeneration in 14 maize lines using MS media without any growth hormone.
Conclusion
In Summary, amendments in different available cultural media were successful in extending the regeneration system to the coleoptilar nodes of elite maize inbred line. In the present investigation, simple and reproducible regeneration system based on initiation of type II embryogenic calli obtained from longitudinal cut sections of coleoptilar node was developed to circumvent the tedious, time consuming, seasonal restricted procedures of raising the immature embryos as a starting material for genetic transformation of maize. The optimized protocol would extend the regeneration system to economically important recalcitrant maize genotypes and would accelerate the work relevant to genetic transformation of maize throughout the year.
Authors Contribution
Ghulam Muhammad Ali: Conceived the idea and technical input at every step. Zaheer Abbas: Conducted Wet Lab experiments and wrote manuscript. Shaukat Ali: Experiments designing and helped in data collection. Jalal-Ud-Din: Helped in data analysis.
References
Abebe, D.Z., W. Teffera and J.S. Machuka. 2008. Regeneration of tropical maize lines (Zea mays L.) from mature zygotic embryo through callus initiation. Afr. J. Biotechnol. 7(13): 2181-2186.
Armstrong, C.L and C.E. Green. 1985. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta.164:207-214. https://doi.org/10.1007/BF00396083
Azad, M.A.K., M.W. Rahman, M. Arifuzzaman and M. Hasanuzzaman, M.A.I. Talukder, R.K. Saha. 2015. Callus Induction and Plant Regeneration from Immature Kernel of Maize Inbred Lines. J. Chem. Biol. Phys. Sci. Sec. B. 5(4):4149-4161.
Carvalho, C.H.S., N. Bohorova, P.N. Bordallo, L.L. Abreu, F.H. Valicente, W. Bressan and E. Paiva. 1997. Type II callus production and plant regeneration in tropical maize genotypes. Plant cell Rep.17:73-76. https://doi.org/10.1007/s002990050355
Che, P., T.M. Love, B.R. Frame, K. Wang, A.L. Carriquiry and S.H. Howell. 2006. Gene expression patterns during somatic embryo development and germination in maize Hi II callus cultures. Plant Mol. Biol. 62(12):1-14. https://doi.org/10.1007/s11103-006-9013-2
Elitriby, H.A., S.K. Assem, E.H.A. Hussein, F.M. Abdelcalil and M.A. Madkour. 2003. Regeneration and transformation of Egyptian maize inbred lines via immature embryo culture and a biolistic particle delivery system. In Vitro Cell Dev. Biol. Plant. 39(5):524-531. https://doi.org/10.1079/IVP2003439
Frame, B.R., H. Shou, K. Rachel, Chikwamba, Z. Zhang and C. Xiang, T.M. Fonger, S.E.K. Pegg, B. Li, D.S. Nettleton, D. Pei, and K. Wang. 2002. Agrobacterium tumefaciens-Mediated Transformation of maize embryos using a standard binary vector system. Plant physiol.129:13-22. https://doi.org/10.1104/pp.000653
Gonzalez, G.A., M.G. Pacheco, C.D. Oneto, V.J. Etchart, M.V. Kandus, J.C. Salerno. G. Eyherabide, D. Presello, D.M. Lewi. 2012. Somatic embryogenesis and plant regeneration capacity in Argentinean maize (Zea mays L.) inbred lines. Electron. J. Biotechnol. 15(1):1-15.
Green, C.E and R.L. Phillips. 1975. Plant regeneration from tissue cultures of maize. Crop Sci. 15 (3):417-421. https://doi.org/10.2135/cropsci1975.0011183X001500030040x
Guruprasad, M., V. Sridevi, V.G. kumar and M.S. Kumar. 2016. Plant regeneration through callus initiation from mature and immature embryos of maize (Zea mays L.) Indian J. Agri. Res. 50(2):135-138. https://doi.org/10.18805/ijare.v0iOF.8435
Huang, X.Q and Z.M. Wei. 2004. High-frequency plant regeneration through callus initiation from mature embryos of maize (Zea Mays L.). Plant Cell Rep. 22:793-800. https://doi.org/10.1007/s00299-003-0748-9
Ishida, Y., H. Saito, S. Ohta, Y. Hiei, T. Komari and T. Kumashiro. 1996. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol. 14(6):745-50. https://doi.org/10.1038/nbt0696-745
Jimenez, V.M and F. Bangerth. 2001. Hormonal status of maize initial explants and of the embryogenic and non-embryogenic callus cultures derived from them as related to morphogenesis in vitro. Plant Sci. 160:247-257. https://doi.org/10.1016/S0168-9452(00)00382-4
Malini, N., C.R. Ananadakumar and S. Hari ramakrishnan. 2015. Regeneration of Indian maize genotypes (Zea mays L.) from immature embryo culture through callus induction. J. Appl. Nat. Sci. 7(1):131–137.
Odour, R.O., E.N.M. Njagi, S. Ndungs, J.S. Machuka. 2006. In vitro regeneration of dryland Kenyan maize genotypes through somatic embryogenesis. Int. J. Bot. 2(2):146-151. https://doi.org/10.3923/ijb.2006.146.151
Pathi, K.M., S. Tula, K.Md.K. Huda, V.K. Srivastava and N. Tuteja. 2013. An efficient and rapid regeneration via multiple shoot induction from mature seed derived embryogenic and organogenic callus of Indian maize (Zea mays L.). Plant Signal Behav. 8(10): 25891-25896. https://doi.org/10.4161/psb.25891
Rafiq, M., T. Fatima, T. Husnain, K. Bashir, M.A. Khan and S. Riazuddin. 2006. Regeneration and transformation of an elite inbred line of maize (Zea mays L.), with a gene from Bacillus thuringiensis. S. Afr. J. Bot. 72:460-466. https://doi.org/10.1016/j.sajb.2005.12.010
Sahoo, K.K., A.K. Tripathi, A. Pareek, S.K. Sopory and S.L. Singla-Pareek. 2011. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods. 7(1):49. https://doi.org/10.1186/1746-4811-7-49
Sidorov, V and D. Duncan. 2009. Agrobacterium mediated maize transformation: immature embryos versus callus. In: Paul Scott M (eds) Methods in Molecular Biology: Transgenic Maize. Humana Press, Totowa, New Jersey. 47-58. https://doi.org/10.1007/978-1-59745-494-0_4
Sidorov, V., L. Gilbertson, P. Addae and D. Duncan. 2006. Agrobacterium mediated transformation of seedling-derivedmaize callus. Plant Cell Rep.25:320–328. https://doi.org/10.1007/s00299-005-0058-5
Songstad, D.D., C.L. Armstrong and W.L. Petersen. 1991. AgNO3 increases type II callus production from immature embryos of maize inbred B73 and its derivatives. Plant Cell Rep. 9: 699-702. https://doi.org/10.1007/BF00235361
Vega, J.M., W. Yu, A.R. Kennon, X. Chen and Z. Zhang. 2008. Improvement of Agrobacterium-mediated transformation in Hi-II maize (Zea mays) using standard binary vectors. Plant Cell Rep. 27(2): 297–305. https://doi.org/10.1007/s00299-007-0463-z
Von Arnold, S., I. Sabala, P. Bozhkov, J. Dyachok and L. Filonova. 2002. Developmental pathways of somatic embryogenesis. Plant Cell Tiss. Org. Cult. 69 (3):233-249. https://doi.org/10.1023/A:1015673200621
Walters, D.A., C.S Vetsch, D.E Potts and C. Lundquist. 1992. Transformation and inheritance of a hygromycin phosphotransferase gene in maize plants. Plant Mol. Biol.18:189–200. https://doi.org/10.1007/BF00034948
Wan, Y., J.M. Widholm and P.G. Lemaux. 1995. Type I callus as a bombardment target for generating fertile transgenic maize (Zea mays L.). Planta.196:7–14. https://doi.org/10.1007/BF00193211
Wang, K., B. Frame, Y. Ishida and T. Komari. Maize transformation. 2009. In: BENNETZEN, J. and HAKE, S. eds. Handbook of maize: genetics and genomics. Springer, New York, USA. 609-640. https://doi.org/10.1007/978-0-387-77863-1_31
Zhong, D.Y., Y.Y. Zhu, Q. Liu, T. Zhou and D.G. Zhao. 2011. Production of embryogenic callus and plant regeneration from elite Guizhou waxy maize inbred lines. Agr. Sci. China. 10(4):490-498. https://doi.org/10.1016/S1671-2927(11)60029-1
To share on other social networks, click on any share button. What are these?








