Immune and Growth Response of Indigenous Pedi Goats Vaccinated with Blanthrax to an Inclusion of Moringa oleifera (Drumstick Tree) in Cenchrus ciliaris (Buffel Grass) Hay-Based Diet
Research Article
Immune and Growth Response of Indigenous Pedi Goats Vaccinated with Blanthrax to an Inclusion of Moringa oleifera (Drumstick Tree) in Cenchrus ciliaris (Buffel Grass) Hay-Based Diet
Lungile Gumede1,2, Thobela L. Tyasi1, Teedzai Chitura1*, Khanyisile R. Mbatha3
1School of Agricultural and Environmental Sciences, Department of Agricultural Economics and Animal Production, University of Limpopo, Private Bag X1106, Sovenga,0727, Limpopo, South Africa; 2Department of Agriculture and Animal Health, University of South Africa, Private Bag X6, Florida, 1709, South Africa; 3UNISA, College of Graduate Studies, School of Interdisciplinary Research and Graduate Students, P.O. Box 392, 0003, South Africa.
Abstract | The study aimed to ascertain the immunomodulatory effects of Moringa oleifera (M. oleifera) leaves supplemented to the diets of wether BaPedi goats following vaccination with blanthrax vaccine. Twelve clinically healthy BaPedi goats with an average body weight of 19 ± 1.47 kg and an average age of 11±0.26 months were randomly selected from the flock at the University of Limpopo experimental farm. The experiment was conducted in three phases which are adaption, vaccination and moringa inclusion over 42 days. At the end of the first week of the trial, all the experimental goats were vaccinated with 2 millilitres of blanthrax vaccine per goat via the subcutaneous route. Three experimental diets were formulated by replacing a conventional supplement of Lucerne with M. oleifera. The inclusion levels varied from 0% to 50%. Data on the growth and haematological parameters of the animals used in the study were analysed using a general linear model (GLM) procedure in a completely randomized design. The results indicated that only platelet counts, monocyte counts and mean corpuscular volume (MCV) showed significant differences (p<0.05) amongst the 11 blood profiles observed in this study. There were significant differences (p<0.05) in body weight gain (BWG), growth rate (GR) and metabolic weight gain (MWG). No significant differences (p>0.05) were observed for feed intake (FI) and feed conversion ratio (FCR). Results of the present study suggest that M. oleifera leaves can be used as a feed supplement at 20% and 50% inclusion levels without having any adverse effects on blood parameters and growth performance.
Keywords | Dietary supplementation, Growth performance, Haematology, Natural forages, Vaccination
Received | November 01, 2021; Accepted | December 31, 2021; Published | January 15, 2022
*Correspondence | Teedzai Chitura, School of Agricultural and Environmental Sciences, Department of Agricultural Economics and Animal Production, University of Limpopo, Private Bag X1106, Sovenga,0727, Limpopo, South Africa; Email: teedzai.chitura@ul.ac.za
Citation | Gumede L, Tyasi TL, Chitura T, Mbatha KR (2022). Immune and growth response of indigenous pedi goats vaccinated with blanthrax to an inclusion of Moringa oleifera (Drumstick tree) in Cenchrus ciliaris (Buffel grass) Hay-based diet. Adv. Anim. Vet. Sci. 10(3): 573-.581.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.3.573.581
ISSN (Online) | 2307-8316
Introduction
Goat production plays an integral role in the world especially in communal areas because they can survive under tough climatic conditions (Fikru and Omer, 2015). The indigenous goats reared in communal areas are commonly perceived as less productive than exotic breeds because successful production in communal areas is often affected by various challenges such as malnutrition, poor health, and welfare-related issues caused by the unavailability of resources (Gwanzura et al., 2011; Abreu et al., 2017). The farmers in communal areas rarely conduct livestock management practices such as vaccinations, moreover, disease cases in animals following vaccination have been reported (Monau et al., 2020). Vaccine negative effects in some cases could be due to underlining conditions such as malnutrition and inappropriate management practices, especially in drought-prone areas (Ayele et al., 2016; Kniffen and Comerford, 2021). An example of a vaccine that is commonly used by farmers in the Limpopo province is blanthrax. It is a combined vaccine that is commonly used worldwide because it is perceived to provide good protection against anthrax and blackleg (Wilson, 2003; Ndeereh et al., 2012).
Although trees and shrubs have been used as a source of nutrients and for their medicinal benefits for livestock, most of these plants do not contain optimal nutrients and components required for ideal performance (Ogbe and Affiku, 2011; Nouman et al., 2014). This often leads to poor immune responses following vaccination. Documentation of the use of plants with herbal and nutritional properties is essential, particularly given the escalating costs of drugs and feed as well as the escalating resistance of pathogens to drugs (Zhang and Ma, 2018).
Interest has developed in the Moringa oleifera (M. oleifera) tree because of its fast growth, higher nutritional traits, medicinal properties, and utilization as a livestock fodder crop (Verma et al., 2009; Nouman et al., 2014). Moringa oleifera thrives best in the tropics and subtropical areas such as eastern and southern Africa (Moyo et al., 2012; Nouman et al., 2014). The levels of harmful substances such as tannins and phytates are very low. Therefore, it is recommended that M. oleifera can be used for its health benefits in livestock (Udom and Idiong, 2011; Nouman et al., 2014). Several studies explored the nutritional and medicinal properties of M. oleifera and found it to be beneficial as a supplement in livestock feeds (Ogbe and Affiku, 2011; Moyo et al., 2012; Nouman et al., 2014). The dried leaves of M. oleifera have average crude protein (CP) levels of up to 30.3%, it is rich in vitamins and minerals needed by the animal’s body to create immune cells for a strong defence system (Moyo et al., 2011; Adouko et al., 2020). Some studies reported trace levels of non-nutritive elements found in M. oleifera leaves, such as Barium (Ba), Cadmium (Cd) and Argentum (Ag) which could have arisen from environmental contaminations (Pakade et al., 2013; Biel et al., 2016). These variations may be due to factors such as the different geographic locations, soil type, stage of growth of the tree, the season of harvesting, and storage conditions before analyses (Mulyaningsih and Yusuf, 2018).
Moringa contains antioxidants such as polyphenols in acceptable ranges that may aid in boosting the immune profile of the animal (Ma et al., 2018). Therefore, this study aimed to ascertain the immunomodulatory and growth effects of M. oleifera supplementation to the diets of castrated indigenous BaPedi goats following vaccination with the blanthrax vaccine. The findings of the present study will aid in decision making on the utilization of M. oleifera as a feed supplement in communal goat production.
MATERIALS AND METHODS
All procedures used in this experiment followed the ethical standards of the University of South Africa (2019_CAES_AREC_131) and the University of Limpopo (AREC/10/2019:1R) Animal Ethics Committees. The experiment proceeded after both ethical clearances were obtained.
The study was conducted at the University of Limpopo experimental farm in the summer season of 2020 (October- December). The farm is situated in the tropic of Capricorn zone, in the southern region of the Limpopo Province. The average temperatures range from 20 to 36 °C during the wet season (November to January) and between 5 and 25 °C during the dry season (May to July) (Brown et al., 2016). Steel, individual well-ventilated pens, with an area of 3.75 m2 were installed at the site for the experiment. The pens were equipped with a feeder and a drinker. The experimental site was thoroughly cleaned and disinfected daily to avoid a build-up of infections such as pneumonia.
Twelve clinically healthy BaPedi goats with an average of 19 ± 1.47 kg body weight and 11±0.26 months of age were randomly selected from the flock at the University of Limpopo experimental farm (Lee et al., 2014; Brown et al., 2016b). Only male goats were used in this study because the sex of the animal can affect haematological values in farm animals (Binta et al., 1996; Tibbo et al., 2004). Health assessments and clinical observations (body temperature, respiratory rate, pulse rate, heart rate, signs of gastrointestinal malfunction such as diarrhoea/constipation, examination of mucous membranes of the conjunctiva to evaluate the circulatory system and signs of anaemia) were done daily throughout data collection to ensure only healthy goats were used. The goats were randomly divided and allocated a treatment diet in a completely randomized design using an individual goat as a replicate (Steel and Torrie, 1980). From the first week until the fourth week of the trial, the goats were fed a control basal diet comprising 90.77% Lucerne (Medicago sativa) and 9.23% Buffel grass (Cenchrus ciliaris) for acclimatization of their rumen microbes (Odenyo et al., 1997). On the fifth and sixth weeks, the goats were fed diets comprising of varying levels of M. oleifera (Table 1). The animals had ad-libitum access to fresh water throughout the data collection period. At the end of the first week of the trial, all the experimental goats were vaccinated with 2 millilitres of blanthrax vaccine per goat via the subcutaneous route as prescribed by the vaccine manufacturer (Intervet South Africa, (Pty) Ltd). The objective of vaccinating the goats with a blanthrax vaccine was to evaluate the immune response following vaccination and compare it with the immune response following supplementation of the experimental diet. One of the goats was removed because it had displayed signs of dietary intolerances in the first week of the trial. Thus, the number of experimental units and animals used for the data analysis was eleven (three animals for the T1C9.23L90.77M0 diet) (Magasa and Mbassa, 1988).
Table 1: Formulation of dietary treatments used for the study.
| Treatment code | Treatment description | Number of animals |
|
T1C9.23L90.77M0 |
Wether goats were fed ad-libitum a basal diet, 9.23 % C. ciliaris grass hay, and 90.77% Lucerne hay without M. oleifera. |
3 |
|
T2C64L16M20 |
Wether goats were fed ad-libitum 64 % C. ciliaris grass hay and 16 % percent Lucerne hay containing 20 % of M. oleifera. |
4 |
|
T3C50L0M50 |
Wether goats were fed ad-libitum 50 % C. ciliaris grass hay and 0 % percent Lucerne hay containing 50 % of M. oleifera. |
4 |
L: Lucerne hay; C: C. ciliaris grass hay; M: M. oleifera
Fresh M. oleifera leaves and Medicago sativa (M. sativa) were harvested and air-dried on a concrete floor at room temperature for 96 hours and turned several times until they were crispy in touch, while retaining their greenish colouration. The dried leaves of M. oleifera and Lucerne were then stored at room temperature until needed for inclusion in the experimental diets. Cenchrus ciliaris (C. ciliaris) which is one of the palatable and perennial grass species that are native to the Limpopo province was used in this study (Smit, 2005). It contains an average of 6.1 % CP. Lucerne hay was used to supplement the insufficient protein content of Buffel grass. Lucerne contains an average of 11.4% CP. Three experimental diets were formulated by replacing a conventional supplement of Lucerne with M. oleifera. The inclusion levels varied from 0% to 50% as presented in Table 1 (Babeker and Bdalbagi, 2015; Su and Chen, 2020). Moringa oleifera leaves utilised in the present study contained traces of non-nutritive elements, such as Ba, Cd, and Ag which could have arisen from environmental contaminations (Pakade et al., 2013). However, there were no signs of toxicity observed in the animals due to the presence of heavy metal trace elements in the leaves. This could be attributed to the low levels of their presence (Pakade et al., 2013; Biel et al., 2016).
Goats were fed experimental diets that were 4% of their body weight daily. Analyses of CP, ash and NDF in dietary treatments were done at the Animal Nutrition Laboratory, University of Limpopo as described by AOAC (1990) (Table 2). The mineral composition was determined at the Limpopo Agro-Food Technology Station (LATS) Laboratory, University of Limpopo as described by ISO 11885 (2007).
The experiment was conducted in three phases for 42 days. The first phase was the adaptation phase which lasted for 7 days. The second phase was the vaccination phase, which lasted for 21 days. The duration of this phase depended on the establishment of immunity in goats which according to the manufacturer takes a minimum of 14 days. The third phase which lasted for 14 days was the inclusion phase of varying levels of M. oleifera.
Five millilitres of blood was collected from each animal via the external jugular vein on day 0 and at the end of each phase. Blood parameter results for day 0 were used as baseline values, this was done to evaluate changes in the haematological responses of the goats (Brown et al., 2016b). Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant were used for blood collection (Daramola et al., 2005; Aikhuomobhogbe and Orheruata, 2006; Brown et al., 2016b). The blood samples were stored in a cooler box containing ice packs and they were immediately transported to the University of Pretoria, Department of Companion Animal Clinical Studies, Gauteng Province for haematological analyses. All blood parameter results were referenced with day 0 as baseline values according to Brown et al. (2016).
Table 2: Nutrient composition of experimental diets used in the study.
| Nutrients |
T1C9.23L90.77M0 |
T2C64L16M20 |
T3C50L0M50 |
| CP % | 11.7 | 10.48 | 15 |
| NDF % | 52.53 | 50.36 | 39 |
| Ash % | 8.41 | 8.86 | 8.5 |
| Zn (mg/kg) | 11.81 | 2.25 | 4.25 |
| Fe (mg/kg) | 449.07 | 605.6 | 434.9 |
| Mn (mg/kg) | 69.57 | 129.21 | 98.78 |
| Cu (mg/kg) | 10.39 | 10.88 | 10.21 |
| Mg (g/kg) | 1.26 | 0.83 | 166.95 |
| Na (g/kg) | 1.22 | 0.52 | 23.55 |
| P (g/kg) | 2.87 | 2.06 | 73.4 |
| Ca (g/kg) | 8.48 | 3.41 | 523.45 |
| K (g/kg) | 13.82 | 13.35 | 550.2 |
T: Treatment; C: C. ciliaris; L: Lucerne; M: M. oleifera; CP: Crude protein; NDF: neutral detergent fibre; Zn: Zinc; Fe: Iron; Mn: Manganese; Cu: Copper; Mg: Magnesium; Na: Sodium; P: Phosphorus; Ca: Calcium; K: Potassium.
Bodyweight data was collected on day 0 from each goat and every seven days in the morning before feeding. The feed offered and refusals were weighed using an electronic scale each day from day 7 until day 42.
Feed intake (FI) was calculated as the difference between feed offered and feed refused. Live body weights were used to calculate growth rate (GR) and feed conversion ratio (FCR). The metabolic weight gain (MWG) is expressed as the bodyweight gain (BWG) in grams (g) to the exponential of 0.75. The FCR was obtained by dividing feed intake by weight gain. The formulae used were:
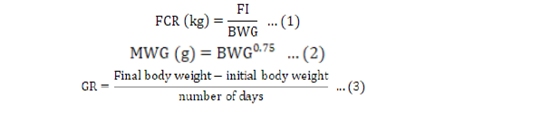
Descriptive statistics such as the mean, standard deviation, and standard error were calculated across different phases and treatments using Procedure of means (Proc mean) of Statistical Analysis Software version 9.4 (SAS, 2020). Data on blood parameters and growth performance was analysed using a general linear model (GLM) procedure. The following model was used:
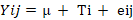
Where;
Yij= blood profiles or growth performance; µ= the overall mean; Ti= dietary treatments and eij = random error. Duncan’s multiple range test was used for any significant differences (p<0.05) amongst treatment means (Duncan, 1955).
Results and Discussion
Measurements on blood parameters before and after vaccination were carried out during the study. There were no significant differences (p>0.05) amongst means for the parameters except for MCV, platelet and monocyte counts (p<0.05) (Table 3). Hb (80-120 g/dL), RBC (17-20 (*10)6 µL), PCV (0.22-0.38 L/L), MCH (5.2-8.0 pg), MCHC (30-36 g/dL), WCC (6.8-20 (*10)3 µL), monocytes (0-1%) and eosinophils (1-7%) for day 0 were within range of healthy goats (Jackson and Cockcroft, 2002; Daramola et al., 2005; Brown et al., 2016b). However, MCV (16-25 fL) and platelet counts (300- 600 (*10)9/L) for day 0 were out of range for healthy goats (Jackson and Cockcroft, 2002).
Table 3: Blood parameters across all phases from day 0- 42 and varying M. oleifera inclusion levels from day 29-42.
| Parameters |
Adaptation phase |
Vaccination phase |
Vaccination + M. oleifera phase |
|||
|
DAY 0 (Mean±SEM)* |
DAY 7 (Mean±SEM)* |
DAY 28 (Mean±SEM)* |
VMO0 | VMO20 | VMO50 | |
|
DAY 42 (Mean±SEM)* |
||||||
| Hb | 92.91±3.30 | 92.67±5.16 | 84.64±4.08 | 79.33±2.40 | 90.00±4.45 | 83.50±8.38 |
| RBC | 17.83±0.70 | 17.42±0.85 | 16.40±0.89 | 15.57±0.88 | 16.92±0.68 | 16.41±1.88 |
| HCT | 0.28±0.01 | 0.28±0.02 | 0.26±0.01 | 0.23±0.01 | 0.28±0.02 | 0.25±0.03 |
| MCV |
15.64±0.22ab |
16.02±0.23ab |
15.62±0.20ab |
15.27±0.41b |
16.28±0.38a |
15.26±0.23b |
| MCH | 5.19±0.10 | 5.70±0.29 | 5.32±0.17 | 5.10±0.15 | 5.23±0.13 | 5.10±0.07 |
| MCH | 33.43±0.35 | 33.14±0.26 | 33.15±0.19 | 33.47±0.27 | 32.65±0.37 | 33.53±0.51 |
| WCC | 16.68±1.07 | 20.03±2.10 | 16.96±1.32 | 14.50±1.28 | 16.29±2.38 | 17.55±1.31 |
| Lymphocyte | 10.87±0.98 | 13.54±1.56 | 13.74±1.07 | 11.64±1.10 | 10.36±2.04 | 10.32±0.80 |
| Monocyte |
0.53±0.10abc |
0.71±0.10ab |
0.27±0.06c |
0.28±0.08bc |
0.48±0.20bc |
0.93±0.29a |
| Eosinophil | 0.20±0.07 | 0.42±0.10 | 0.40±0.09 | 0.48±0.17 | 0.27±0.06 | 0.35±0.16 |
| Platelet count |
643.27±65.74ab |
1372.89±392.01a |
693.55±102.33ab |
140.0±53.11b |
503.50±192.01b |
554.25±65.03b |
a,b,c Means with different superscripts on same row differ significantly (p < 0.05). SEM: Standard error of the means. WCC: white blood cell count; RBC: blood erythrocytes count; MCV: mean corpuscular volume; HCT: Haematocrit; MCH: mean corpuscular haemoglobin; MCHC: mean corpuscular haemoglobin concentration; Hb: haemoglobin. Day 0: initial values, Day 7: interval 1, Day 28: interval 2, Day 42 (VMO0): interval 3 Vaccination+ 0% M. oleifera inclusion, Day 42 (VMO20): interval 3 Vaccination+ 20% M. oleifera inclusion, Day 42 (VMO50): interval 3 Vaccination+ 50% M. oleifera inclusion.
Table 4: Comparison of growth performance parameters across phases from day 0–42.
| Variable | Adaptation phase | Vaccination phase |
Vaccination+M. oleifera phase (Day 29-42) |
||
|
Day 1-6 (Mean±SEM) |
DAY 7-28 (Mean±SEM) |
VMO0 (Mean±SEM) |
VMO20 (Mean±SEM) |
VMO50 (Mean±SEM) |
|
| BW (Kg) |
19.27±1.47b |
20.24±7.51b |
25.17±1.7a |
19.63±0.73b |
22.25±1.28ab |
| BWG |
0.55±0.25ab |
0.52±0.27ab |
0±0.26b |
1±0.27ab |
1.38±0.32a |
| GR |
0.08±0.04ab |
0.07±0.04ab |
0±0.04b |
0.14±0.04ab |
0.20±0.05a |
| MWG |
0.70±0.18ab |
1.13±0.13a |
0.33±0.21b |
0.92±0.23ab |
1.21±0.24a |
| FI | 7.13±2.05 | 7.42±3.57 | 6.57±4.88 | 6.59±3.93 | |
| FCR | 0.04±0.00 | 0.03±0.00 | 0.03±0.00 | 0.03±0.00 | |
a,b Means with different superscripts on the same row differ significantly (p< 0.05). SEM: Standard error of means.
There were significant differences (p<0.05) amongst growth performance parameters and no differences were observed (p>0.05) for feed efficiency parameters (Table 4). VMO50 displayed higher mean values on BWG, GR, and MWG (p<0.05) and there were no significant differences (p>0.05) in FI and FCR for VMO0, VMO20 and VMO50 in the M. oleifera supplementation phase (Table 4). VMO0 had 0 GR and BWG.
Haematological profiles may be utilised to assess the immunological status in goats (Al-Seaf and AlHarbi, 2012), however, factors such as age, nutrition, stress, management and environmental factors are known to have an effect on blood profiles in small ruminants (Mohammed et al., 2016). In comparison across the phases, there were no significant differences for total white blood cell counts (WCC) throughout the study. This varies from the findings of Jo et al. (2014), who observed an increase in total WCC following vaccination of indigenous goats. However, it agrees with Kumar et al. (2017), who found no significant differences in total WCC but observed significant differences in monocyte counts. Furthermore, in agreement with Kumar et al. (2017), monocyte counts significantly reduced following vaccination, this could have been due to a mild inflammation following vaccination. Useh et al. (2010), reported a significant decrease in monocyte count following blackleg infection, however, there was no significant difference in total WCC. Therefore, it may be useful to observe the specific leukocytes rather than WCC in separation (Cornell University, College of Veterinary Medicine, 2020). The results of the current study also revealed a significant increase of monocyte counts on VMO50 compared to monocyte counts following the vaccination phase. The significant differences in monocyte counts observed in the literature and the present study show that monocytes act as first-line mediators that prepare the immune system for defence. Inclusion of M. oleifera commenced on day 22 post-vaccination to allow sufficient time for the animals to develop immunologic protection (Hoebe et al., 2004). Therefore, the significant increase in monocyte count for VMO50 suggests an enhanced immune response to vaccination which could have been caused by 50 % M. oleifera inclusion as compared to 0% and 20% inclusion levels. This observation could also be associated with the higher content of heavy metals such as Ag and Cd in the experimental diet due to supplementation with 50% M. oleifera as reported by Kar et al. (2015). However, Shen et al. (2019) stated that toxicity from heavy metals such as Cd affects the bones, thus, causing anaemia in the animals. However, there was no evidence of anaemia in animals that were fed a diet containing 50% M. oleifera although MCV and RBC levels were lower as compared to VMO20.
Higher levels of platelet count outside the reference intervals as seen on day 0 are not of pathological importance especially in young animals but sometimes maybe bought about by an infection or anaemia (Evstatiev et al., 2014). According to Kumar et al. (2017), platelet count does not play any role in immune response following vaccination. However, in the present study, mean platelet counts increased during the adaptation period and significantly reduced following vaccination towards the recommended reference ranges of healthy goats as recommended by Jackson and Cockcroft (2002). Although there were no significant differences in mean platelet counts during the M. oleifera supplementation phase amongst all treatments, 0% M. oleifera supplementation decreased platelets counts to below the recommended reference interval and supplementation with 20% and 50% M. oleifera leaves decreased the platelet counts to within the recommended reference interval of healthy goats. This could be due to the M. oleifera immune-boosting effects as reported Adouko et al. (2020). Metcalf-Pate et al. (2013) and Ali et al. (2015), revealed that platelets may play a role in the immune response in animals which agrees with the results of the present study. According to Brown et al. (2016b), low levels of Hb and RBC may also be an indication of anaemia, however, the results in this study showed values within the reference intervals. Therefore, we can safely rule out the possibility of anaemia in the present study and assume that supplementing with 20% and 50% of M. oleifera assisted in boosting the immune system of the animals.
Mean corpuscular volume (MCV) is a measurement of the average size of RBC. However, in the present study, MCV levels on day 0 were slightly below the reference range of healthy goats of (16-25 fL), this was also observed by Mohammed et al. (2016) in different goat breeds. The causes of these discrepancies are not clear, since the goats did not any show signs of anaemia. A significant increase to within the reference range of healthy goats in MCV for VMO20 was expected due to higher levels of Cu and Fe minerals in the treatment diet which aid in red blood cell formation compared to treatment diets for VMO0 and VMO50 (Gaston et al., 2021). Therefore, it seems that anaemia may develop in the absence of these minerals. However, according to Wada (2004), high levels of Ca in the diet reduce the occurrence of anaemia even when Cu is deficient, therefore justifying the absence of anaemia in goats used in the present study.
Furthermore,the goats in VMO20 were observed to be healthy, indicating that the higher Fe content than the recommended requirement for indigenous goats of 50-100mg Fe/kg did not cause any toxicity (Souza, 2014; Alfaro et al., 2021). The results of the present study are similar to the findings of Jiwuba et al. (2017), who observed significant differences in MCV values for M. oleifera inclusion levels and MCV levels were maintained within the range of healthy goats, suggesting that M. oleifera may be used as a supplement without compromising the nutritional quality of the feed.
Balanced nutrition is vital in maintaining a healthy immune status in animals (Brown et al., 2016). Insignificant differences in most of the blood parameters among VMO0, VMO20, and VMO50 observed in this study agree with some previous studies which reported insignificant differences in blood parameters of animals fed M. oleifera as a supplement (Moyo et al., 2012; Osman et al., 2012). Results of this study show that most of the blood parameters were within the range of healthy indigenous goats (Jackson and Cockcroft, 2002; Brown et al., 2016b; Daramola et al., 2005). However, VMO0 had the lowest mean platelet and monocyte counts while VMO50 had the highest counts. This could have been caused by a high content of minerals such as Ca in M. oleifera leaves which supports the formation of platelets, thus alleviating the occurrence of anaemia (Wada, 2004). According to Ali et al. (2015), there is a positive relationship between platelets and monocyte counts because platelets influence the immune response by activating immune cells such as monocytes. This is evident in the present study because as the level of M. oleifera inclusion increased, the means of platelet and monocyte counts increased as well. With this in mind, we can conclude that the presence of heavy metals and the low mineral content of Cu and Fe in the diets for VMO50 in the present study did not bring about any toxicity or anaemia to the animals. Therefore, the possibility of anaemia in the present study can be ruled out and accept that supplementing with 20% to 50% of M. oleifera may assist in boosting the immune system of the animals.
Moringa oleifera is one of the alternative forages that are utilized for livestock nutrition in South Africa due to its high nutrient content (Qwele et al., 2013). Results from the present study showed that the growth performance parameters were influenced significantly by vaccination and varying M. oleifera inclusion levels. The mean values obtained for body weight gain, growth rate, and metabolic weight gain for VMO50 were significantly higher than those in the control (VMO0) but similar to VMO20. The higher values for VMO50 and VMO20 on BWG, GR, and MWG could have been influenced by the lower levels of NDF and higher protein content in the diet, making it to be more digestible resulting in higher growth rates (Moyo et al., 2012). The significant decrease in BWG and GR following vaccination agrees with the findings from Jo et al. (2014), who reported a significant decrease in growth performance following vaccination of goats, which could be due to some of the side effects such as inflammation that may arise from vaccination. However, the introduction of varying levels of M. oleifera to the diets of the goats, increased BWG, GR, and MWG, suggesting that M. oleifera due to its composition of elements such as antioxidants may aid in reducing inflammation and thus improving growth performance. This study agrees also with Mahfuz and Piao (2019); Aregheore (2002), who reported an improved growth performance in indigenous goats after supplementing with varying levels of M. oleifera. However, BWG and GR for VMO0 were zero without affecting the FI, this agrees with the findings of Jo et al. (2014), who reported that vaccination decreases BWG without affecting FI. It could also be due to the significantly higher bodyweight of the goats in VMO0 due to random grouping. The current findings disagree with the results of Yusuf et al. (2018), who reported insignificant differences in growth performance of indigenous goats fed 5 to 10% M. oleifera supplementation, therefore, it can be assumed that the minimum levels of M. oleifera that can be used as a supplement are 10% for improved growth performance in indigenous goats.
Conclusions and Recommendations
In conclusion, supplementing with M. oleifera leaves in indigenous goat diets had a positive effect on growth performance and immunological response in goats following vaccination. Twenty percent and 50% M. oleifera supplementation yielded improved results as compared to the control diet following vaccination. Therefore, supplementation with 20% to 50% of M. oleifera may be used by farmers to overcome shortages of good quality feeds that may lead to malnutrition and poor immune responses in livestock. However, more research-based knowledge transfer is required for awareness to farmers especially in communal areas to produce M. oleifera in abundance for a more affordable protein and mineral source for their livestock.
Acknowledgements
The authors would like to thank the University of Limpopo and the University of South Africa for the financial support received throughout the trial period.
Scientific contribution
The potential of Moringa oleifera as a feed supplement and
an immunomodulatory in indigenous Pedi goats was discovered. The problem of poor immune responses in livestock following vaccination has been cited as one of the reasons for vaccine failure in communal livestock production systems. The study pursues an alternative feed source that may help to ease the challenges of communal livestock productivity after vaccination. Thereby, alleviating poverty levels and improving household food security.
Novelty Statement
The study explored the use of Moringa oleifera as a local resource to enhance the performance of the indigenous BaPedi breed as compared to the majority of previous studies which evaluated M. oleifera in exotic breeds.
Author’s Contribution
LG, KRM and TC conceptualization, visualization, funding acquisition, methodology, writing-review, and editing. LG and TC data collection and writing original draft preparation. LG, TLT and TC methodology investigation, formal analysis, visualization, writing-review, and editing. LG, KRM, TLT and TC writing-review, and editing, formal analysis, validation, resource.
Data availability
The data that supports the findings of this study is available upon request.
Conflict of interest
The authors have declared no conflict of interest.
References
Adouko SJ, Arnaud SSS, Fréjus OOH, Jacques DT (2020). Review on biological and immunomodulatory properties of Moringa oleifera in animal and human nutrition. J. Pharmacogn. Phytother., 12: 1-9. https://doi.org/10.5897/JPP2019.0551
Alfaro GF, Rodriguez-Zas SL, Southey BR, Muntifering RB, Rodning SP, Pacheco WJ, Moisá SJ (2021). Complete blood count analysis on beef cattle exposed to fescue toxicity and rumen-protected niacin supplementation. Animals (Basel). 11:988. https://doi.org/10.3390/ani11040988.
Al-Seaf AM, Al-Harbi KB (2012). Variability of disease resistance, hematological parameters and lymphocyte proliferation in two goat breeds and their F1 and F2 crosses. Int. J. Food Agric. Vet. Sci., 2: 47-53.
AOAC (1990). Official methods of analysis. 15th edition. AOAC, Association of official analytical chemists. Arlington, VA, USA.
Aregheore EM (2002). Intake and digestibility of Moringa oleifera batiki grass mixtures by growing goats. Small Rum. Res., 46: 23-28. https://doi.org/10.1016/S0921-4488(02)00178-5
Ayele B, Tigre W, Deressa B (2016). Epidemiology and financial loss estimation of Blackleg on smallholder cattle herders in Kembata Tambaro zone, southern Ethiopia. Springer Plus, 5: 1-14. https://doi.org/10.1186/s40064-016-3541-2
Babeker EA, Bdalbagi YMA (2015). Effect of feeding different levels of Moringa oleifera leaves on performance, haematological, biochemical and some physiological parameters of Sudan Nubian goats. Online J. Anim. Feed Res. 5: 50-61.
Biel W, Jaroszewska A, Łysoń E (2016). Nutritional quality and safety of moringa (Moringa oleifera lam., 1785) leaves as an alternative source of protein and minerals. J. Elem., 22: 569-579. https://doi.org/10.5601/jelem.2016.21.3.1249
Brown D, Ng’ambi JW, Norris D, Mbajiovgu FE (2016). Blood profiles of indigenous Pedi goats fed varying levels of Setaria verticillata hay-based diet. S. Afr. J. Anim. Sci., 46: 432- 440. https://doi.org/10.4314/sajas.v46i4.11
Brown D, Ngámbi JW, Norris D (2016). Voluntary intake and palatability indices of Pedi goats fed different levels of Acacia karoo leaf meal by cafeteria method. Indian J. Anim. Res., 50: 41-47. https://doi.org/10.18805/ijar.5542
Duncan DB (1955). Multiple range and multiple F tests. Biometrics, 11: 1- 42. https://doi.org/10.2307/3001478
Evstatiev R, Bukaty A, Jimenez K, Kulnigg-Dabsch S, Surman L, Schmid W, Eferl R, Lippert K, Scheiber-Mojdehkar B, Kvasnicka HM, Khare V, Gasche C (2014). Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol., 89(5): 524–529. https://doi.org/10.1002/ajh.23682
Fikru S, Omer AA (2015). Traditional small ruminant production and management practices in Awbare district of Ethiopian Somali regional state. J. Anim. Prod. Adv., 5: 697-704. https://doi.org/10.5455/japa.20150626043822
Gaston FA, Sandra LR, Bruce RS, Russell BM, Soren PR, Wilmer JP, Sonia JM (2021). Complete blood count analysis on beef cattle exposed to fescue toxicity and rumen-protected niacin supplementation. Animals, 11: 1-15. https://doi.org/10.3390/ani11040988
Gwanzura T, Ngambi JW, Norris D (2011). Effects of selected species and forage sorghum hay grown in Limpopo province on voluntary intake and relative palatability indices of Pedi goats. Asian J. Anim. Vet. Adv., 6: 1249-1255. https://doi.org/10.3923/ajava.2011.1249.1255
Hoebe K, Janssen E, Beutler B (2004). The interface between innate and adaptive immunity. Nat. Immunol., 5: 971-974. https://doi.org/10.1038/ni1004-971
ISO 11885 (2007). Water quality determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES). [online] Available from: https://www.iso.org/standard/36250.html [Accessed 7 July 2021].
Jackson PGG, Cockcroft PD (2002). Clinical examination of farm animals. Blackwell science, Iowa. pp. 302. https://doi.org/10.1002/9780470752425
Jiwuba PC, Ahamefule FO, Ogbuewu IP, Ikwunze K (2017). Blood chemistry and haematology of West African Dwarf goats fed Moringa oleifera leaf meal (MOLM) in their diet. Comp. Clin. Pathol., 26: 621–624. https://doi.org/10.1007/s00580-017-2434-2
Jo NC, Jung J, Kim JN, Lee J, Jeong SY, Kim W, Sung HG, Seo S (2014). Effect of vaccination against foot-and-mouth disease on growth performance of Korean native goat (Capra hircus Coreanae). J. Anim. Sci., 92: 2578-2586. https://doi.org/10.2527/jas.2014-7190
Kar I, Mukhopadhayay SK, Patra AK, Pradhan S (2015). Metal concentrations and histopathological changes in goats (Capra hircus) reared near an industrial area of west Bengal, India. Arch. Environ. Contam. Toxicol., 69: 32–43. https://doi.org/10.1007/s00244-015-0130-2
Kniffen DM, Comerford JW (2021). Causes of vaccine failure in beef cattle. [online] Penn State Extension, Available from: https://extension.psu.edu/causes-of-vaccine-failure-in-beef-cattle [Accessed 7 July 2021].
Kumar A, Gupta VK, Verma AK, Kumar A, Yadav SK (2017). Assessment of hematological bio Markers during vaccination and challenge of Brucella melitensis in goats. Int. J. Vaccines Vaccin, 4: 78- 83. https://doi.org/10.15406/ijvv.2017.04.00079
Lee JJ, Seo J, Jung JK, Lee J, Lee JH, Seo S (2014). Effects of β-mannanase supplementation on growth performance, nutrient digestibility, and nitrogen utilization of Korean native goat (Capra hircus Coreanae). Livest. Sci., 169: 83-87. https://doi.org/10.1016/j.livsci.2014.08.018
Ma ZF, Ahmad J, Zhang H, Khan I, Muhammad S (2018). Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. S. Afri. J. Bot., Available from: https://www.sciencedirect.com/science/article/pii/S0254629918315060?via%3Dihub [Accessed: 26/ 02/2020].
Magasa MN, Mbassa GK (1988). Tolerance of goats to experimental grain engorgement and intraruminal lactic acid injection. Vet. Res. Commun., 12: 143-147. https://doi.org/10.1007/BF00362793
Mahfuz S, Piao XS (2019). Application of moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals, 9: 431. Available from: https://www.mdpi.com/2076-2615/9/7/431/htm [Accessed: 05/05/2021]. https://doi.org/10.3390/ani9070431
Metcalf-Pate KA, Lyons CE, Dorsey JL, Shirk EN, Queen SE, Adams RJ, Gama L, Morrell CN, Mankowski JL (2013). Platelet activation and platelet-monocyte aggregate formation contribute to decreased platelet count during acute Simian immunodeficiency virus infection in pig-tailed Macaques. J. infect. Dis., 208: 874–883. https://doi.org/10.1093/infdis/jit278
Mohammed SA, Razzaque MA, Omar AE, Albert S, Al-Gallaf WM (2016). Biochemical and hematological profile of different breeds of goat maintained under intensive production system. Afr. J. Biotechnol., 15: 1253-1257. https://doi.org/10.5897/AJB2016.15362
Monau P, Raphaka K, Zvinorova-Chimboza P, Gondwe T (2020). Sustainable utilization of indigenous goats in southern Africa. Divers, 12: 1-9. https://doi.org/10.3390/d12010020
Moyo B, Masika BP, Hugo A, Muchenje V (2011). Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afri. J. Biotechnol., 10: 12925- 12933. https://doi.org/10.5897/AJB10.1599
Moyo B, Masika BP, Muchenje V (2012). Effect of supplementing crossbred Xhosa lop-eared goat castrates with Moringa oleifera leaves on growth performance, carcass and non-carcass characteristics. Trop. Anim. Health Prod., 44: 801-809. https://doi.org/10.1007/s11250-011-9970-6
Mulyaningsih RTH, Yusuf S (2018). Determination of minerals content in leaves of Moringa oleifera by neutron activation analysis. Ganendra J. Nucl. Sci. Technol., 21: 11-16. https://doi.org/10.17146/gnd.2018.21.1.3683
Nouman W, Basra SMA, Siddiqui MT, Yasmeen A, Gull T, Alcayde, MAC (2014). Potential of Moringa oleifera leaves as livestock fodder crop. Turk. J. Agric. For., 38: 1- 14. https://doi.org/10.3906/tar-1211-66
Odenyo AA, Osuji PO, Karanfil O, Adinew K (1997). Microbiological evaluation of Acacia augustissima as a protein supplement for sheep. Anim. Feed Sci. Technol., 65: 99-112. https://doi.org/10.1016/S0377-8401(96)01087-5
Osman HM, Shayoub ME, Babiker EM (2012). The Effect of Moringa oleifera Leaves on blood parameters and body weights of Albino rats and rabbits. Jordan J. Biol. Sci., 5: 147–150.
Pakade V, Cukrowska E, Chimuka L (2013). Metal and flavonol contents of Moringa oleifera grown in South Africa. S. Afr. J. Sci., 109: 1-7. https://doi.org/10.1590/sajs.2013/835
Qwele K, Hugo A, Oyedemi SO, Moyo B, Masika PJ, Muchenje V (2013). Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 93: 455–462.
SAS (2020). Statistical analysis system user’s guide 9.4. Statistical Analysis System Institute, Inc. Cary, North Carolina, USA.
Shen X, Chi Y, Xiong K (2019). The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. Plos One. 14:1- 15. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0207423 [Accessed: 18/04/2021]. https://doi.org/10.1371/journal.pone.0207423
Steel RGD, Torrie JH (1980). Principles and procedures of statistics. McGraw-Hill, New York. pp. 20-90.
Souza AP, Medeiros AN, Carvalhoc FFR, Costa RG, Ribeiro LPS, Bezerra AB, Branco GLC, Silva Jr CG (2014). Energy requirements for maintenance and growth of Canindé goat kids. Small Rumin. Res. 121: 255–261.
Su B, Chen X (2020). Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 7:53. https://doi.org/10.3389/fvets.2020.00053
Tibbo M, Jibril Y, Woldemeskel M, Dawo F, Aragaw K, Rege, JEO (2004). Factors affecting haematological profiles in three Ethiopian indigenous goat breeds. J. Appl. Res. Vet. Med., 2: 297–309.
Useh NM, Nok AJ, Esievo KAN (2006). Blackleg in ruminants. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour., 1: 1-8. https://doi.org/10.1079/PAVSNNR20061040
Verma AR, Vijayakumar M, Mathela CS, Rao CV (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol., 47: 2196-2201. https://doi.org/10.1016/j.fct.2009.06.005
Wilson RT (2003). Animal health and disease control in the Usangu wetland of southern Tanzania. Trop. Anim. Health Prod., 35: 47- 67.
Yusuf AO, Mlambo V, Iposu SO (2018). A nutritional and economic evaluation of Moringa oleifera leaf meal as a dietary supplement in west African Dwarf goats. S. Afr. J. Anim. Sci., 48: 81-87. https://doi.org/10.4314/sajas.v48i1.10
Zhang H, Ma Z (2018). Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients, 10: 116. https://doi.org/10.3390/nu10020116
To share on other social networks, click on any share button. What are these?






