Genetic Variation in Chickpea Genotypes against Fusarium Wilt (Fusarium oxysporum F. sp. Ciceris) and their Management
Research Article
Genetic Variation in Chickpea Genotypes against Fusarium Wilt (Fusarium oxysporum F. sp. Ciceris) and their Management
Khalid Hussain1, Muhammad Younas1*, Niaz Hussain1, Abdul Ghaffar1, Muhammad Raheel2, Anees Akhtar3, Muhammad Irshad1, Munir Abbas1 and Farah Shabir4
1Arid Zone Research Institute (AZRI), Bhakkar, Punjab, Pakistan; 2Department of Plant Pathology, The Islamia University of Bahawalpur, Pakistan; 3Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan; 4Government Associate College for Women, Layyah, Pakistan.
Abstract | Chickpea (Cicer arietinum L.) is an important food legume. In Pakistan, yield potential of chickpea is low due to the prevalence of Fusarium wilt. Present investigations were conducted at Arid Zone Research Institute, Bhakkar, Punjab, Pakistan during winter 2021. Experiment was laid out in Randomized Complete Block Design (RCBD) following three replications. Thirty chickpea genotypes were examined for their resistant levels against Fusarium wilt caused by Fusarium oxysporum ciceris (FOC). Six exhibited resistant response with <10% disease incidence and seven genotypes were moderately resistant (11-20% DI) against wilt pathogen. However, five genotypes recorded moderately susceptible response (21-29% DI) and five genotypes showed susceptible response (30-50% DI) moreover; the remaining seven genotypes expressed highly susceptible response with maximum percent disease index (PDI) (>50%). Maximum and minimum disease incidence was recorded on CH-32/10 (7.26%) and D-15024 (69.61%) genotypes, respectively. It is concluded that chickpea resistant genotypes including CH-32/10, TG-1410 identified in present study might be helpful in different breeding programs against wilting pathogen. Among six fungicides (Fosetyle aluminium, Derosal, Shinkar, Ridomil gold, Cabrio Top, Acrobate) Fosetyle aluminium caused maximum disease reduction (75.16%) at the concentration of 3 g/liter of water followed by Derosal carbendazim) (65.76), Shinkar (59.44), Ridomil gold (52.41), Cabrio Top (44.17) and acrobat (41.86) respectively on comparison to control. Results are also helpful for the farmers for timely management of fusarium wilt.
Received | January 28, 2022; Accepted | July 25, 2022; Published | October 19, 2022
*Correspondence | Muhammad Younas, Arid Zone Research Institute (AZRI), Bhakkar, Punjab, Pakistan; Email: younasali643@gmail.com
Citation | Hussain, K., M. Younas, N. Hussain, A. Ghaffar, M. Raheel, A. Akhtar, M. Irshad, M. Abbas and F. Shabir. 2022. Genetic variation in chickpea genotypes against Fusarium wilt (Fusarium oxysporum F. sp. Ciceris) and their management. Sarhad Journal of Agriculture, 38(4): 1519-1525.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.4.1519.1525
Keywords | Cicer arietinum, Fosetyle aluminium, Fungicides, Disease resistance, Percent disease index (PDI)
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Chickpea (Cicer arietinum L.) an important pulse crop of Pakistan belonging to leguminosae family was originated from West Asia. It is now cultivated due to nutritive and health protective values. It is used as an important source of protein in human diet (Jendoubi et al., 2017). It has occupied a prominent position among legumes due to its superior nutritional contents. However, due to numerous biotic stresses, average global production of chickpea is still limited (Tarafdar et al., 2017, 2018).
Chickpea is attacked by numerous fungal diseases but Fusarium wilt caused by Fusarium oxysporum ciceris (FOC), is one of the most common diseases of chickpea. It is the potential threat to the successful cultivation of chickpea (Navas-Cortés et al., 2000) and causes severe yield losses ranging from 10 to 100% depending upon the varietal susceptibility and climatic circumstances (Patil et al., 2015; Haqqani et al., 2000). In Pakistan, it causes 10 to 50% yield losses annually (Khan et al., 2005). It is mainly reported in Ethopia, Australia, Syria, Iran and United States (Iqbal et al., 2005). The FOC is seed as well as soil born pathogen which remains viable in soil for six years (Ayub et al., 2003; Haware et al., 1996). All stages of plant growth particularly flowering and pod development are severely affected by fusarium wilt disease and it leads to the complete defoliation with in few weeks of infection. Disease development is favored by the high relative humidity and drought (Govil and Rana, 1994).
Numerous management strategies including the application of fungicides, cultural practices, use of resistant resources and bio-control agents have been tested against Fusarium wilt (Chandel and Deepika, 2010). Among all strategies, use of resistant resource is the best suited and economical strategy to overcome the potential maladies of FOC. Therefore, screening of available chickpea germplam is prerequisite to identify the source of resistance against FOC (Bakhsh et al., 2007). Thus, present study was aimed to identify resistant genotypes of chickpea against FOC. However, when disease appears in epidemic form, farmers don’t have any option except chemical fungicides. Fungicides with novel chemistry are being used for controlling plant diseases. Application of such fungicides can only be recommended against pathogen after their successful assessment against these diseases (Jameel and Kumar, 2010). Thus, present study was also designed to evaluate fungicides at different concentrations to select the most effective fungicide with least toxicity to environment against fusarium wilt (FOC).
Materials and Methods
Research site
Present study was conducted in the field area of Arid Zone Research Institute, Bhakkar, Punjab, Pakistan (31.6344° N, 71.1202° E). Experiment was planned during winter season in the month of November 2021. The climate of study area is arid where average temperature remains 24.6°C whereas, the annual rainfall is 213 mm. November was the driest month with 2 mm rainfall.
Research design
Experiment was laid out in Randomized Complete Block Design (RCBD) following three replications. Thirty chickpea genotypes were cultivated in single row sub-plot of four meter length with row to row and plant to plant distance of 30 and 15cm, respectively. The genotype AUG-424 served as repeated checks among all genotypes.
Data collection
Experimental data of the number of wilted plants in each row for each genotype were collected on weekly basis and wilt disease incidence was determined by using the following formula:
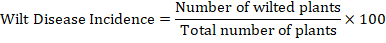
Assessment of fungicides against FOC
Six chemical fungicides Fosetyle aluminium, Derosal, Ridomil gold, Cabrio Top, Shinkar, and Acrobate were collected from market and evaluated against FOC at three different concentrations (1.5, 2.5 and 3 g/liter of water) (Table 3). IHT-401 Hand sprayer was used for the application of fungicides on genotypes. Application of fungicides was started after the appearance of initial disease symptoms. Disease data were recorded by following visual observation and rating scale as described by Iqbal et al. (2005) and Toker et al. (1999).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and treatments were compared by using Fisher’s Least Significant Difference (LSD) test. All the statistical tests were performed by using SAS statistical software (SAS Institute, 2011).
Results and Discussion
Disease severity ranged from 7.26 to 69.61% among thirty chickpea genotypes. Resistant levels were observed among tested genotypes (Table 1). The results revealed that there was not even a single genotype that showed immune/highly resistant response against fusarium wilt. However, among all the genotypes, six (PARB-913/CH03, PAR-913/CH01, TG-1305, Bhakkar-2011, TG-1410, CH-32/10) exhibited resistant response with PDI 7.26 to 9.85% whereas, seven genotypes (D-13036, NIAB-ch-2016, PARB-913/CHO4, CH-29/11, TG-1427, Bittle-2016, PARB/CH02) exhibited moderately resistant response with PDI 11.64 to 19.68% against FOC (Table 2).
Table 1: Rating scale (Iqbal et al., 1993).
|
Ratings |
Reaction |
Description |
|
1 |
Immune |
No symptoms |
|
2 |
Highly Resistant |
Spot or depression on small tissue |
|
3 |
Resistant |
Elongated spot |
|
4 |
Moderately Resistant |
Coalescent spot |
|
5 |
Tolerant |
Girdling of stem |
|
6 |
Moderately susceptible |
Breaking of stem |
|
7 |
Susceptible |
Downward lesion growth from stem breaking point |
|
8 |
Highly Susceptible |
Complete plant is nearly to die |
|
9 |
Highly susceptible |
Complete plant died |
Result revealed that five genotypes (D-14005, D-13011, BRC-448, CH-10/11, and TG-1620) expressed moderately susceptible response with PDI ranging from 23.02 to 29.48%. However, five genotypes including TG-1829, TGX-220, TGX-228, TG-1812, and TG-1801 showed susceptible responsible response with PDI 34.21 to 49.73% against fusarium wilt. Maximum values of PDI ranging from 51.80 to 69.61% were recorded in D-15024, TG-1714, TG-1415, Thal-2006, TG-1815, TG-1814 and TG-1806 respectively (Table 2). Results of contemporary study are supported by the findings of Nazir et al. (2012) who assessed one hundred and seventy-eight chickpea genotypes against fusarium wilt and recorded none of the tested genotypes as immune/highly resistant. Similarly results of present study are also in line with the findings of various researchers Bakhsh et al. (2007) and Dubey and Singh (2004). Ahmad et al. (2010) also evaluated 321 chickpea genotypes against fusarium wilt and reported nonetheless of genotypes immune to FOC and found some genotypes with resistant response.
Table 2: Evaluation of Chickpea genotypes against Fusarium oxysporum ciceris (Foc) under field conditions.
|
Sr. |
Genotypes |
Disease mean (%) |
Response |
Rating scale |
|
1 |
CH-32/10 |
7.26y |
R |
1 |
|
2 |
TG-1410 |
7.68xy |
R |
1 |
|
3 |
Bhakkar-2011 |
8.63wx |
R |
1 |
|
4 |
TG-1305 |
9.16vw |
R |
1 |
|
5 |
PAR-913/CH01 |
9.55vw |
R |
1 |
|
6 |
PARB-913/CH03 |
9.85v |
R |
1 |
|
7 |
PARB-913/CH02 |
11.64u |
MR |
3 |
|
8 |
Bittle-2016 |
13.27t |
MR |
3 |
|
9 |
TG-1427 |
14.85s |
MR |
3 |
|
10 |
CH-29/11 |
15.85s |
MR |
3 |
|
11 |
PARB-913/CH04 |
17.05r |
MR |
3 |
|
12 |
NIABC-2016 |
18.49q |
MR |
3 |
|
13 |
D-13036 |
19.68p |
MR |
3 |
|
14 |
TG-1620 |
23.02o |
MS |
4 |
|
15 |
CH-10/11 |
24.24n |
MS |
4 |
|
16 |
BRC-448 |
25.31n |
MS |
4 |
|
17 |
D-13011 |
27.86m |
MS |
4 |
|
18 |
D-14005 |
29.48l |
MS |
4 |
|
19 |
TG-1801 |
34.21k |
S |
5 |
|
20 |
TG-1812 |
39.49j |
S |
5 |
|
21 |
TGX-228 |
43.99i |
S |
5 |
|
22 |
TGX-220 |
46.09h |
S |
5 |
|
23 |
TG-1829 |
49.73g |
S |
5 |
|
24 |
TG-1806 |
51.80f |
HS |
6 |
|
25 |
TG-1814 |
53.94e |
HS |
6 |
|
26 |
TG-1815 |
54.80e |
HS |
6 |
|
27 |
Thal-2006 |
56.00d |
HS |
6 |
|
28 |
TG-1415 |
58.77c |
HS |
6 |
|
29 |
TG-1714 |
62.46b |
HS |
6 |
|
30 |
D-15024 |
69.61a |
HS |
6 |
|
31 |
LSD |
1.6881 |
||
*Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P<0.05).
Among all genotypes D-15024 and TG-1714 recorded highly susceptible response against FOC with maximum values. Therefore, these genotypes were further used for determining the efficacy of fungicides towards Fusarium oxysporum ciceris (Foc) under field conditions. Analysis of Variance for the management of Fusarium wilt expressed through fungicides showed significant results (Table 4). Among all treatments Fosetyle aluminium expressed maximum (75.16%) reduction in disease severity (Figure 1) at the rate of 3 g/liter of water followed by Derosal (65.76%), Shinkar (59.44%), Ridomil gold (52.41%), Cabrio Top (44.17%) and Acrobat (41.86%), respectively on comparison to control (Table 5).
Table 3: Chemicals description used during investigations.
|
Sr. |
Commercial name |
Molecule |
Chemical formula |
Manufacturer’s |
|
1 |
Fosetyle aluminium |
Fosetyl-Al |
[C₂H₅OPO₂]₃Al |
Engro Pesticides Pakistan |
|
2 |
Derosal |
Carbendazim |
C9H9N3O2 |
Bayer (Pvt,) ltd |
|
3 |
Ridomil Gold |
Matalaxyl + Mancozeb |
C15H21NO4 + C8H12MnN4S8Zn |
Sygenta (Pvt.) Pakistan |
|
4 |
Cabrio Top |
Pyraclostrobin + Metiram |
C19H18ClN3O4 |
FMC Pvt. Pakistan |
|
5 |
Shincar |
Carbendazim |
C9H9N3O2 |
FMC Pvt. Pakistan |
|
6 |
Acrobate |
Mancozeb + Dimethomorph |
C8H12MnN4S8Zn + C21H22ClNO4 |
FMC Pvt. Pakistan |
Table 4: Analysis of Variance (ANOVA) Table for management of Fusarium wilt.
|
Source |
DF |
SS |
MS |
F |
P |
|
Rep |
2 |
2.5 |
1.26 |
||
|
Fungicides |
6 |
32029.4 |
5338.23 |
9441.46 |
0.0000* |
|
Error rep × Fungicides |
12 |
6.8 |
0.57 |
||
|
Genotypes |
2 |
2444.4 |
1222.22 |
1802.06 |
0.0000* |
|
Fungicides × Genotypes |
12 |
749.3 |
62.44 |
92.06 |
0.0000* |
|
Error Rep× Fungicides× Genotypes |
28 |
19.0 |
0.68 |
||
|
Total |
62 |
35251.4 |
Table 5: Evaluation of Fungicides against Fusarium oxysporum f. sp. ciceris (Foc) under field conditions at Arid Zone Research Institute (AZRI) Bhakkar, Punjab during winter 2021.
|
Treatment |
Disease reduction (%) |
SD% |
CV% |
|
Fosetyle aluminium |
75.16a |
13.02 |
17.32 |
|
Derosal |
65.76b |
7.85 |
11.93 |
|
Shinkar |
59.44c |
8.61 |
14.49 |
|
Ridomil Gold |
52.41d |
5.89 |
11.23 |
|
Cabrio Top |
44.17e |
5.32 |
12.04 |
|
Acrobat |
41.86f |
5.85 |
13.99 |
|
Control |
0.00g |
00 |
00 |
|
LSD |
0.77 |
||
*Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P<0.05).
During impact of interaction between treatments and concentrations on the development of fusarium wilt of chickpea under field conditions (Figure 2), Fosetyle aluminium showed maximum disease reduction at all application rates (60.51, 74.46, 90.50%) followed by Derosal (57.50, 64.38, 75.40%), Shinkar (50.33, 58.00, 70.00%), Ridomil gold (45.91, 52.00, 59.33%), Cabrio Top (38.00, 44.33, 50.20%) and Acrobat (35.25, 41.66, 48.66%), respectively in comparison to control (0.00%) (Table 6). Results are supported by the Maitlo et al. (2014) who evaluated fourteen fungicides against wilting and reported Carbendazim as the most effective against FOC. Results of contemporary study are also favored by the Mengist et al. (2018) and Mahmood et al. (2015) who assessed different chickpea genotypes and fungicides against the fusarium wilt of chickpea. Results of the present investigation are supported by various researcher (Jamil and Ashraf, 2020; Harshita et al., 2019; Wavare et al., 2017; Sahar et al., 2013; Iqbal et al., 2010; Sinha and Sinha, 2004).
Table 6: Impact of the concentrations on suppression of Fusarium oxysporum f. sp. ciceris (Foc) at Arid Zone Research Institute (AZRI) Bhakkar, Punjab during winter 2021.
|
Fungicides |
Reduction in disease severity (%) |
||
|
Concentrations |
|||
|
1.5g/liter of water |
2.5g/liter of water |
3g/liter of water |
|
|
Fosetyle aluminium |
60.51e |
74.46b |
90.50a |
|
Derosal |
57.50d |
64.38d |
75.40b |
|
Shinkar |
50.33i |
58.00fg |
70.00c |
|
Ridomil gold |
45.91k |
52.00h |
59.33ef |
|
Cabrio Top |
38.00n |
44.33l |
50.20i |
|
Acrobate |
35.25o |
41.66m |
48.66j |
|
Control |
0.00p |
0.00p |
0.00p |
|
LSD |
1.3637 |
||
*Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P<0.05).
Based on the aforementioned screening results, assessed resistant genotypes can be employed as a basis of resistance in different breeding projects against fusarium wilt of chickpea. Accessions with complete agronomic attributes can be introduced at the commercial level. It is also concluded that fungicides Fosetyle aluminium and Derosal has the best potential against fusarium wilt of chickpea.
Conclusions and Recommendations
Resistant chickpea genotypes (CH-32/10, TG-1410) found in contemporary study against Fusarium wilt might be helpful for future breeding programs to develop resistant chickpea genotypes which could be further released at commercial level. Based on the above findings, it is also concluded that fungicide Fosetyle alauminium at the rate of 3.00g/liter of water has the best efficacy against fusarium wilt.
Acknowledgements
The authors are highly grateful to Punjab Agricultural Research Borad (PARB), Lahore for financial support under PARB Project No. 913 ‘Enhancement of mungbean and gram production in Thal through development of improved genotypes and technologies to reduce pulse import bill’. The authors are also thankful to Arid Zone Research Institute Bhakkar, Punjab, Pakistan for research activities in its experimental area.
Novelty Statement
Determination of resistant source is the best way to control Fusarium oxysporum F.sp. Ciceris. Moreover, Fosetyle-Al and Carbendazim may be used against Fusarium wilt of chickpea.
Author’s Contribution
Khalid Hussain: Provide resources
Muhammad Younas: Conceived the idea
Niaz Hussain: Project administration
Abdul Ghaffar: Conducted research trial and wrote the paper
Anees Akhtar: Analyzed and compiled the data
Muhammad Irshad: Corrected the paper
Muneer Abbas: Supervised the research
Fariha Shabir: Data Interpretation
Conflict of interest
The authors have declared no conflict of interest.
References
Ahmad, M.A., S.M. Iqbal, N. Ayub, Y. Ahmad and A. Akram. 2010. Identification of resistant sources in chickpea against Fusarium wilt. Pak. J. Bot., 42: 417-426.
Ayyub, M.A., S.M. Khan, R. Ahmad and K. Iftikhar. 2003. Screening of chickpea germplasm for the sources of resistance against chickpea wilt (F. oxysporum f. sp. ciceris). Pak. J. Phytopathol., 15: 25-27.
Bakhsh, A., S.M. Iqbal and I.K. Haq. 2007. Evolution of chickpea germplasm for wilt resistance. Pak. J. Bot., 39: 583-593.
Chandel, S. and R. Deepika. 2010. Recent advances in management and control of Fusarium yellows in Gladiolus species. J. Fruit Ornament. Plant Res., 18: 361-380.
S.C., and B. . 2004. Reaction of chickpea genotypes against Fusarium oxysporum f. sp. Cicero causing vascular wilt. Ind. Phytopathol., 57(2): 233–237.
Govil, J.N. and B.S. Rana. 1994. Stability of host plant resistance to wilt (Fusarium oxysporum f. sp. ciceri) in chickpea. Int. J. Trop. Plant Dis., 2: 55-60.
Haqqani, A.M., M.A. Zahid and M.R. Malik. 2000. Legumes in Pakistan. Legumes in rice cropping system of the Indo-Genetic Planes-constraints and opportunities. ICRISAT India, 230: 98-128.
A.S., J.B. , U.K. , S. , A. A. . 2019. In vitro evaluation of systemic fungicides against Fusarium oxysporum f. sp. lycopersici and their compatibility with bioagents. J. Pharmacogn. Phytochem., 8(3): 3117–3123.
Haware, M.P., Y.L. Nene and M. Natarajan. 1996. The survival of Fusarium oxysporum f. sp. ciceri in the soil in the absence of chickpea. Phytopathol. Mediterr., pp. 9-12.
Iqbal, S.M., I.U. Haq, A. Bukhari, A. Ghafoor and A.M. Haqqani. 2005. Screening of chickpea genotypes for resistance against Fusarium wilt. Mycopath, 3: 1-5.
Z., M.A. , S. , Y. , M. , A. , M.U. , A.A. . 2010. Determination of minimum inhibitory concentrations of fungicides against fungus Fusarium mangiferae. Pak. J. Bot., 42(5): 3525–3532.
Iqbal. M.J., K. Iftikhar and M.B. Ilyas. 1993. Evaluation of the chickpea germplasm for resistance against wilt disease. J. Agric. Res. 31(4): 449-453.
Jamil, S. and M. Kumar. 2010. Evaluation of fungicides against phyllosphere mycoflora of foliage plants. Biol. Forum, 2: 56-59.
Jendoubi, W., M. Bouhadida, A. Boukteb, M. Béji and M. Kharrat. 2017. Fusarium wilt affecting chickpea crop. Agriculture, 7(3): 23. https://doi.org/10.3390/agriculture7030023
Khan, M., R.U. Khan, A. Wahab and A. Rashid. 2005. Yield and yield components of wheat as influenced by intercropping of chickpea, lentil and rapeseed in different proportions. Pak. J. Agric. Sci., 42: 1-3.
Mahmood, Y., M.A. Khan, N. Javed and M.J. Arif. 2015. Comparative efficacy of fungicides and biological control agents for the management of chickpea wilt caused by Fusarium oxysporum f. sp. ciceris. J. Anim. Plant Sci., 25(4).
Maitlo, S.A., R.N. Syed, M. Rustamani, R. Khuhro and A. Lodhi. 2014. Comparative efficacy of different fungicides against fusarium wilt of chickpea (Cicer arietinum L.). Pak. J. Bot., 46: 2305-2312.
Mengist, Y., S. Sahile, A. Sintayehu and S. Singh. 2018. Evaluation of chickpea varieties and fungicides for the management of chickpea fusarium wilt disease (Fusarium oxysporum f. sp. ciceris) at adet sick plot in Northwest Ethiopia. Int. J. Agro., 2018. https://doi.org/10.1155/2018/6015205
Navas-Cortés, J.A., B. Hau and R.M. Jiménez-Díaz. 2000. Yield loss in chickpeas in relation to development of Fusarium wilt epidemics. Phytopathol., 90: 1269-1278. https://doi.org/10.1094/PHYTO.2000.90.11.1269
Nazir, M.A., M.A. Khan and S. Ali. 2012. Evaluation of national and international chickpea germplasm for resistance against Fusarium wilt (Fusarium oxysporum f. sp. ciceris) in Pakistan. Pak. J. Phytopathol., 24: 149-151.
Patil, M., G. Om, M. Pawar and D.R. Chobe. 2015. Effect of culture media, pH and temperature on the mycelial growth and sporulation of Fusarium oxysporum f. sp. ciceris isolates of chickpea from Central Zone of India. JNKVV Res. J., 49: 54-60.
P., S.T. , A. , A. , K. A. . 2013. Chemical and biological management of Fusarium oxysporum f. sp melongenae. Pak. J. Phytopathol., 25(2):155–159.
SAS Institute, 2011. SAS/IML 9.3 user’s guide. Sas Institute.
R.K.P., B.B.P. . 2004. Effect of potash, botanicals and fungicides against wilt disease complex in lentil. Ann. Plant Prot. Sci., 12(2): 454–455.
Tarafdar, A., T.S. Rani, U.S. Chandran, R. Ghosh, D.R. Chobe and M. Sharma. 2018. Exploring combined effect of abiotic (soil moisture) and biotic (Sclerotium rolfsii Sacc.) stress on collar rot development in chickpea. Front. Plant Sci., 9: 1145-1154. https://doi.org/10.3389/fpls.2018.01154
Tarafdar, A., T.S. Rani, U.S.S. Chandran, R. Ghosh and M. Sharma. 2017. Impact of moisture stress on collar rot (Sclerotium rolfsii Sacc.) development in chickpea (Cicer arietinum L.). In: Proceedings of Ismpp International Conference on “Plant health for human welfare.” Organized by Indian Society of Mycology and Plant Pathology, Jaipur, Vol. 119.
Toker, C., B. Uzun and M.I. Cagirgan. 1999. Screening and selection for resistance to Ascochyta blight [Ascochyta rabiei (Pass). Labr.] of chickpea (Cicer arietinum L.) under field conditions. J. Turk. Phytopathol., 28: 101-110.
S.H., R.M. A.V. . 2017. Antifungal efficacy of floral extracts, biocontrol agents and fungicides against Fusarium oxysporum f. sp. ciceri. ip. Indian Agric. Res. J., 70(2): 191–199. https://doi.org/10.24838/ip.2017.v70.i2.70615
To share on other social networks, click on any share button. What are these?








