Ganoderma lucidum as a Biocontrol agent for Management of Alternaria solani, A Pathogen of Early Blight of Tomato
Research Article
Ganoderma lucidum as a Biocontrol agent for Management of Alternaria solani, A Pathogen of Early Blight of Tomato
Muhammad Asif1*, Ahmad Ali Shahid 1,2 and Nasir Ahmad3
1Insititute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan; 2Center of Excellence and Molecular Biology, University of the Punjab, Lahore, Pakistan; 3School of Biological Sciences, University of the Punjab Lahore, Pakistan.
Abstract | Tomato is a beneficial, economically important and highly consumable crop. Several fungal diseases may affect its yield. Early blight is one of its most destructive diseases. Biocontrol agents are the best-known source to minimize the attack of pathogen. Tomato leaves infected with early blight symptoms were collected from Lahore. Pathogen was isolated on PDA and its morphological characters were studied. All the characters used for identification of pathogen confirmed the culture as Alterneria solani. Ganoderma lucidum was collected from University of the Punjab, Lahore associated with dead oaks of Dalbergia sisso. Macro and microscopic identification was performed to confirm the characteristics of G. lucidum. Interaction of G. lucidum with A. solani was also observed to measure the disease inhibiting potential. Crude protein extract was also prepared to observe the inhibitory effect of G. lucidum against A. solani. The results for interaction of mycelia of G. lucidum with pathogen A. solani reveal that G. lucidum is an efficient biocontrol agent to reduce the disease incidence. Crude protein extract was also found beneficial to control the pathogen. The results for minimum inhibitory concentration (MIC) of crude protein extract reveal that 5mg/ml is the concentration, which inhibited 100% growth of A. solani. It was concluded that either mycelia or crude protein extract of G. lucidum could be used for pathogen inhibition.
Received | April 12, 2020; Accepted | November 03, 2021; Published | May 20, 2022
*Correspondence | Muhammad Asif, Insititute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan; Email: ali_asif514@yahoo.com
Citation | Asif, M., A.A. Shahid and N. Ahmad. 2022. Ganoderma lucidum as a biocontrol agent for management of Alternaria solani, a pathogen of early blight of tomato. Sarhad Journal of Agriculture, 38(2): 734-741.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.2.734.741
Keywords | Ganoderma lucidum, Alternaria solani, Biocontrol agent, Minimum inhibitory concentration, Morphology, Crude protein extract
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Tomato is amongst the economically important and health beneficial vegetable crops worldwide. It was grown on about 52300 ha area in 2016-2017, giving 529.6 million tons production (GoP, 2017). There are approximately two hundred known diseases of tomato caused by viruses, bacteria, nematodes and fungi. Among them, fungi are much complex organisms than viruses or bacteria. They might develop various diseases in plants that cause loss of a considerable portion of the crop each year (Juan, 2015). Early blight caused by Alterneria solani (Ellis and Gibson, 1975) is the most common and disparaging disease of tomato crop that affect 5 to 50% of the crop yield and cause loss of millions of dollars worldwide (Saleem et al., 2016, Kumar et al., 2018). This disease is developed in warm temperatures and prolonged phases of leaf moisture due to heavy rain, overhead irrigation, or dews. The pathogen might be present on susceptible tomato plants or on solanaceous weeds (Foolad et al., 2008). This fungus attacks on leaves, shoot and fruit of tomato plant and causes complete defoliation in case of severe outbreak (Sobia et al., 2019). A number of chemical fungicides are available in the market for the crop protection. Some of them are excellent in terms of efficacy and cost benefit. However, their indiscriminate use has created the problems of air, soil and water pollution, development of resistance in target organisms and serious health hazards due to toxicity of their residues. Satisfying consumer needs and taking care of human health and environmental safety can be achieved either by reducing the usage of synthetic pesticides or by supplementing with biological pesticides. Synthetic pesticides could be supplemented with biopesticides as alternative pest and disease management products (Engindeniz et al., 2013).
Ganoderma lucidum (Curtis) P. Karst is a medicinal mushroom in the group polypores and family Ganodermataceae. Due to its capability of medication it acknowledged terms like “Elixir of life”, “Food of Gods”, “Mushroom of the Universe”. The extracts derived from G. lucidum have antimicrobial effect due to lysozyme and acid protease (Klaus and Nikšić, 2007, Garuba et al., 2020). Its extract has been used against Bacillus subtilis and Pseudomonas syringes which are plant pathogen (Ofodile, 2006).
The current investigation was adopted to observe the potential of G. lucidum to manage the early blight of tomato caused by A. solani. For this purpose, mycelia of G. lucidum were used for interaction against A. solani. Crude protein extract was also used to assess the fungal inhibiting potential of G. lucidum.
Materials and Methods
Collection of infected tomato
Surveys of tomato fields at Lahore were conducted during February-March 2016 to collect the infected leaves with symptoms of early blight. Infected leaves were cut into 1 cm2 small pieces and surface sterilized with 1% NaOCl for 1 min. Pieces of infected leaves were inoculated on PDA plates and incubated at 25℃ for three days (Shoaib et al., 2019).
Collection of Ganoderma lucidum
Isolates of G. lucidum were collected associated with Dalbergia sisso from STC (Student Teacher Center), Botanical Garden and PU Graveyard in Quaid-e-Azam Campus, University of the Punjab, Lahore. Specimens were collected based on morphological features such as size of basidiocarps and pores, color of upper and lower surface, type of stipe attachment with pileus and presence or absence of any ornamentations on the surface. Collected specimens were cut into small pieces, surface sterilized with 1% NaOCl, inoculated on PDA media and incubated for at 25℃ for three days.
Morphological identification of isolated cultures
Morphological and microscopic characteristics of the cultures isolated from tomato leaves and basidiocarps were studied for identification. Colony characters of the fungal isolates were recorded with respect to color, shape and nature of the colony (Burkholder et al., 1954). Microscopic features of fungi such as type of hyphae, type of spores or conidia, color and shape of spore or conidia etc. were also observed. Microscopic features of basidiocarps were also observed directly by cutting their small pieces.
Interaction of Ganoderma lucidum with Alternaria solani
Antifungal potential of G. lucidum against early blight of tomato was determined by Dual Culture Method. An agar disc of 6mm diameter was plugged out from periphery of agar media and replaced with the disc of A. solani. The plug of G. lucidum was also inoculated in the center of media plate. The inoculated plates were incubated at 28℃ for 7 days. Antagonistic activity was calculated after 5 days of incubation by measuring the radius of the A. solani colony in the direction of the G. lucidum (T) and the radius of the A. solani colony in the control plate (C). The two readings were transformed into percentage inhibition of radial growth (PIRG) using the formula developed by Skidmore and Dickinson (Anupama et al., 2015).
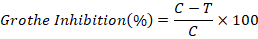
Where;
C: Control; T: Treatment.
Isolation of crude protein extract
One hundred gram mycelia of G. lucidum prepared in 1000 ml potato dextrose broth were extracted with 250 mL extraction buffer (pH 6.5) containing 10 mM NaH2PO4, 15 mM Na2HPO4, 100 mM KCI, 2 mM EDTA, 2 mM thiourea. Cell disrupter was used for lysis of cell walls and membranes at 20000 pa pressure to isolate proteins. Cocktail protease inhibitor (Sigma fast S-3830) was added before cell disruption. Disrupted material was centrifuged at 10,000 rpm for 20 min and supernatant was obtained (Miguel et al., 1996). This supernatant was used as crude protein extract and quantified by Bradford assay. The crude extract with different concentration of 0.5 mg/mL, 1 mg/mL and 2 mg/mL were spread in agar media plate while the fungal pathogen was inoculated in center of the plate. Control with pathogen was also used for comparison of results. These plates were incubated for 5 days at 28℃ and then percentage growth inhibition was observed (Anupama et al., 2015).
Minimum inhibitory concentration (MIC)
MIC is the concentration, which inhibits more than 90% growth of pathogens. Several doses with increasing concentration (100 µg/mL, 200 µg/mL, 400 µg/mL, 600 µg/mL, 800 µg/mL,1 mg/mL, 1.25 mg/mL, 1.5 mg/mL, 2 mg/mL, 2.5 mg/mL, 5 mg/mL, 7.5 mg/mL, 10 mg/mL) of crude protein extract were applied to determine the MIC. The activity assay was performed for crude extract to observe MIC against A. solani by using micro spectrophotometry (Broekaert et al., 1990). In this protocol, 20 µl of fungal spore suspension (2×105 spores/ml) and 50 µl of crude extract with different concentrations were added in 100 µl potato dextrose broth. A. solani and Tris-Cl (pH 7.5) were used as positive and negative controls respectively. Plate was incubated for 30 min to settle down the materials. After 30 min, the absorbance at 595 nm wavelength was observed with microspectrophotometer. Further incubation was done at 28℃ for 72 h and then its absorbance was observed again at 595 nm. Percentage growth inhibition was observed with following formula by subtracting the absorbance value of 72 h with absorbance value of 30 min.
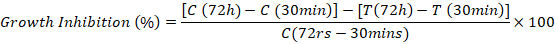
Where:
C: Control; T: Treatment.
Thermal stability of crude protein extract
The temperature stability of the crude protein extract (5mg/mL) was determined by incubating protein specimen at high temperatures 80℃, 90℃ and 100℃ for 15 min. After incubation the protein fractions were rapidly cooled in ice water and antifungal activity assay was performed to measure the thermal stability of extract by microspectrophotometry.
Statistical ansalysis
Data from invitro experiments were subjected to a one-way analysis of variance (ANOVA). Mean separation of treatments was accomplished using LSD (least significant difference) and the probability of P-value was taken P ≤ 0.05 to indicate the significance of data. All statistical analyses were conducted using Statistix 8.1.
Results and Discussion
Morphological identification of Pathogen
The fungal isolates had concentric zonation with regular and smooth margin, colony was matt like in appearance and greyish-brown at early stage which become black in color as mature. Conidiophores were dark brown, usually grow single or in groups and 35-80 µm in size. Conidia were single, slender, muriform, olivaceous brown in color with 1-5 longitudinal and 4-9 transverse septation and measured as 55 - 95 µm × 12-20 µm with a tapering end (Figure 1B). Mycelium was containing septate, branched and pale brown hyphae which turned dark with maturity (Figure 1A).
Morphological identification of G. lucidum
Fruiting bodies of G. lucidum were collected from University of the Punjab, Lahore Pakistan growing and associated with the base of D. sissoo. Basidiocarp consisted of three main parts namely; cap (pileus), stem (stalk) and pores. Cap was kidney or bracket shaped, upper side had rough, irregular, corky, tough and varnished surface, yellowish towards margins, edges were off white at early stages. Cap was 37 cm in diameter and 5-7 cm thick, soft, moist and leathery at early stages which became tough and corky with maturity. Lower surface consisted of minute tubes of 3-18 mm length, appeared as dense pores (3-8 per µm) which were whitish yellow at earlier and became bruising brown with maturity. Stalk was 2-15 cm long, 1-4 cm wide, sometimes straight or twisted having the same pigmentation as of upper surface. Overall basidiocarp remained soft and corky but after maturity it became hard like wood.
Microscopic study has shown three types of hyphae (generative, binding and skeletal) with a number of basidia and basidiospores. Elongated tubes on lower surface were of brown color and having hymenium all over its inner surface, producing dark brown double walled basidiopores which were elliptical and about 9 µm in length. A number of ellipsoidal and thin walled basidia were also observed (Figure 2).
Antagonistic effect of G. lucidum against A. solani
Results revealed that the maximum growth inhibition of A. solani in dual culture method was calculated up to 80% in comparison with control. Zone of inhibition was separating two cultures exhibiting the antifungal potential of G. lucidum against the mycelial growth of A. solani (Figure 3).
Activity assay of crude protein extract
In results of activity, assay of crude protein against A. solani significant growth inhibition was observed at three different concentrations (0.5 mg/ml, 1 mg/ml, 2 mg/ml. Among all the applied concentrations of crude protein extract 2 mg/ml exhibited about 94% growth inhibition potential followed by 1mg/ml (72%) against A. solani while minimum inhibitory effectiveness was observed with the 0.5 mg/ml concentration which was measured as 43% in combating with A. solani. Thus the antifungal effectiveness of crude protein extract of G. lucidum was confirmed (Figure 4 and 5).
Table 1: Thermal stability of crude extract.
|
Treatment |
%age inhbition |
|
80ºC., 15min |
100 |
|
90ºC., 15min |
100 |
|
100ºC., 15min |
100 |
MIC of crude protein extract
In result of MIC of crude extract against A. solani significant growth inhibition was also observed with increasing concentration of crude protein extract. As a result, maximum value for the MIC of crude protein extract was calculated in case of 2 mg/ml which was recorded as 92% followed by 5, 7.5 and 10 mg/ml which have exhibited a stationary phase while inhibiting the mycelial growth of A. solani up to 100% (Figure 6).
Thermal stability
Crude protein extract (5 mg/mL) of G. lucidum was heated at 80℃, 90℃ and 100℃ for 15 min and
found that the crude protein extract has a tremendous thermal stability even at very high temperatures like 100 ℃ and exhibited 100% inhibition in the mycelial growth of A. solani at this very temperature (Table 1).
Food consumption and its demand is increasing day by day with the increase of world population, while crops are threatened by pests and pathogens. Similarly, need and application of fungicides is also increased because of its instant effect on pest population. Frequent application of fungicides is responsible for environmental hazards, residual toxicity and development of resistance in pathogens against these fungicides (Sobia et al., 2019). To minimize the effect of chemical fungicides, there is dore need to find a biocontrol agent. For this purpose G. lucidum was used to check as a biocontrol agent against early blight of tomato. The tomato leaves with early blight symptoms were collected with their proper symptoms. Light brown to black spots with necrotic lesions on the older leaves of tomato with diameter of 1-2 mm were observed, enlarged lesions with concentric rings were also observed. These symptoms agreed on the study of Singh (1987) while he was working with early blight of tomato. Datar and Mayee (1985) also observed symptoms with the black lesions on stem and fruit of tomato while working with the same infectious disease. The isolated fungus was morphologically identified on the basis of its smooth and regular margins with concentric zonation and greyish-brown color were also observed (Figure 1). The results of the present study were synchronized with findings of previous investigation of A. solani (Ellis and Gibson, 1975; Nafisa et al., 2017). Single, slender, muriform, beaked and olivaceous brown conidia with longitudinal and transverse septa were observed. Septate, branched and pale brown-dark hyphae were observed on mycelia (Figure 1). Ahmad (2002) and Nafisa et al. (2017) found the same observations during identification of pathogen isolated from early blight of tomato. Thus, the pathogen causing early blight of tomato was identified as A. solani.
G. lucidum collected from University of the Punjab, Lahore Pakistan was associated with the base of D. sissoo. Ying et al. (1987) have also collected G. lucidum from the roots and stumps of dead oaks and other hardwood trees. Kidney or bracket shaped cap
with rough, irregular, corky and varnished upper side surface and off-white edges were observed. Wide, straight or twisted stalk with white pigmentation was observed (Figure 2). Kapoor and Sharma (2014) also observed the same features on G. lucidum. Mycelial colony of G. lucidum was white and hyphae with clamp connection were observed. Three types of hyphae (generative, binding and skeletal) with a number of basidia and basidiospores were observed in case of microscopic identification. Elongated tubes on lower surface and hymenium over its inner surface, producing basidiopores with thin walled basidia were also observed (Figure 2). Roy et al. (2006) wrote the same identification key for G. lucidum in their book “American Herbal Pharmacopoeia and therapeutic compendium” by analyzing the variability of G. lucidum with other species of Ganoderma. Singh et al. (2007) also observed the same findings to identify the G. lucidum by macro and microscopically. G. lucidum is a medicinal mushroom and has been used as biocontrol agent to restrict the growth of several fungal and bacterial pathogens. Interaction between G. lucidum (antagonistic) and A. solani (pathogenic fungi) reveals that G. lucidum has the potential to inhibit the pathogens. Ofodile et al. (2005) revealed this potential of restricting the mycelial growth of other fungi is due to the presence of inhibiting metabolites which G. lucidum releases after inoculation at its substrate. Yihuai et al. (2003) found that G. lucidum releases some secondary metabolites which could be responsible for its antifungal activity. In this research it is revealed that crude protein extracted from G. lucidum is also a potential inhibitor against the growth of A. solani. Uma et al. (2014) reported that extract prepared from basidiocarp of G. lucidum could be employed to combat several diseases caused by pathogenic microorganisms (Figure 3 and 4). Anita et al. (2016) also used extracts of Ganoderma to control pathogens. Shahid et al. (2016) also reported that crude extract prepared from fruiting body of G. lucidum has the potential to control the fungal pathogens of Calendula officinalis. Woo-Sik et al. (2011) reported that G. lucidum secretes some enzymes as extracellular in broth media that has the potential to reduce the diseases. Hadda et al. (2015) also found that G. lucidum secretes highly specific extracellular enzymes. Wan et al. (2017) used mycelial extract to isolate glucan sulfate to cure some diseases. A lectin has also been isolated from mycelia of G. lucidum by Kawagishi et al. (1996). Baig et al. (2015) also showed that the extract of G. lucidum inhbiting growth of Fusarium oxysporum, Aspergilus flavus and Alterneria alternata.
The results from this study conclude that the mycelia and crude protein extract of G. lucidum has potential to control the growth of A. solani, a causal pathogen of early blight of tomato. Thus G. lucidum may be an effective biological control agent for early blight of tomato as well as a prudent replacement to chemical pesticides.
Novelty Statement
This research is initial of our NRPU Project Funded by HEC to determine the antifun-gal proteins against early blight of tomato. To use antifungal fungal proteins for man-agement of crop diseases is a novelty.
Author’s Contribution
Muhammad Asif: Performed experimental work and wrote the article.
Ahmad Ali Shahid: Provided funding for experiment and finalized the manuscript.
Nasir Ahmad: Helped in experimental design and material collection and reviewed the manuscript.
Conflict of Interest
Authors have declared no conflict of interest.
References
Ahmad, S. 2002. Conidial morphology of Alternaria solani, its variations in tomato leaf blight. Ann. Agric. Res., 23: 514-515.
Anita S.K., S.K. Maja, Đ.V. Jovana, M.P. Predrag, P.N. Miomir. 2016. Antibacterial and antifungal potential of wild basidiomycete mushroom ganoderma applanatum. Lek. Sirov. 36: 37 – 46.
Anupama, S., Manali, M. and Sonali, R. 2015. Antifungal Activity of a Fungal Isolates against Pomegranate Wilt Pathogen Fusarium. Int. J. Curr. Microbiol. App. Sci., 2:48-57.
Baig, M.N., Shahid, A.A. and Ali, M. 2015. In Vitro Assessment of Extracts of the Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Higher Basidiomycetes) Against Different Plant Pathogenic Fungi. Int. J. Med. Mushrooms., 17:407-11. https://doi.org/10.1615/IntJMedMushrooms.v17.i4.90
Broekaert, W.F., Terras, F.R.G., Cammue, B.P.A. and Vanderleyden, J., 1990. An automated Quantitative assay for fungal growth inhibition. FEMS Microbiol. Lett., 69: 55-60. https://doi.org/10.1111/j.1574-6968.1990.tb04174.x
Burkholder, P.R., S.H. Sun, J. Ehrlich and L. Anderson. 1954, Criteria of speciation in the genus Streptomyces. Annal. N.Y. Acad. Sci., 60: 102- 123.
Datar, V.V. and Mayee, C.D. 1985. Chemical management of early blight of tomato. J. Maharashtra Uni., 3: 278-280.
Ellis, M.B. and I.A.S. Gibson. 1975. Alternaria solani. CMI Descriptions of Pathogenic Fungi and Bacteria No. 475.
Engindeniz, S. and Ozturk G.C. 2013. An economic comparison of pesticide application for processing and table tomatoes, a case study for Turkey. J. Plant Prot. Res., 53: 230-237. https://doi.org/10.2478/jppr-2013-0035
Foolad, M.R., Merk, H.L. and Ashrafi, H. 2008. Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit. Rev. Plant. Sci., 27: 75-107. https://doi.org/10.1080/07352680802147353
Garuba, T., Olahan, G.S. Lateef, A.A. Alaya, R.O. Awolowo, M. and Sulyman, A. 2020. Proximate composition and chemical profiles of Reishi mushroom (Ganoderma lucidum (Curt: Fr.) Karst). J. Sci. Res., 12: 103–110. https://doi.org/10.3329/jsr.v12i1.42059
Government of Pakistan. Agricultural Statistics of Pakistan 2016-2017, 2017. Islamabad, Ministry of Food, Agriculture and Co- Operatives.
Hadda, M., Djamel, C. and Akila, O. 2015. Screening of Extracellular Enzyme Activities of Ganoderma and Fomes Species Collected from North East Algeria. Res. J. Pharma. Biol. Chem. Sci., 6: 1455.
Juan, Y., Su-su, Y., Luan-luan, J., Xiu-juan, Y., Tzi, B.N. and Zu-jian, W. 2015. Plant antifungal proteins and their applications in agriculture. Appl. Microbiol. Biotechnol. 99:4961-4981. https://doi.org/10.1007/s00253-015-6654-6
Kapoor, P. and Sharma, B.M. 2014. Studies on different growth parameters of Ganoderma lucidum. Int. J. Sci. Environ. Tech., 3: 1515-1524.
Kawagishi, H., Mitsunaga, S.I., Yamawaki, M., Ido, M., Shimada, A., Kinoshita, T., Murata, T., Usu1, T., Kimura, A. and Chiba C. 1997. A Lectin from Mycelia of the Fungus Ganoderma lucidum. Phytochem, 44: 7-10. https://doi.org/10.1016/S0031-9422(96)00492-X
Klaus, A. and Miomir, N. 2007. Influence of the extracts isolated from Ganoderma lucidum mushroom on some microorganisms. Proceedings of National Science, Matica Srpska Novi Sad, 113: 219-226. https://doi.org/10.2298/ZMSPN0713219K
Klaus, A.S., Maja, S.K., Jovana, Đ.V., Predrag, M.P. and Miomir, P.N. 2016. Antibacterial and antifungal potential of wild basidiomycete mushroom Ganoderma applanatum. Lek. Sirov. 36: 37-46. https://doi.org/10.5937/leksir1636037K
Kumar, N., Bhardwaj, M.L., Kumari, S., Sharma, A., and Kansal, S. 2018. Screening of tomato (Solanum lycopersicum L.) germplasm for growth, yield, resistance against buckeye rot and Alternaria blight severity under mid-hills conditions of Himachal Pradesh. J. Pharmacogn. Phytochem. 7 :2098-2103.
Loper, J.E., Henkels, M.D., Roberts, R.G., Grove, G.G., Willet, M.J., and Smith, T.J, 1991. Evaluation of streptomycin, oxytetracycline, and copper resistance of Erwinia amylovora isolated from pear orchards in Washington State. Plant Dis. 75: 287-290. https://doi.org/10.1094/PD-75-0287
Miguel, D.B., Willem, F., Broekaert, Bruno, P.A., Cammue S.B. and Jozef, V. 1996. Biocidal Proteins. United States Patent.
Nafisa, Shoaib, A. and Iqbal, J. 2017. Cultural, morphological, molecular comparison and pathogenicity of Alternaria solanicausing early blight disease in tomato. Mycopath, 15: 7-11.
Ofodile, L.N., 2006. Taxonomy and Antimicrobial Activity of some Basidiomycetous Fungi in Southern Nigeria. PhD Thesis, Department of Botany and Microbiology, University of Lagos. Akoka, Lagos, 6-44.
Ofodile, L.N., Kokubum, N.U., Grayer, O.R.J., Ogundipe, O.T. and Simmonds, M.S.J. 2005. Antimicrobial Activity of Some Ganoderma Species from Nigeria. Phytother. Res., 19: 210-213. https://doi.org/10.1002/ptr.1641
Roy, U.H., Cathirose, P.A.G. and Diana, S. 2006. Reishi Mushroom Ganoderma lucidum Standards of Analysis, Quality Control, and Therapeutics. American Her Pharma Herapeutico, 1538-0297.
Saleem, M.Y., Akhtar, K.P., Iqbal, Q., Asghar, M., Hameed, A. and Shoaib, M. 2016. Development of tomato hybrids with multiple disease tolerance. Pak. J. Bot., 48: 771-778.
Shahid, A.A., Asif, M., Shahbaz, M. and Ali, M. 2016. Antifungal Potential of Ganoderma Lucidum Extract Against Plant Pathogenic Fungi of Calendula Officinalis L. 5th International Conference on Biological, Chemical and Environmental Sciences (BCES-2016) March 24-25, London (UK).
Shoaib, A., Awan, Z.A. and Khan, K.A. 2019. Intervention of antagonistic bacteria as a potential inducer of disease resistance in tomato to mitigate early blight. Sci. Horticult., 252:20-28. https://doi.org/10.1016/j.scienta.2019.02.073
Singh, R.S. 1987. Diseases of vegetable crops. Oxford and IBH Pub. Co. Pvt. Ltd., New Delhi, Bombay, Culcutta, p. 419.
Singh, A., J.P.S. Gautam, J. Upadhyay and A. Joshi. 2007. Heterosis for yield and quality characters in tomato. Crop Res., 29: 285-287.
Sobia, C., Rashida, P., Anees, M., Muhammad, A. and Abid. M. 2019. Estimation of secondary metabolites of indigenous medicinal plant extracts and their in vitro and in vivo efficacy against tomato early blight disease in Pakistan. J. Plant Dis. Prot. 126:553–563. https://doi.org/10.1007/s41348-019-00252-6
Uma, G.S., Chathurdevi, G. and Rani, K. 2014. Evaluation of Bioactive Potential of Basidiocarp Extracts of Ganoderma lucidum. Int. J. Pharm. Res. Allied. Sci., 3:36-46.
Wan, A.Q., Imad, W.M., Christina, V., Anita, K. and Sarina, A.H.L. 2017. Antifungal-demelanizing properties and RAW 264.7 macrophages stimulation of glucan sulfate from the mycelium of the mushroom Ganoderma lucidum. Food Sci. Biotechnol., 26: 159-165. https://doi.org/10.1007/s10068-017-0021-6
Woo-Sik, J., Ha-Na, P., Doo-Hyun, C., Young-Bok, Y. and Seung-Chun, P. 2011. Detection of Extracellular Enzyme Activities in Ganoderma neo-japonicum. Mycobiol. 39: 118-120. https://doi.org/10.4489/MYCO.2011.39.2.118
Yihuai, U., Zhau, S., Huang, M. and Xu, A. 2003. Antibacterial and Antiviral Value of the Genus Ganoderma Species (Amphyllophoromycetideae): A Review. Int. J. Med. Mushrooms., 5: 235-246. https://doi.org/10.1615/InterJMedicMush.v5.i3.20
Ying, J., Mao, X., Ma, Q., Zong, Y. and Wen, H. 1987. Icons of Medicinal Fungi from China. Yuehan X, translator. Beijing: Sci. Pr., 575.
To share on other social networks, click on any share button. What are these?








