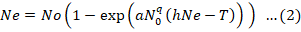Functional Response of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) to Lipaphis erysimi (Kaltenbach) and Diuraphis noxia (Kurdjumov)
Functional Response of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) to Lipaphis erysimi (Kaltenbach) and Diuraphis noxia (Kurdjumov)
Yasir Hameed, Muhammad Rizwan, Muhammad Shahzaib, Muhammad Sarmad, Muhammad Farrukh Hamid, Syed Muhammad Zaka*, Khalid Abbas and
Muhammad Zakria
Department of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan.
ABSTRACT
Green lacewing, Chrysoperla carnea S. (Neuroptera: Chrysopidae), is an important predator of many aphid species such as Lipaphis erysimi and Diuraphis noxia. In biological control C. carnea is most effective predator in controlling of aphid species. The functional response of all larval stages of C. carnea is calculated over a time of 24h at different prey densities (10, 20, 30, 40, and 50). Functional response of three larval instars was calculated using a logistic regression model, where handling time (Th) and attack rate (a) that calculated by using Roger random predator equation. The results revealed that all the predatory stages show a type II functional response and consumption rate increased with the increase in prey density for the first, second and third larval instar of C. carnea on both L. erysimi and D. noxia. It is concluded that as the handling time increases the attack rate decreases. When the third instar of C. carnea fed on L. erysimi its handling time was 0.029h and its attack rate was 1.55h-1, while when fed on D. noxia its handling time was 0.14h and its attack rate was 0.72h-1. This study revealed that third larval instar of C. carnea has great predatory potential for L. erysimi and D. noxia.
Article Information
Received 05 July 2019
Revised 23 August 2021
Accepted 07 September 2021
Available online 14 April 2022
(early access)
Published 07 November 2022
Authors’ Contribution
YH and MR did experiments. YH analyze the data. M Shahzaib and M Sarmad edited the manuscript. MFH helped in field collection and experimentation. SMZ supervised the work. KA helped in data collection and making graph. MZ helped in rearing and data collection.
Key words
Predatory potential, Chrysoperla carnea, Aphids, Biological control
DOI: https://dx.doi.org/10.17582/journal.pjz/20190705190700
* Corresponding author: zaka_ento@bzu.edu.pk
0030-9923/2023/0001-385 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Many aphid species are dengerous pest of different agricultural crops including wheat (Triticum aestivum L.), mustard (Brassica campestris L.) and other crops (Singh et al., 1988; Arora, 1999; Oerke, 2006). Research studies have indicated that biocontrol agents are effective against aphids (Tassan et al., 1979; Bugg et al., 2008). Green lacewing Chrysoperla carnea S. (Neuroptera; Chrysopidae) is a cosmopolitan polyphagus predator (Nordlund et al., 1991; Singh and Manoj, 2000; Atlihan et al., 2004) and a key predator for the management of various insect pests in agriculture system (Mulligan et al., 2010). The larval stages of C. carnea are voracious feeder and they can eat more than 487 aphids before pupation (Afzal, 1978). High searching capability of C. carnea in prey finding is a good adoption in defferent environment, while wide range of prey and resistance to mostly applied insecticides, makes it an important natural control agent (Sablon et al., 2013). The relationship between density of prey and consumption of predator is one of the main fundamental characteristic of predator prey interaction, this is termed as numerical and functional response (Holling, 1961). Functional response specifically is termed as a key element in regulation dynamics of a population in predator prey system. This is also described as the rate of killing of a predator to its prey at different given densities (Khan and Mir, 2008). Attack rate (a) and handling time (Th), time spent to dominate, eat and digest prey are the important components of functional responses (Hassell et al., 1976). Therefore, the studies on functional response are important to understand the underline mechanism of predator prey interaction, to explain the practicle role in coevolutionary realtionships and to contribute in bio-control (Houck and Strauss, 1985).
The objective of this study was to determine predatory potential of C. carnea on L. erysimi and D. noxia by functional response. Little work has been done on predatory efficiency of C. carnea on L. erysimi and D. noxia, as these are serious pests of wheat and mustard. There is a dire need to explore more predatory efficiency of C. carnea as a bio-control tool against these aphid species through fucntional response estimation.
Materials and methods
The adults of C. carnea were collected in start of January to end of February, 2019 from Bahauddin Zakariya University fields of B. campestris L. and T. aestivum L. The adults were collected in plastic jars (15×15×25 cm) with the help of aerial net and transferred to a plastic cage (23×38×38 cm) with ventilation holes on both sides. The adults were reared and maintained under laboratory conditions (25±2˚C and 65±5% R.H.) with photoperiod L12:D12 h. Artificial diet (yeast honey and distilled water with the ratio of 1:2:4 ml, respectively) was provided once a day as a food for C. carnea adults on plastic sticks having pores. Black glossy paper was hung along sealing of cage for eggs laying. Next day eggs were removed and collected separately with the help of forceps from cage and kept in 6cm plastic petri dishes until hatching. Moist filter papers were placed at the bottom of the Petri dish to maintain humidity level.
Aphids were collected from infested fields of Triticum aestivum L. and Brassica campestris L. present at University.
Functional response of all larval stages of C. carnea on L. erysimi and D. noxia was evaluated by providing different densities (10, 20, 30, 40, and 50) of aphids. Each starved predatory stage was transferred to 6cm plastic petri dishes having a specific density of both aphid species. Dead or alive aphids were removed from petri dishes (6cm) and provide newly collected aphids on daily basis. Second and third instars of C. Carnea were provided with 3rd instar aphids to know the precise predatory potential. Whole experiment was consisted on 5 treatments and each treatment included 10 replications for each predatory stage. Lastly aphids consumed were counted after 24h of treatment applied.
All data were analyzed by using a statistical software R v.3.5.3 (Team, 2019). Functional response was calculated by using frair_test present in an integrated FRAIR package in statistical R software. Determining shape of functional response clearly predicts that either it is type II functional response or type III (Trexler et al., 1989). The functional response shape was determined through a logistic regression model (Juliano, 2001). Following is an equation having polynomial function (Equation 1) describes the relationship between the prey number consumed (Ne/No) and the prey number provided (No):
Coefficient of above equation are Po= intercept P1= linear P2= quadratic and P3= cubic. A maximum likelihood method is used to estimate these coefficients. Confirmation of type III response obtains when P1 > 0 and P2 < 0 which shows prey consumed is positively density dependent. Whereas if P1 < 0, this shows prey consumed is negatively density dependent and it confirms a type II response (Juliano, 2001). FRAIR package include ‘frair_responses’ this function have different responses from which rogers II was used for functional response parameters calculation (Pritchard et al., 2017). Preforming above analysis resulted in type II response to our data. So further parameters of functional response were estimated through using (Equation 2) Roger’s random predator equation (Rogers, 1972):
Here initial prey density is No and prey number eaten is Ne where T is for experimental time (in hours or days) a is for instant capture rate of consumer per unit area or volume per unit time; time spent to consume prey is denoted by h and its unit is same as T (Jeschke et al., 2002; Sentis et al., 2013) and q is a scaling exponent that defines to which extent the functional response will change from a decelerating hyperbola (Type II: q = 0) to a sigmoidal form (Type III: q > 0). As Roger’s random predator equation has solved the issue of prey depletion so to calculate attack rate (a) and handling time (Th) data was fitted to this equation.
Results
Maximum likelihood estimate data by using logistic regression showed type II functional response for all the larval predatory stages of C. carnea on both the aphid species (Table I). Results showed that consumption rate of all the larval stages of C. carnea significantly increased by increase in prey densities. Attack rate (a) of first second and third instar recorded for L. erysimi and D. noxia was 0.16h, 0.43h, 1.56h, and 0.20h, 0.53h, 0.72h, respectively (Table II). Similarly, handling time (Th) recorded was 0.12h, 0.057h, 0.029h, and 0.17h, 0.050h, 0.015h, respectively (Table II). When different densities (10, 20, 30, 40, and 50) of L. erysimi were given to 1st instar of C. carnea the mean consumption rates were 1.8, 3.4, 5.9, 7.3, and 9, respectively, and for 2nd instar it was 4.2, 7.3, 12.1, 12.8, and 13.1, respectively, while for 3rd instar it was 9, 18.7, 22, 23.9, and 38.2. Similarly, when the same densities of D. noxia was provided to 1st instar the mean consumption rate was 1.7 3.8, 4.1, 4.7, and 8, respectively, and for 2nd instar it was 4.8, 9.2, 14.3, 14.7, and 16.7, respectively, while for 3rd instar it was 7.3, 11.8, 20, 24.1, and 29.6, respectively. The functional response-curves for all the instars of C. carnea to the various prey densities are presented in Figure 1, showing increase in prey consumption by larvae with increase in aphid densities.
Table I. Maximum likelihood estimates from logistic regression (frair) of the proportion of L. erysimi and D. noxia eaten by three predatory stages of C. carnea.
|
Aphid species |
Predator stage |
Estimate |
S.E. |
z value |
Pr (>|z|) |
|
Lipaphis erysimi |
First instar |
-0.0180471 |
0.0053457 |
-3.476 |
0.0007354 *** |
|
Second instar |
-0.0171718 |
-3.9646 |
7.353e-05 *** |
||
|
Third instar |
-0.0263583 |
0.0051237 |
-5.1444 |
2.684e-07 *** |
|
|
Diuraphis noxia |
First instar |
-0.015553 |
0.009782 |
-2.6016 |
0.009278 ** |
|
Second instar |
-0.0187132 |
0.0042358 |
-4.4179 |
9.967e-06 *** |
|
|
Third instar |
-0.0091162 |
0.0043117 |
-2.1143 |
0.03449 * |
Note: Significance codes: ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05
Table II. Estimated attack rate (a) and handling time (Th) of C. carnea different predatory stages on L. erysimi and D. noxia.
|
Aphid species |
Predator stage |
Coefficient |
Estimate |
S.E. |
z value |
Pr (z) |
|
Lipaphis erysimi |
First instar |
a |
0.194726 |
0.041438 |
4.6992 |
2.612e-06*** |
|
Th |
0.118579 |
0.035953 |
3.2981 |
0.0009733*** |
||
|
Second Instar |
a |
0.429184 |
0.066317 |
6.4717 |
9.692e-11*** |
|
|
Th |
0.056637 |
0.012983 |
4.3624 |
1.287e-05*** |
||
|
Third Instar |
a |
1.553613 |
0.01829358 |
8.4927 |
˂2.2e-16*** |
|
|
|
Th |
0.0287669 |
0.0039117 |
7.354 |
1.924e-13*** |
|
|
Diuraphis noxia |
First instar |
a |
0.155205 |
0.044099 |
3.5194 |
0.0004325*** |
|
Th |
0.173346 |
0.060669 |
2.8572 |
0.0042734** |
||
|
Second Instar |
a |
0.526254 |
0.074198 |
7.0925 |
1.317e-12*** |
|
|
Th |
0.049791 |
0.010032 |
4.9632 |
6.933e-07*** |
||
|
Third Instar |
a |
0.7188259 |
0.0774432 |
9.282 |
˂2e-16*** |
|
|
|
Th |
0.0144063 |
0.0061845 |
3 |
0.01984* |
Note: Significance codes: ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05
Discussion
All the larval instars of C. carnea have shown linear parameter (density) as negative. The prey number consumed increased by increasing prey density which predicts a type II functional response. The present results are in agreement with the studies where functional response of type II was calculated when aphids were predated by ladybird beetle (Kabissa et al., 1996; Mushtaq and Khan, 2010; Starn and Whitford, 1987; Zada et al., 2016). In our result all larval instars of C. carnea showed good predation potential against L. erysimi and D. noxia but third instars larvae of C. carnea were more predacious on these two aphid prey species. Previous studies also proved that the last larval instar of C. carnea have higher predation as compared to early instars (Atlıhan et al., 2004; El-Gawad et al., 2010). Higher predation of third instar larvae of C. carnea is a valid expression because of its large size and resulting higher rapacity. The reason of this higher predation might be due to fixed time starvation given in the begging of trial. Moreover, the age factor of larvae of C. carnea plays a vital role in increasing the movement speed (Zada et al., 2016).
Attack rate was observed higher of third instar of C. carnea on both the aphid species followed by second and first instar (Fig. 1). Less handling time was observed in respective manner as third instar having high attack rate and lesser handling time but in case of L. erysimi least handling time observed in first instar rather than third instar. In general handling time was more for first and second instar as compared to third instar this is because of high consumption rate of aphids this might also be because of higher levels of hunger power of digestive system and speeds of searching similar results were observed by C. carnea on different aphid species (Mushtaq and Khan, 2010).
Conclusion
Type II functional response is established in the presence of L. erysimi and D. noxia by all three larval stages of C. carnea. Third instar on both aphid species is more voracious feeder and higher attack rate with less handling time than second and first instars. The most preferable host for C. carnea larvae is L. erysimi as compared to D. noxia aphid specie. The results of this study will provide functional response information of C. carnea to scientific society in designing a bio-control tool for aphid pests.
Acknowledgements
The authors wish to acknowledge the Department of Entomology, Central Cotton Research Institute for coordinating in the present research work and undergraduate students for helping in experiments.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Afzal, M., 1978. Life history and feeding behavior of green lacewing Chrysopa carnea Steph (Neuroptera: Chrysopidae). Pakistan J. Zool., 10: 83-90.
Arora, R., 1999. Major insect pest of rapessed-mustard and their management. In: Practical guide series (eds. R.K. Upadhyay, K.G. Mukerji and R.L. Rajak). vol 5. Aditya books, New Delhi, pp. 35–75.
Atlıhan, R., Kaydan, B. and Özgökçe, M., 2004. Feeding activity and life history characteristics of the generalist predator, Chrysoperla carnea (Neuroptera: Chrysopidae) at different prey densities. J. Pest. Sci., 77: 17-21. https://doi.org/10.1007/s10340-003-0021-6
Bugg, R.L., Colfer, R.G., Chaney, W.E., Smith, H.A. and Cannon, J., 2008. Flower flies (Syrphidae) and other biological control agents for aphids in vegetable crops. ANR Publication 8285. pp. 1–25.
El-Gawad, H.A., Sayed, A. and Ahmed, S., 2010. Functional response of Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae) larvae to Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae) eggs. Aust. J. Basic appl. Sci., 4: 2182-2187.
Hassell, M., Lawton, J. and Beddington, J., 1976. The components of arthropod predation: I. The prey death-rate. J. Anim. Ecol., 23: 135-164. https://doi.org/10.2307/3772
Holling, C., 1961. Principles of insect predation. Annu. Rev. Ent., 6: 163-182. https://doi.org/10.1146/annurev.en.06.010161.001115
Holling, C.S., 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol., 91: 385-398. https://doi.org/10.4039/Ent91385-7
Houck, M.A. and Strauss, R.E., 1985. The comparative study of functional responses: Experimental design and statistical interpretation. Can. Entomol., 117: 617-629. https://doi.org/10.4039/Ent117617-5
Jeschke, J.M., Kopp, M. and Tollrian, R., 2002. Predator functional responses: Discriminating between handling and digesting prey. Ecol. Monogr., 72: 95-112. https://doi.org/10.1890/0012-9615(2002)072[0095:PFRDBH]2.0.CO;2
Juliano, S.A., 2001. Non-linear curve fitting: predation and functional response curve. Design Ecol. Exp., 26: 178-196. https://doi.org/10.1002/col.1015
Kabissa, J., Yarro, J., Kayumbo, H. and Juliano, S., 1996. Functional responses of two chrysopid predators feeding on Helicoverpa armigera (lep.: noctuidae) and Aphis gossypii (Homoptera: Aphididae). Entomophaga, 41: 141-151. https://doi.org/10.1007/BF02764242
Khan, A. and Mir, R., 2008. Functional response of four predaceous coccinellids, Adalia tetraspilota (Hope), Coccinella septempunctata L., Calvia punctata (Mulsant) and Hippodamia variegata (Goeze) feeding on the green apple aphid, Aphis pomi De Geer (Homoptera: Aphididae). J. biol. Contr., 22: 291-298 https://doi.org/10.1177/0748730407303387.
Mulligan, E.A., Ferry, N., Jouanin, L., Romeis, J. and Gatehouse, A.M., 2010. Characterisation of adult green lacewing (Chrysoperla carnea) digestive physiology: impact of a cysteine protease inhibitor and a synthetic pyrethroid. Pest Manage. Sci., 66: 325-336. https://doi.org/10.1002/ps.1879
Mushtaq, T. and Khan, A.A., 2010. Functional and aggregational response of Chrysoperla sp. (carnea-group) (Neuroptera: Chrysopidae) on Brevicoryne brassicae (Linnaeus) (Hemiptera: Aphididae). J. biol. Contr., 24: 28-34.
Nordlund, D.A., Vacek, D.C. and Ferro, D.N., 1991. Predation of Colorado potato beetle (Coleoptera: Chrysomelidae) eggs and larvae by Chrysoperla rufilabris (Neuroptera: Chrysopidae) larvae in the laboratory and field cages. J. entomol. Sci., 26: 443-449. https://doi.org/10.18474/0749-8004-26.4.443
Obrycki, J., Hamid, M., Sajap, A. and Lewis, L., 1989. Suitability of corn insect pests for development and survival of Chrysoperla carnea and Chrysopa oculata (Neuroptera: Chrysopidae). Environ. Ent., 18: 1126-1130. https://doi.org/10.1093/ee/18.6.1126
Oerke, E.C., 2006. Crop losses to pests. J. agric. Sci., 144: 31–43. https://doi.org/10.1017/S0021859605005708
Pritchard, D.W., Paterson, R.A., Bovy, H.C. and Barrios-O’Neill, D., 2017. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol., 8: 1528-1534. https://doi.org/10.1111/2041-210X.12784
Rogers, D., 1972. Random search and insect population models. J. Anim. Ecol., 369-383.
Sablon, L., Haubruge, E. and Verheggen, F., 2013. Consumption of immature stages of Colorado potato beetle by Chrysoperla carnea (Neuroptera: Chrysopidae) larvae in the laboratory. Am. J. Potato Res., 90: 51-57. https://doi.org/10.1007/s12230-012-9275-y
Sentis, A., Hemptinne, J.L. and Brodeur, J., 2013. Parsing handling time into its components: implications for responses to a temperature gradient. Ecology, 94: 1675-1680. https://doi.org/10.1890/12-2107.1
Singh, H., Singh, Z. and Yadava, T., 1988. Post harvest losses in rapeseed caused by aphid pests. 7th International Rapeseed Congress/convened under the patronage of Stanislaw Zieba; by the Plant Breeding and Acclimatization Institute under the auspices of the Group Consultatif International de Recherche sur le Colza. Poznan: Panstwowe Wydawnictwo Rolnicze i Lesne.
Singh, N. and Manoj, K., 2000. Potentiality of Chrysoperla carnea (Stephens) in suppression of mustard aphid population. Indian J. Ent., 62: 323-326.
Starn, S. and Whitford, F., 1987. Functional response of Chrysopa carnea (Neuroptera: Chrysopidae) larvae feeding on Heliothis virescens (Lepidoptera: Noctuidae) eggs on cotton in field cages. Entomophaga, 12: 227-231.
Tassan, R., Hagen, K. and Sawall, Jr.E., 1979. The influence of field food sprays on the egg production rate of Chrysopa carnea. Environ. Ent., 8: 81-85. https://doi.org/10.1093/ee/8.1.81
Team, R.C., 2019. R Foundation for statistical computing; Vienna, Austria: 2014. R: A language and environment for statistical computing.
Trexler, J.C., McCulloch, C.E. and Travis, J., 1989. How can the functional response best be determined? Oecologia, 78: 571-571. https://doi.org/10.1007/BF00378751
Zada, H., Ahmad, B., Nadeem, M., Huma, Z. and Salim, M., 2016. Functional response of Chrysoperla carnea Stephen (Neuroptera: Chrysopidae) fed on cabbage aphid, Brevicoryne brassicae (Linnaeus) under laboratory conditions. Pakistan J. Zool., 48: 165-169.
To share on other social networks, click on any share button. What are these?