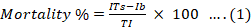Exploring Enhanced Insecticidal Activity of Mycelial Extract of Trichoderma harzianum against Diuraphis noxia and Tribolium castaneum
Exploring Enhanced Insecticidal Activity of Mycelial Extract of Trichoderma harzianum against Diuraphis noxia and Tribolium castaneum
Sadaf Rahim and Mudassar Iqbal*
Department of Agricultural Chemistry, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Fungi are important organisms that produce potent secondary metabolites with antimicrobial, herbicidal, insecticidal and other such beneficial activities. Production of such compounds can be increased or decreased depending on growth conditions. For these reasons a Trichoderma harzianum was selected for its growth optimization conditions and extraction of insecticidal compounds effective against two major insects pests including Diuraphis noxia and Tribolium castaneum. The pure fungal strain of Trichoderma was grown using two different nutrient media including Glucose Peptone Yeast Broth (GPYB) and Potato Dextrose Broth (PDB) and the effect of organic extract was investigated against selected insects’ pest. From this study it was found that the mycelial extract of T. harzianum fermented on PDB showed enhanced insecticidal mortality (73% and 76%) against Diuraphis noxia and Tribolium castaneum respectively while the extract obtained from GPYB media showed merely 46% and 56% mortality against Diuraphis noxia and Tribolium castaneum respectively at highest concentration after 2 hours exposure. Increased potency of extracts was observed with the increased exposure time. The LC50 value calculated for organic extract obtained from T. harzianum grown on PDB against Diuraphis noxia was 398.69 μg mL-1 after 3 hours with increased potency (237.46 μg mL-1) after 24 hours, whereas against Tribolium castaneum it was 213.85 μgmL-1 after 24 hours while its decreased potential was recorded (1153.5 μg mL-1) after 3 hours. Similar pattern of toxicity level was observed for organic extract exposure of T. harzianum grown on GPYB viz increasing exposure time increased the mortality rate, The LC50 value against Diuraphis noxia was reduced to 1083.49 μg mL-1 after 24 hours from 2269.08 μg mL-1 after 3 hours, whereas against Tribolium castaneum it reduced significantly from 1849.69 μg mL-1 after 3 hours to 677.21 μg mL-1 after 24 hours. From the results it is concluded that T. harzianum grown on PDB produced potent secondary metabolites that could be used for the development of target specific insecticides against Diuraphis noxia and Tribolium castaneum. Further studied towards the isolation and structural elucidation of lead compounds is recommended for the development of local agro-chemical product as environmental friendly biodegradable organic insecticides.
Received | March 21, 2019; Accepted | May 22, 2019; Published | July 09, 2019
*Correspondence | Mudassar Iqbal, Department of Agricultural Chemistry, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Rahim, S. and M. Iqbal. 2019. Exploring enhanced insecticidal activity of mycelial extract of Trichoderma harzianum against Diuraphis noxia and Tribolium castaneum. Sarhad Journal of Agriculture, 35(3): 757-762.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.757.762
Keywords | Fungal extract based insecticides, Diuraphis noxia, Tribolium castaneum, Trichoderma harzianum, Biological insecticides
Introduction
Agricultural productivity is adversely affected by insects’ pathogen as they damage both standing crops as well as stored commodities. These insect pests can be controlled by various ways, among them use of insecticides is very common. These insecticides generally contain an active ingredient along with a carrier substance, the active ingredient can be from chemical or/and biological source. Chemically synthesized pesticides do have some advantage but possess serious threat to environment and can have serious side effects as well, pesticides like Aldrin and Azodrin have been banned and their application limit have reduced in most countries. For this reason, a work towards the development of natural products based agrochemicals is needed. The advantage of naturally occurring bioactive metabolites over synthetic molecules is that they are environmental friendly and least harmful to human health. Natural products or bioactive secondary metabolites are considered as most effective ingredients of potent drugs. These molecules could be obtained from various sources including, plants animals and microbes.
Fungi are diverse group of organisms found in both terrestrial and aquatic environment and can produce variety of bioactive metabolites (Iqbal et al., 2014). Metabolites from microbes are host specific and having broad range of activity (Berdy, 2005). The derivatives of secondary metabolites are used as pesticides having antiparasitic, antihelminthic agents, antifeedant, insecticides, nematocides, cestocides, antiworm compounds, acaricides, phytotoxins, phytohormones (Cole and Rolinson, 1972), plant growth regulators, chlorosis inducers, algicide, germination inhibitors, and as biocontrol agents (El-Hasan et al., 2009). The most useful microbial compounds are monensin, bialaphos and avermectin isolated from different species of Streptomyces. Commercially various agricultural products are available which are produced from microorganisms like Beauveria speices, Bacillus thuringiensis, Metarhizium species, Baculovirus, Trichoderma species and Verticillium species (Dayan et al., 2009).
Trichoderma spp are free living fungi found in root, soil as well as in foliar environments. It has numerous antibiotic producing and bio-controlling characteristics (Reino et al., 2008a). The isolates of Trichoderma are good source of antibiotics having diverse effects against various pathogenic microbes (Chruma et al., 2009). The azaphilone and butenolide isolated from Trichoderma harzianum were reported with potent antifungal activities against different yeast (Reino et al., 2008b). Its use as a biocontrol agent was proposed against soil borne fungal and other bacterial pathogens along with its use as a potent bio-pesticide (Osmanova et al., 2010).
The biosynthesis of secondary metabolites depends primarily on growth conditions. This current study was designed to identify cost effective growth medium for the biosynthesis of insecticidal secondary metabolites of Trichoderma harzianum effective against Diuraphis noxia and Tribolium castaneum.
Materials and Methods
Collection of soil samples
Surface level soil samples (from up to a 6 cm soil depth) were collected randomly from Peshawar, Khyber Pakhtunkhwa using standard protocol (Rohilla and Salar, 2012). The collected samples were packed in a sterilized polyethylene bag, labelled properly and stored at 4 °C further use (Gaddeyya et al., 2012).
Isolation of Trichoderma harzianum from soil sample
The separation of fungal species from soil samples were carried out using standard serial dilution method under aseptic conditions. The seed suspension was prepared by adding 1 g of soil sample in 10 mL potato dextrose broth (PDB) and the solution was further diluted to 1 × 10-4 concentration suspension. To avoid bacterial infection Streptomycin sulfate (1 μg mL-1) was added to each test tube and incubated at 28 °C for 05 days. The obtained fungal species were transferred to the petri dishes containing pre-sterilized potato dextrose agar media (PDA) and streptomycin sulfate (1 μg mL-1) and incubated at 30 °C in dark for 7 days. (Gupte et al., 2002) (Atalla et al., 2008). The obtained fungal biomass contained variety of fungal species which was further purified by transferring and growth a colony of each fungi using repeated growth on potato dextrose agar (PDA) as described above till the growth of one specie per petri dish was achieved. The fungal strains were identified by observing it under binocular microscopic through microscopic characteristics (color, texture appearance and diameter of colonies) (Rohilla and Salar, 2012). The Trichoderma harzianum selected for growth, extraction of bioactive metabolites and their insecticidal activities against Diuraphis noxia and Tribolium castaneum was further purified and identified by mycologist at department of plant pathology, The University of Agriculture-Peshawar.
Growth and extraction of fungal biomass
Two different media i.e. potato dextrose broth (PDB) and glucose peptone yeast broth (GPYB) were used for the fermentation of Trichoderma harzianum (Iqbal et al., 2018). The biomass of Trichoderma harzianum obtained from both PDA and GPYB was extracted separately by following the procedure described by (Begum et al., 2018). The fermented biomass of Trichoderma harzianum was separated from the broth through filtration, washed with distilled water and 10 g was transferred to conical flasks containing 100 mL ethyl acetate (EtOAc). The fungal biomass was homogenized through blender and kept on magnetic stirrer for 24 hours for maximum extraction of biomolecules. The fungal cells were separated via filtration, 50 mL distilled water was added to liquid fraction and transferred to separating funnel. Both aqueous and organic fractions were separated and the aqueous fraction was re-extracted with EtOAc (100 × 3 mL). The combined organic fractions were dried over anhydrous MgSO4, filtered and concentrated under reduced pressured to obtain brown oil as crude extract. The extract was stored at 4 °C in glass vial for further use.
Insecticidal assay
Organic extract of Trichoderma harzianum obtained from both media was separately screened for insecticidal activity (Noshad et al., 2015) against Diuraphis noxia and Tribolium castaneum . The stock solution (1000 µg mL-l) from crude of each extract was prepared (8 mg of crude per 8 mL of EtOAc) and further diluted to 500 µg mL-1 and 250 µg mL-1 concentration. Each concentration (3 mL) was added to clean petri dish containing a filter paper and solvent was allowed to evaporate at room temperature. Ten insects were transferred in each petri dish along with their normal feed (fresh wheat leaves for Diuraphis noxia and grounded wheat flour for Tribolium castaneum). The experiments were performed in triplicate and the mortality of insects was recorded at 3 hr and 24 hr interval and the data was compared with both positive control (Malathion 1000 μg mL-1) and negative control (Blank, 0 μg mL-1) experiment. The LC50 was calculated by probit analysis (Finney and Stevens, 1948) and percent mortality was calculated using following formula (Equation 1) (Begum et al., 2018).
Where;
ITs= Killing of insects by test solution; Ib= Killing of insects by blank; Ti= Total insects.
Statistical analysis
The data collected during the experimental analysis was analyzed using Statistix 8.1® and the result is presented as mean of triplicate. Statistical descriptive comparison of more values for experiment were analyzed by CRD design and ANOVA followed by LSD at 5% level of significance (Steel et al., 1997).
Results and Discussion
Effect of mycelial extract of Trichoderma harzianum against Diuraphis noxia
Aphids as a vector of plant viruses are considered serious insect pest of various crops including, wheat, tomato, pepper, cucumber etc all over the world. Indiscriminative use of insecticides have also caused resistance amoung various insects pests. Biological control of aphids using entomopathogenic fungi is an emerging strategy. Fungi produce important bio molecules that can be used for the development of bio-insecticides for the control of insect pests. The organic extract of T. harzianum grown on PDB media was tested against Diuraphis noxia in three different concentrations. Thre results clearly showes both time and concentration dependent effect on the mortality of aphids (Table 1), although the results were non-significant in comparison to positive control experiment (Melathion, 1000 µg mL-1) while significantly increased mortality (73% after 24 hours using 1000 μg mL-1 concentraiton of organic extract of T. harzianum grown on PDB meida) of Diuraphis noxia was recorded in comparison to blank experiment (3.3% after 24 hours). The effect of organic extract of T. harzianum fermented on GPYB media showed appreciate mortality against aphids (Table 1). In first 3 hours increased aphids’ mortality from 6.66% to 23% was recorded with the increase in concentration of organic extract from 250 µg mL-1 to 1000 μg mL-1. Similarly increase in exposure time of organic extract from 3 hours to 24 hours also showed increased mortality. The highest percent mortality (23.3%) was observed with 1000 µg. mL-1 crude extract after 3 hours, which significantly increased (46.6%) after 24 hours. The LC50 calculated after 24 hours (237.46 µg mL-1) clearly shows the high aphicidial potential of organic extract of Trichoderma harzianum (Table 1). The LC50 calculated (1083.49 µg mL-1) clearly showed that the organic extract have very least effect on aphids mortality after 24 hours, while as the mortality increased significantly (2269.05 µg mL-1) after three hours. From the results it is concluded
Table 1: Effect of organic extract of T. harzianum obtained from PDB and GPYB media against Diuraphis noxiavalues in parenthesis show in percentage.
| Concentrations (µg mL-1) | PDB* | GPYB * | |||||||
| After 3hr | After 24hr | After 3hr | After 24hr | ||||||
| Negative ** | 0.33f (3.33%) | 0.33f (3.33%) | 0.33f (3.33%) | 0.33f (3.33%) | |||||
| 250 | 4.33d (43.3%) |
LC50 : 398.69 μg mL-1 |
5.00c (50%) |
LC50 : 237.46 μg mL-1 |
0.66f (6.66%) |
LC50 : 2269.08 μg mL-1 |
2.33e (23.3%) |
LC50 : 1083.49 μg mL-1 |
|
| 500 | 5.33c (53.3%) | 6.33b (63.3%) | 1.66e (16.6%) | 3.66d (36.6%) | |||||
| 1000 | 6.66b (66.6%) | 7.33b (73.3%) | 2.33e (23.3%) | 4.66c (46.6%) | |||||
| Positive *** | 6.66b (66.6%) | 9.66a (96.6%) | 7.66b (76.6%) | 9.66a (96.6%) | |||||
* Media used for the growth of T. harzianum; ** Blank experiment was run same as all except use of organic extract of T. harzianum; *** A known insecticide Malathion (1000 μg mL-1) was used in positive control experiment; Values with different letters are significantly different (P<0.05).
Table 2: Effect of organic extract of T. harzianum obtained from PDB and GPYB media against Tribolium castaneum values in parenthesis show in percentage.
| Concentrations (µg mL-1) | PDB* | GPYB* | ||||||
| After 3hr | After 24hr | After 3hr | After 24hr | |||||
| Negative** | 0.00h (0%) | 0.00h (0%) | 0.00h (0%) | 0.00h (0%) | ||||
| 250 | 2.33g (23.3%) |
LC50 : 1153.5 μg mL-1 |
5.33d (53.3%) |
LC50 : 213.85 μg mL-1 |
1.66g (16.6%) |
LC50 : 1849.69 μg mL-1 |
3.33e (33.3%) |
LC50 : 677.21 μg mL-1 |
| 500 | 3.33f (33.3%) | 6.00c (60%) | 2.33f (23.3%) | 4.66d (46.6%) | ||||
| 1000 | 4.66e (46.6%) | 7.66b (76.6%) | 3.66e (36.6%) | 5.66c (56.6%) | ||||
| Positive*** | 7.66b (76.6%) | 9.66a (96.6%) | 7.66b (76.6%) | 9.66a (96.6%) | ||||
* Media used for the growth of T. harzianum; ** Blank experiment was run same as all except use of organic extract of T. harzianum; *** A known insecticide Malathion (1000 μg mL-1) was used in positive control experiment; Values with different letters are significantly different (P<0.05).
that the organic extract of T. harzianum fermented on GPYB media possess statistically less aphidicidal potential as compare to T. harzianum grown on PDB media. This is due to the production of more potent bioactive compounds when T. harzianum was fermented on PDB. The carbon source available in nutrient media is responsible for the bio-synthesis of secondary metabolites having increases or decreased biological activities. The use of soil borne fungus Beauveria bassiana Conidia is reported for its effective use to control aphids (Mingguang and Shenghua, 2003). Similarly, Feng has discribed the formulation of various fungi including Omuraea rileyi, Paecilomyces fumosoroseus, and Verticillium lecanii for the control of insects’ pests (Dun et al., 2003). Copping and Duke have reported the efficient use mycelium of Myrothecium verrucaria as a potent namaticide highly effective against the plant parasitic nematodes residing the field of tobacco, grapes, citrus etc (Copping and Duke, 2007).
Effect of mycelial extract of Trichoderma harzianum against Tribolium castaneum
Many of the existing insecticides and other pesticides were originated initially from various biological sources. Even now natural products have been considered as an important source for the development of new agrochemicals. Recently Sparks et al have reported the importance of fungal natural products towards the discovery and formulation of new insecticidal agrochemicals (Sparks et al., 2017). The effect of crude extract of T. harzianum mycelia against red flour beetle (Tribolium castaneum) is presented in Table 2. Results showed that the mortality rate of red flour beetle by fungal extract (all tested concentrations) had shown significantly increased mortality as compare to negative control experiment. The ethyl acetate extract of T. harzianum obtained from the mycelia cells cultured on PDB media showed overall improved insecticidal activity against Tribolium castaneum in comparison to mycelia cells cultured on GPYB media (Table 2). The mortality of Tribolium castaneum significantly increased with the increase in concentration (from 250 μg mL-1 to 1000 μg mL-1) and exposure time (3 hours to 24 hours). The organic extract of T. harzianum grown on GPYB culture media showed 56.6% mortality of Tribolium castaneum (at 1000 μg mL-1 concentration) whereas significantly improved mortality (76.6%) was recorded for mycelium extract of T. harzianum fermented using PDB media. The LC50 values clearly shows that the insecticidal effect of extract obtained from T. harzianum grown on PDB media increased from 1153.5 µg mL-1 after 3 hours to 213.85 µg mL-1 after 24 hours which suggested that the bioactive molecules present in the extract possess appreciable activity against red flour beetle. In comparison the LC50 efficacy (Table 2) of organic extract of T. harzianum grown on GPYB media was 3 fold less (677.21 µg mL-1) at highest tested concentration after 24 hours of exposure. Two fungal strains i.e. Beauveria bassiana and Verticillium were reported for the mortality of red flour beetles, where Beauveria bassiana showed 61.67% mortality through infection via adhesion, spore germination and mycelium colonization in cadavers of Tribolium castaneum (Hanan, 2017). Another study shows the significant effect of B. bassiana isolate 22292A against three stored grain pests including red flour beetles (Cogburn and Rice, 1999) where up to 100% mortality of pests was achieved.
Conclusions and Recommendations
Secondary metabolites are bio-molecules with significant importance in routine life, these compounds are produced by the living organisms such as plants, animals and microbes. The exploration of biologically potent secondary metabolites where fungi is known to produce more potent compounds. These bioactive molecules can be used for the development of various pharmaceuticals as well as Agro chemicals. From present study it is concluded that the biosynthesis of insecticidal molecules by T. harzianum depends on growth media. The organic extract of T. harzianum grown on potato dextrose broth in comparison to glucose peptone yeast broth showed improved insecticidal activities against both Diuraphis noxia and Tribolium castaneum. The organic extract of T. harzianum might contain more potent biomolecule as a good candidate towards the development of environmental friendly insecticides for the control of both Diuraphis noxia and Tribolium castaneum. To our knowledge this is the initial report for the control of Diuraphis noxia and Tribolium castaneum using organic extract of T. harzianum. Further studies on the isolation of targeted compound(s) is needed towards the development of bio-based insecticides.
Acknowledgements
The Authors acknowledge the Higher Education Commission (HEC), Pakistan through indigenous PhD program and The Directorate of Science and Technology (DoST), Govt. of Khyber Pakhtunkhwa, for provision of funding for this project. This work is a part of PhD desertion submitted to the University of Agriculture, Peshawar.
Novelty Statement
The use of biological extract towards the control of various pathogens is the need of the day. To our knowledge this is the first report where the organic extract of Trichoderma harzianum is used for the control of apids and red flour beetles.
Author’s Contribution
Mudassar Iqbal: Principal author, conceive the idea, planned and supervised the research.
Sadaf Rahim Awan: Researcher, conducted the research, collected the data.
References
Atalla, M.M., H.K. Zeinab, R.H. Eman, A.Y. Amani and A. Abeer. 2008. Production of some biologically active secondary metabolites from marine-derived fungus varicosporina ramulosa. Malays. J. Microbiol. 4: 14-24. https://doi.org/10.21161/mjm.01608
Begum, S., M. Iqbal, Z. Iqbal, H.U. Shah and M. Numan. 2018. Assessment of mycelia extract from Trichoderma harzianum for its antifungal, insecticidal and phytotoxic importance. J. Plant Biochem. Physiol. 6. https://doi.org/10.4172/2329-9029.1000209
Berdy, J. 2005. Bioactive microbial metabolites. J. Antibiot. 58: 1. https://doi.org/10.1038/ja.2005.1
Chruma, J., S.J. Moon, Sanford and E. William. 2009. Azaphilone î±-bromoacetates (azî±bs): Fluorescent linchpin reagents for the inter-and intramolecular cross-linkage of primary amines to thiols. Lett. Org. Chem. 6: 367-371. https://doi.org/10.2174/157017809788681428
Cogburn, R.R. and W.C. Rice. 1999. Activity of the entomopathogenic fungus beauveria bassiana (deuteromycota: Hyphomycetes) against three coleopteran pests of stored grain. J. Econ. Entomol. 92: 691-694. https://doi.org/10.1093/jee/92.3.691
Cole, M. and G.N. Rolinson. 1972. Microbial metabolites with insecticidal properties. Appl. Environ. Microbiol. 24: 660-662.
Copping, L.G. and S.O. Duke. 2007. Natural products that have been used commercially as crop protection agents. Pest Manage. Sci. 63: 524-554. https://doi.org/10.1002/ps.1378
Dayan, F.E., C.L. Cantrell and S.O. Duke. 2009. Natural products in crop protection. Bioorg. Med. Chem. 17: 4022-4034. https://doi.org/10.1016/j.bmc.2009.01.046
Dun, Y., M. Feng and S. Ying. 2003. Evaluation for enhanced aphidicidal activity of a noval emulsifiable formulation of beauveria bassiana conidia. Wei sheng wu xue bao= Acta Microbiol. Sin. 43: 781-787.
El-Hasan, A., F. Walker, J. Schöne and H. Buchenauer. 2009. Detection of viridiofungin a and other antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 124: 457-470. https://doi.org/10.1007/s10658-009-9433-3
Finney, D.J. and W.L. Stevens. 1948. A table for the calculation of working probits and weights in probit analysis. Biometrika. 35: 191-201. https://doi.org/10.1093/biomet/35.1-2.191
Gaddeyya, G., P.S. Niharika, P. Bharathi and P.K.R. Kumar. 2012. Isolation and identification of soil mycoflora in different crop fields at salur mandal. Adv. Appl. Sci. Res. 3: 2020-2026.
Gupte, M., P. Kulkarni and B. Ganguli. 2002. Antifungal antibiotics. Appl. Microbiol. Biotechnol. 58: 46-57. https://doi.org/10.1007/s002530100822
Hanan, B.A. 2017. The electron microscopic examination of fungal distortions in the adult red flour beetle, Tribolium castaneum h.(coleoptera: Tenebrionidae). J. Entomol. Nematol. 9: 1-8. https://doi.org/10.5897/JEN2017.0175
Iqbal, M., M. Amin, Z. Iqbal, H. Bibi, A. Iqbal, Z. Din, M. Suleman and H.U. Shah. 2014. Antimicrobial, cytotoxic and phytotoxic potency of ethyl acetate extract of rhizopus stolonifer culture. Trop. J. Pharm. Res. 13: 87-92. https://doi.org/10.4314/tjpr.v13i1.13
Iqbal, M., R. Ullah, H. Bibi, Z. Iqbal, M. Numan, S.R. Awan, Z. Din, I. Khan and M.I. Khan. 2018. Mycelial extract of Acremonium sp.: A potent target specific bio herbecide against Echinochloa crus-galli and Asphodelus tenuifolius. Fresenius Environ. Bull. 27: 1778-1785.
Mingguang, D.Y.F. and Y. Shenghua. 2003. Evaluation for enhanced aphidicidal activity of a noval emulsifiable formulation of Beauveria bassiana conidia [j]. Acta Microbiol. Sin. 6.
Noshad, A., M. Iqbal, Z. Iqbal, H. Bibi, S. Bibi and H.U. Shah. 2015. Aphidicidal potential of ethyl acetate extract from Pleurotus ostreatus. Sarhad J. Agric. 31. 101-105. https://doi.org/10.17582/journal.sja/2015/31.2.101.105
Osmanova, N., W. Schultze and N. Ayoub. 2010. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 9: 315-342. https://doi.org/10.1007/s11101-010-9171-3
Reino, J.L., R.F. Guerrero, R. Hernández-Galán and I.G Collado. 2008a. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 7: 89-123. https://doi.org/10.1007/s11101-006-9032-2
Reino, J.L., F. Raul, Guerrero, R. Hernández-Galán and I.G. Collado. 2008b. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 7: 89-123. https://doi.org/10.1007/s11101-006-9032-2
Rohilla, S.K. and R.K. Salar. 2012. Isolation and characterization of various fungal strains from agricultural soil contaminated with pesticides. Res. J. Recent Sci. 2277: 2502.
Sparks, T.C., D.R. Hahn and N.V. Garizi. 2017. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manage. Sci. 73: 700-715. https://doi.org/10.1002/ps.4458
Steel, R.G.D., James H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A biological approach: McGraw-Hill. 73(4): 700-715.
To share on other social networks, click on any share button. What are these?