Evaluation of the Gastrointestinal Parasite Burden of Goats in Traditional Breeding in Benin
Research Article
Evaluation of the Gastrointestinal Parasite Burden of Goats in Traditional Breeding in Benin
Kétomon Pierre Challaton1*, Coovi Guénolé Akouedegni1, Kadoéito Cyrille Boko2, Goué Géorcelin Alowanou1,3, Pascal Venant Houndonougbo4, Aboudou Habirou Kifouly1, Mawulé Sylvie Hounzangbé-Adoté1
1Laboratoire d’Ethnopharmacologie et de Santé Animale, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, Cotonou, Bénin; 2Unité de Recherche sur les Maladies Transmissibles du Laboratoire de Recherches en Biologie Appliquée, Ecole Polytechnique d’Abomey-Calavi, Université d’Abomey-Calavi, Cotonou, Bénin; 3Laboratoire des Recherches Pluridisciplinaires de l’Enseignement Technique, Ecole Normale Supérieure de l’Enseignement Technique, Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques, Abomey, Bénin; 4Laboratoire de Recherches Avicoles et de Zoo Économie, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, Cotonou, Benin.
Abstract | Goat farming in Benin is traditional. In this system, animals harbor parasites that cause economic losses related to growth and reproduction performance, which strongly affect farm productivity. The objective of this study was to evaluate the gastrointestinal parasite burden of goats in Benin. Thus, feces were sampled from 572 and 497 goats in the rainy and dry seasons, respectively, in southern, central and northern Benin. The parasite inventory was performed using the Mini-FLOTAC technique for the quantitative study and the Baermann method for the qualitative research. The results showed an overall prevalence of gastrointestinal parasites of 96.82 %. Furthermore, goats were mainly infected with coccidia (92.24 %); strongyles (83.91 %), of which the critical genera were Haemonchus sp., Trichostrongylus sp., and Oesophagostomum spp. ; Strongyloides spp. (73.25 %) followed by Moniezia spp. (21.8 %). Other gastrointestinal parasites retrieved were Trichuris sp. (0.94%) and Toxocara sp. (0.28%). Infestation rates and the number of Eggs Per Gram of feces (EPG) or Oocysts Per Gram of feces (OPG) were higher in the wet season than in the dry season. During the wet season, infestations were severe for strongyles, Moniezia spp., coccidian, and moderate for Strongyloides spp. and light during the dry season except for coccidia, where they were intense. Infestation rates and egg excretion of gastrointestinal parasites were shaped by age, sex, breed, and study areas. This knowledge of gastrointestinal parasites will help guide for the surveillance of goat parasitosis in Benin.
Keywords | Benin, Breeding, Gastrointestinal parasites, Strongyles, Goats
Received | September 27, 2022; Accepted | February 25, 2023; Published | April 15, 2023
*Correspondence | Ketomon Pierre Challaton, Laboratoire d’Ethnopharmacologie et de Santé Animale, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, Cotonou, Bénin; Email: pierrechallaton@yahoo.fr
Citation | Challaton KP, Akouedegni CG, Boko KC, Alowanou GG, Houndonougbo PV, Kifouly AH, Hounzangbe-Adote MS (2023). Evaluation of the gastrointestinal parasite burden of goats in traditional breeding in benin. J. Anim. Health Prod. 11(2): 144-154.
DOI | http://dx.doi.org/10.17582/journal.jahp/2023/11.2.144.154
ISSN | 2308-2801

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In Benin, goat farming is practiced throughout the country, with a national population in 2019 estimated at 1,955,811 heads (FAOSTAT, 2021). Highly adapted to the different agro-ecological conditions of Benin, goats are an essential source of meat products for urban and peri-urban markets. They are present in the daily life of herders for savings, consumption, and ceremonies (Dossa et al., 2007). However, several constraints limit their productivity. Diseases in general, and more particularly parasitosis, are bottlenecks (Challaton et al., 2022). Thus, helminth diseases have been identified as limiting factors to expressing the productive potential (meat and milk) of goats (Wesongah et al., 2005). In tropical countries, gastrointestinal parasites are the most involved in these helminthiases of small ruminants (Githiori et al., 2004). Terefe et al. (2012) observed that the overwhelming majority of small ruminants in the tropics are infected with gastrointestinal parasites. In Benin, parasitological investigations on small ruminants revealed the presence of strongyle polyparasitism (Salifou, 1996). The prevalence rates of strongyles in small ruminants are high and vary between 4.4 and 92.6% (Salifou, 1996). A gastrointestinal (GI) parasite study carried out by Faihun et al. (2017) on small ruminants (sheep and goats) and a wild ruminant species (harnessed guib) revealed the presence of six parasites in sheep and goats (strongyles, Strongyloides, Coccidia, cestodes, Capillaria, and trematode) with rates ranging from 60 to 100%. Salifou (1996) carried out a study in South Benin more than 26 years ago, and the most recent one was carried out in the riparian camps at the Wari-Mar classified forest by Faihun et al. (2017) in North-Benin were not exhaustive. Moreover, the previous study did not highlight the strongyles genera involved in gastrointestinal parasitosis of small ruminants.
According to Challaton et al. (2022), the GI parasites treatment by farmers is based on synthetic chemical products. Waller (2006), has shown that the ever-increasing and uncontrolled usage of chemical products are the leading cause of anti-parasites resistant issues. This could increasingly lead to a high prevalence of gastrointestinal parasites in livestock.
In addition, climatic variables can affect the prevalence, intensity, and geographical distribution of helminths, directly influencing the free-living larval stages and indirectly influencing mainly the hosts (Mas-Coma et al., 2008). Since 1970s, Benin has experienced pronounced climatic variations (Tidjani and Akponikpé, 2012), affecting ecosystems with an increased frequency of new infectious diseases (Gnanglè et al., 2011). Hence, farmers could face a high prevalence of gastrointestinal parasites.
All this requires a new diagnosis by extending the studies to other country regions to identify the new species involved by focusing on goats to implement an effective and cost-efficient treatment plan in the farms. This diagnosis is the interest of this study which aims to evaluate the gastrointestinal parasite burden of goats in Benin.
Materials and methods
Study environment
The studies were conducted in Benin. With an area of 114763 km2, Benin is located in West Africa between latitudes 6°10’N and 12°25’N and longitudes 0°45’E and 3°55’E. The Atlantic Ocean borders Benin on the south, Togo on the west, Nigeria on the east, Niger on the northeast, and Burkina Faso on the northwest. Benin Republic has a tropical continental climate characterized by two seasons in the north (one rainy and one dry) and four seasons more or less marked in the south (two rainy and two dries). In terms of agriculture, the national territory in the current strategic context of development of its agricultural sector, is divided into seven Agricultural Development Poles (PDAs), which take into account the agroclimatic conditions and the potentialities of each region of the country. PDA 1 covers the Niger Valley: an area of rice cultivation, flood plains, and lowlands. PDA 2 covers Alibori (South), Borgou (North), and the 2KP (Kandi-Kouandé-Péhunco): this is the leading cotton area. The PDA 3 considers Atacora (West), Borgou South, and Donga: an agricultural diversification zone (cotton and food products are grown there). PDA 4 encompasses the mountain region (South Borgou, Donga, and mountain region) with cotton, food products, and cashew nuts as crops (diversification). Zou and Couffo represent PDA 5: a zone dedicated to food crops. PDA 6 is represented by the Plateau (specialized in agricultural diversification - oil palm and food products). PDA 7 is made up of Ouémé, Atlantique, and Mono: a fishing and market gardening zone.
Goat feces were collected in PDAs 1, 2, 4, and 5 (Figure 1) due to the concentration of livestock.
Coprological examinations were carried out at the Laboratory of Ethnopharmacology and Animal Health (LESA), located at the Faculty of Agronomic Sciences (FSA) of the University of Abomey-Calavi (UAC).
Sampling period
The first round of sampling was performed in the rainy season (August to October 2019) and a second one in the dry season (January to March 2020).
Choice of farms and animals
Nine (9) traditional farms were randomly selected in the communes of Malanville, Kandi, Gogounou, Kérou, Kouandé, Péhunco, Lalo, Klouékanmè, Zogbodomey Bohicon Djidja, Glazoué, Savè, Bantè, Parakou and N’Dali, divided into four PDAs (Figure 1). The animals roam freely during the day while sharing the natural range. At night, most of them are reared in straw or tin-roofed pens, some of which are adjacent to the rooms. These shelters are also used to serve food supplements such as cassava peelings, soybean bran, millet bran, maize bran, and kitchen waste.
Animals over two months of age are eligible and were selected randomly from the sampled farms regardless of their general condition. All animals were tested for farms with less than six heads of goats, and six goats were sampled for farms with more than six heads. The age, sex, and breed of the tested animals were recorded.
Fecal samples collection
For fecal collection, breeches were worn by the animals in night and were removed early the next day (Figure 2). The feces in the breeches were collected in bags and labeled. The samples were then transported under cold-cover (mini dry icebox) to the Laboratory of Ethnopharmacology and Animal Health of the Faculty of Agronomic Sciences where they were immediately analyzed, or kept in the refrigerator at +4°C for a maximum of two days to be diagnosed later. A total of 1069 fecal samples were taken from 572 and 497 goats, respectively, in the wet and dry seasons on 144 traditional farms per season. The difference in fecal samples is justified by the movement of animals within the farms during different seasons.
Quantitative coprology
Quantitative coprology was performed by the Mini-Flotac technique (Cringoli et al., 2010) following standard operating procedures. The flotation fluid was a saturated solution of Sodium chloride (NaCl) of density (d=1.20). Two (02) grams of fresh fecal material was introduced into the Fill-Flotac vessel, and 18 ml of NaCl of specific gravity 1.2 was added. The mixture’s solution was then homogenized adequately with the Fill-Flotac homogenizing stick. The fecal suspension was then filtered through the Fill-Flotac and used to fill the two chambers of the Mini-Flotac. After 10 minutes, the upper part of the flotation chambers was translated and the Mini-Flotac (10 x 40 microscope). Fecal egg or oocysts counts, expressed as the average number of eggs or oocysts per gram of feces, were obtained by multiplying the total number of eggs or oocysts by 5. The identification of the parasite eggs was made based on the morphological characteristics described by Thienpont et al. (1995).
Coproculture
The study was completed by characterizing gastrointestinal strongyles encountered in each farm, coprocultures were performed for each farm. Fecal samples from individual farms were mixed and cultured at room temperature for ten days. The larvae were then extracted from the fecal mass using the Baermann device, which is based on the hygrotropism of the larvae. The coproculture was performed with feces from 288 goat farms (144 farms per season). Extracts from larval cultures were counted. One hundred (100) larvae were counted per sample and killed by the combined action of a 2% formaldehyde solution and heating at 55°C. Larvae were collected and placed on a slide, and a drop of Lugol’s solution was added to the transparent slide. Identification of the larvae was made under light microscopy at x10 and x40 magnification using the identification key described by Van Wyk and Mayhew (2013).
Statistical analysis
PDA was performed to determine the infestation rate of the animals, the rate of the type of infestation, and the parasites’ prevalence; the infestation rate by parasite type was estimated using the formula:
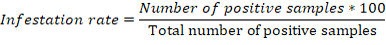
In addition, Pearson’s Chi-squared test was performed to determine the influence of PDA, season, age, or breed on the infestation rate of the animals and the infestation rate of the farms by digestive strongyles. Finally, factorial correspondence analyses were performed to determine the distribution of these parasites according to PDA, sex age, and breed using the FactoMineR package of R.
To study the variation of eggs per gram of feces (EPG) or oocysts per gram of feces (OPG) with PDA and the degree of infestation of the farms with different strongyles species and to see if the season, age, sex, and breed influence EPG or OPG, means and standard errors were calculated. The effects of PDA and breed were identified using one-factor analysis of variance (ANOVA) with Tukey’s post hoc test for the season, sex, and age; Student’s t-test on independent data was used.
Results
Infestation of livestock with gastrointestinal parasites
After analyzing the fecal samples from goats (n=1069), the infestation rate of the animals was 96.82%. The eggs observed belonged to nematodes, cestodes. The Coccidian oocysts were also observed. Nematodes were represented by Strongyles (83.91%), Strongyloides spp. (73.25%), whipworms (0.28%) and Toxocara sp. (0.28%). Eggs of cestodes belonging to the genus Moniezia spp. (21.8%) were found as well as coccidian oocysts (Eimeria spp.) (92.24%). Figure 3a, Figure 3b and Figure 3c show the eggs of Strongyles sp., Strongyloides sp. and Moniezia sp. The parasite burden in terms of average EPG was 405.5 ± 29.9 for Strongyles spp.; 287.8 ± 20.7 for Strongyloides spp.; 211 ± 148 for Moniezia spp.; 15 ± 2.36 for Trichuris sp.; 16.67 ± 3.33 for Toxocara sp. and 1984 ± 138 OPG for coccidia (Table 1).
The main strongyles genera found in the fecal cultures were Haemonchus sp. (98.95%), Trichostrongylus sp. (98.95%), and Oesophagostomum spp. (35.42%) (Figure 4). Figure 5a and Figure 5b show the images of Haemonchus sp. and Trichostrongylys sp. larvae. The proportions of Haemonchus sp. and Trichostrongylus sp. larvae were significant in all seasons. The ratio of Haemonchus sp. in the fecal samples in the dry season was decreased and was dominated by Trichostrongylus sp. (Table 2).
Zootechnical parameters influence the parasitic fauna
The difference between Strongyles, Strongyloides spp. and Moniezia spp. infestation rate was significant in the rainy season (p < 0.0001). The EPG parasite burden was considerably higher in the wet season than in the dry season (p < 0.0001) for all three types of parasites (Table 3).
Females were susceptible to undergo gastrointestinal parasites infestation than males. However, these differences were not significant (p > 0.05). In addition, the prevalence of Strongyloides spp. infestation was significantly higher in females than males (P < 0.05). In contrast, egg excretion of the parasites was higher in males (Table 4).
Age influenced the infestation rate and gastrointestinal
Table 1: Prevalence, mean EPG, and extreme egg shedding of gastrointestinal parasites
| Parasites | Fecal samples from goats (n=1069) | ||
| % | Mean EPG/OPG | EPG/OPG extremes | |
| Strongyles | 83.91 | 405.5 ± 29.9 | 10 - 10080 |
|
Strongyloides spp. |
73.25 | 287.8 ± 20.7 | 10 - 6630 |
|
Moniezia spp. |
21.80 | 211 ± 148 | 5 - 34540 |
|
Trichuris sp. |
0.94 | 15 ± 2.36 | 5 - 25 |
|
Toxocara sp. |
0.28 | 16.67 ± 3.33 | 10 - 20 |
| Coccidian oocysts | 92.24 | 1984 ± 138 |
20 - 78500 |
EPG: Eggs per gram; OPG: Oocysts per gram
Table 2: Intensity of strongyle larvae in fecal samples
| Variables | Overall average | Rainy season | Dry season | P-value |
|
Haemonchus sp. |
44.7 ± 1.07 | 47.28 ± 1.51 | 42.12 ± 1.52 |
0.0162 |
|
Trichostrongylus sp. |
40.23 ± 1.07 | 32.13 ± 1.4 | 48.32 ± 1.51 | 7.92e-14 |
|
Oesophagostomum spp. |
4 ± 0.48 | 4.51 ± 0.83 | 3.49 ± 0.47 | 0.29 |
|
Cooperia spp. |
2.54 ± 0.31 | 2.05 ± 0.36 | 0.6 ± 0.23 | 0.000863 |
|
Bunostomum sp. |
0.54 ± 0.19 | 0.71 ± 0.28 | 0.37 ± 0.27 |
0.372 |
Table 3: Effect of season on goat infestation and egg excretion of gastrointestinal parasites
| Season | Strongyles |
Strongyloides spp. |
Moniezia spp. |
Trichuris sp. |
Toxocara sp. |
Coccidian oocysts |
||||||
| % | ME | % | ME | % | ME | % | ME | % | ME | % | MO | |
| Rainy (n=572) | 88.81 | 638.4 ± 50 | 85.49 | 424 ± 31.4 | 11.19 | 631 ± 539 | 1.40 | 16.25 ± 2.8 | 0.52 |
16.67 ± 3.33 |
93.36 | 2798 ± 230 |
|
Dry (n=497) |
78.27 | 101.38 ±7.76 | 59.15 | 61.31 ± 4.67 | 34.00 | 52.43 ± 7.75 | 0.40 | 10 ± 0 | 0.00 | - | 90.95 | 1022 ± 116 |
| Pvalue | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.2865 | 0.171 | 0.06043 | 0.2996 | 0.1755 |
< 0.0001 |
|
ME: Mean eggs per gram; MO: Mean oocysts per gram
Table 4: Effect of sex on goat infestation and egg excretion of gastrointestinal parasites
| Sex | Strongyles |
Strongyloides spp. |
Moniezia spp. |
Trichuris sp. |
Toxocara sp. |
Coccidian oocysts | ||||||
| % | ME | % | ME | % | ME | % | ME | % | ME | % | MO | |
| Female (n=750) | 85.07 | 391.9 ± 33.5 | 75.47 | 277.4 ± 23 | 22.40 | 53.87 ± 5.87 | 0.80 | 15.63 ± 2.9 | 0.27 | 15 ± 5 | 92.48 | 1726 ± 156 |
| Male (n=319) | 81.19 | 439.5 ± 62.7 | 68.03 | 315.2 ± 44.6 | 20.38 | 619 ± 530 | 1.25 | 10.83 ± 0.83 | 0.31 | 20 ± 0 | 92.13 | 2596 ± 279 |
| Pvalue | 0.137 | 0.5029 |
0.01471 |
0.4514 | 0.5141 | 0.291 | 0.7202 | 0.1505 | 1 | > 0.999 | 0.9466 |
0.006866 |
ME : Mean eggs per gram; MO: Mean oocysts per gram
Table 5:Effect of age on goat infestation and egg excretion of gastrointestinal parasites.
| Category | Strongyles |
Strongyloides spp. |
Moniezia spp. |
Trichuris sp. |
Toxocara sp. |
Coccidian oocysts |
||||||
| % | ME | % | ME | % | ME | % | ME | % | ME | % | MO | |
|
Adult (n= 560) |
85.71 | 451.4 ± 44 | 76.96 | 315.3 ± 29.9 | 18.39 | 54.17 ± 7.61 | 0.89 | 17.14 ± 2.86 | 0.18 | 20 ± 0 | 93.04 | 2149 ± 190 |
|
Young (n=509) |
81.93 | 352.7 ± 39.4 | 69.16 | 254.1 ± 27.8 | 25.54 | 336 ± 265 | 0.98 | 10 ± 1.09 | 0.39 | 15 ± 5 | 91.36 | 1837 ± 199 |
| Pvalue | 0.1095 | 0.0949 | 0.004933 | 0.1346 | 0.00591 | 0.2904 | 1 | 0.03068 | 0.934 | > 0.999 | 0.3624 | 0.2576 |
Table 6: Effect of breed on goat infestation and egg excretion of gastrointestinal parasites
| Breed | Strongyles |
Strongyloides spp. |
Moniezia spp. |
Trichuris sp. |
Toxocara sp. |
Coccidian oocysts | ||||||
| % | ME | % | ME | % | ME | % | ME | % | ME | % | MO | |
|
Dw (n=962) |
83.16 | 405.8 ±31.8 | 74.01 | 286 ± 21.8 | 21.93 | 222 ± 164 | 1.04 | 15 ± 2.36 | 0.31 | 16.67 ± 3.33 | 92.00 | 1991 ± 147 |
| Dw* Sah (n=64) | 87.50 | 424 ± 113 | 59.38 | 448 ± 114 | 21.88 | 152.5 ± 73.3 | 0.00 | - | 0.00 | - | 93.75 | 2198 ± 752 |
|
Sah (n=43) |
95.35 | 375 ± 135 | 76.74 | 142.1 ± 42.9 | 18.60 | 23.75 ± 6.25 | 0.00 | - | 0.00 | - | 95.35 | 1732 ± 415 |
| Pvalue | 0.07505 | 0.965 | 0.0327 | 0.0821 | 0.8747 | 0.966 | 0.5704 | 0.8459 | 0.6492 |
0.859 |
||
DW: Dwarf goat ; Sah: Sahelian goat; ME: Mean eggs per gram; MO: Mean oocysts per gram
Table 7: Effect of PDA on goat infestation and egg excretion of gastrointestinal parasites
| PDA |
Strongyles |
Strongyloides spp. |
Moniezia spp. |
Trichuris sp. |
Toxocara sp. |
Coccidian oocysts | ||||||
| % | ME | % | ME | % | ME | % | ME | % | ME | % | MO | |
|
PDA1 (n=43) |
95.35 | 375 ± 135 | 76.74 | 142.1 ± 42.9 | 18.60 | 23.75 ± 6.25 | 0.00 | - | 0.00 | - | 95.35 | 2198 ± 752 |
|
PDA2 (n=283) |
78.09 | 415.7 ± 67.7 | 63.25 | 242.6 ± 41.7 | 33.57 | 75.1 ± 10.6 | 1.06 | 10 ± 0 | 0.00 | - | 89.75 | 2207 ± 358 |
| PDA4 (n=416) | 80.05 | 523.4 ± 61.5 | 69.23 | 421.4 ± 44.4 | 14.18 | 652 ± 584 | 1.68 | 17.17 ± 3.06 | 0.24 | 20 ± 0 | 87.98 | 2014 ± 201 |
| PDA5 (n=327) | 92.35 | 272.3 ± 20 | 86.54 | 197.5 ± 20.2 | 21.71 | 48.9 ± 15.4 | 0.00 | - | 0.61 | 15 ± 5 | 99.39 | 1749 ± 194 |
| Pvalue | < 0.0001 | 0.00555 | < 0.0001 | < 0.0001 | < 0.0001 | 0.393 | 0.1093 | 0.178 | 0.5277 | 0.667 | < 0.0001 | 0.628 |
PDA i: Agricultural Development Pole; ME: Mean eggs per gram; MO: Mean oocysts per gram
parasite burden. In general, adults were the most infested with Strongyles, Strongyloides spp., Moniezia spp., and Coccidian oocysts with higher nematode EPG (Table 5).
The chi-square test showed that the breed of the animal had no influence on the infestation rate and parasite burden except for Strongyloides spp., where the infestation rate was significant (p < 0.05). However, the Sahelian breeds had higher infestation rates for strongyles Strongyloides spp. and coccidian oocysts, but the burden were lower than those of the other breeds. There was an absence of Toxocara sp. and Trichuris sp. eggs in the Metis (Dwarf Goat*Sahelian breed) and Sahelian breeds (Table 6).
Strongyles, Strongyloides spp., Moniezia spp., and coccidian oocysts infestation rates were significantly different between PDAs (p < 0.0001) (Table 7, Figure 6). The discharge of parasite eggs was more significant in PDA 4, the lack of Trichirus sp. eggs in PDA 1 and PDA 5 and the absence of Toxocara sp. eggs in PDA 1 and PDA 2 were observed (Table 7, Figure 6).
Distribution of identified parasites within gender-age
Correspondence Factor Analysis (CFA) results revealed that the three factorial axes summarize 79.85, 12.98, 7.17% distribution of parasite species within sex-age, respectively
(Table 8). Strongyles, Strongyloides spp., Moniezia spp., and Coccidian oocysts were much more characteristic of sex age. Toxocara sp. and Trichirus sp. affected goats less regardless of sex age (Figure 7).
Table 8: Eigenvalues, percent variability, cumulative percent variability explained by factorial axes for sex-age
| Eigenvalue | Percentage of variability | Cumulative percentage of variability | |
| dim 1 | 0.0052035625 | 79.853179 | 79.85318 |
| dim 2 | 0.0008456422 | 12.977112 | 92.83029 |
| dim 3 | 0.0004672078 | 7.169709 | 100 |
Table 9: Eigenvalues, percent variability, cumulative percent variability explained by factorial axes for PDA
| Eigenvalue | Percentage of variability |
Cumulative percentage of variability |
|
| dim 1 | 0.0114623104 | 77.260199 | 77.26020 |
| dim 2 | 0.0031096776 | 20.960374 | 98.22057 |
| dim 3 | 0.0002639954 | 1.779426 | 100 |
Table 10: Eigenvalues, percent variability, cumulative percent variability explained by factorial axes for the breed
| Eigenvalue | Percentage of variability | Cumulative percentage of variability | |
| dim 1 | 0.001075577 | 80.47648 | 80.47640 |
| dim 2 | 0.000260934 | 19.52352 | 100 |
Distribution of parasites identified according to the PDA
CFA results showed that the three factorial axes summarize 77.26, 20.96, 1.78% of the distribution of parasite species in the PDAs, respectively (Table 9). Strongyles, Strongyloides spp., Moniezia spp., and Coccidian oocysts were much more characteristic of all PDAs. Toxocara sp. and Trichirus sp. species affected goats more miniature regardless of PDA (Figure 8).
Species based parasites distribution
CFA outcome showed that the two factorial axes summarize 80.48%, 19.52% distribution of parasite species between breeds, respectively (Table 10). Strongyles, Strongyloides spp., Moniezia spp., and coccidian oocysts were much more characteristic of all breeds. Toxocara sp. and Trichirus spp. affected goats regardless of the species bred (Figure 9).
Discussion
This study identified the different gastrointestinal parasites in traditional goat farms in Benin and evaluated their prevalence and parasite intensity in Eggs Per Gram of feces (EPG).
The high parasitism rate (96.82%) can be explained on the one hand by the traditional type of farming where animals are free and share the same grazing area and, on the other hand, by the lack of routine deworming. Similar prevalences have been reported by Salifou (1996) in Benin, Sylvia et al. (2015) in Nigeria, Ntonifor et al. (2013) in Cameroon in goats farming.
The high prevalence of coccidian oocysts, strongyles, and Strongyloides spp. could be attributable to their life cycle. L3 larvae are the causative agent of the parasitic infestation. These L3 larvae undergo an automatic translation of the feces towards the grass. On the other hand, the cestodes found (Moniezia) have an obligatory intermediate host represented by certain arthropods that harbor the cysticercoid larvae. The contamination of animals requires prior ingestion of these infected arthropods. This outcome could justify that the infestation of animals by natural cycle and monoxenous parasites would be more frequent than that by parasites with an obligatory intermediate host. Furthermore, the low prevalence of whipworms among nematodes could be related to the fact that it is the embryonated egg containing the L3 larva that is the infesting form of this parasite.
Similar observations for the highest prevalences of Strongyles, Strongyloides spp. or coccidial oocysts in small ruminant farms, have also been made in most tropical countries, Ethiopia (Dagnachew et al., 2011), Cameroon (Ntonifor et al., 2013), India (Dixit et al., 2016), Nigeria (Paul et al., 2016), Laos (Gueguen et al., 2016).
Prevalences of nematodes, as well as fecal egg excretion, are significantly higher in the rainy season. According to Nwosu et al. (2007), environmental conditions are generally favorable for developing, survival, and translocation of pre-parasitic stages of parasitic nematodes during the rainy season. As a result, there is an accumulation of adult worm populations in grazing animals such that maximum worm burden are recorded during the rainy season. After that, worm populations decrease in the dry season. This funding justifies a higher prevalence of animal infestation and levels of parasite egg excretion in the wet season than in the dry season. The high prevalence rates of gastrointestinal parasites observed in this study indicate the risks of economic losses for goat farmers in Benin.
The mean degrees of infestation in animals were 638.4; 424; 631; 2798 EPG in the rainy season and 101.38; 61.31; 52.43; 1022 EPG in the dry season for strongyles, Strongyloides spp., Moniezia spp., and Coccidian oocysts, respectively. According to the severity scales, based on the EPG limits revealed by Alowanou et al. (2021), it could be concluded that infestations were severe during the rainy season for strongyles, Moniezia spp., Coccidia oocysts and moderate for Strongyloides spp. During the dry season, infestations were light for strongyles, Strongyloides spp., Moniezia spp., and severe for Coccidia. This present case requires systematic deworming of all animals in the herd for according to Bosco (2014), an infestation rate above 200 EPG already requires treatment when the fecal analysis technique is Mini-FLOTAC.
Moreover, egg production still has no relation to the population of worms harbored, as many factors influence the EPG value. Thus, an animal may only host male worms or even adults who have not yet reached sexual maturity or worms whose females are in oviposition inhibition conditions (Salifou, 1996). This result shows that the animals would be heavily parasitized.
Sex had no statically significant effect on infestation with gastrointestinal parasites except Strongyloides spp.. However, on all the animals studied, the proportion of females affected by nematodes was higher than males. This observation is in opposite of the conclusion of Tariq et al. (2010) who revead that both sexes are susceptible to nematode infestation. During the peripartum period, females temporarily lose their naturally acquired immunity to gastrointestinal parasites (Schoenian, 2012), and therefore, they could be easily infected. Thus the slight differences in prevalence observed in females could be due to pregnant and breast-feeder females among the females sampled in this study.
The parasites prevalence and egg excretion level by age showed that adult animals were more infected with gastrointestinal nematodes than the young animales. This observation is not in agreement with the findings of some authors for whom juveniles are more susceptible to infestations than adults due to their low immunity levels (Tariq et al., 2010; Khan et al., 2010; Zeryehun, 2012; Nabi et al., 2014). This difference may be related to physiological status (gestation and breastfeeding stage) and malnutrition, making adults more susceptible to infestation and excreting more eggs than non-pregnant or non-breast-feeder ones (Suarez et al., 2017). In addition, young animals are fed milk by their mothers until adulthood (Saidi et al., 2020), limiting their grazing exposure. Therefore they would be less infested, hence the finding of higher prevalence in adults than in young.
The higher infestation rates in Sahelian rears than in dwarf and mixed breeds indicate that they are more susceptible to gastrointestinal parasites than the other breeds. Their EPG burden is lower than that of the other breeds despite their higher infestation rates. The difference in EPG burden would be due to the limited infestation by infesting L3 larvae during grazing, as Sahelian goats were more commonly encountered in PDA 1 and PDA 2, which are characterized by a Sahelian climate. This hot and dry climate is not conducive to the development and survival of infesting L3 larvae.
Parasite burden in EPG were higher in PDA 4 than in PDA 1, PDA 2, and PDA 5. This outcome could be explained by the fact that free-grazing is the more common method of rearing in the dry season than in the wet season in PDA 4, whereas in the other PDAs, during fieldwork in the wet season, animals were put on fixed or mobile stakes. This freedom, especially in the rainy season when conditions are favorable for the survival and translocation of the pre-parasitic stages of helminths, means that the animals would ingest enough infesting larvae on the grazing ground. As a result, the gastrointestinal burden of adult worms would be high and, consequently, a high rate of egg-laying expelled by the animals. On the other hand, the risk of infestation of the staked animals by a high burden of infesting larvae would be low even if they are fed fresh fodder. Furthermore, Trichirus sp. eggs were not observed in PDA 1 and PDA 5 and Toxocara sp. eggs in PDA 1 and PDA 2. This could indicate the absence of these parasites in these areas; helminthological autopsies could give more details.
The coprocultures showed that strongyles were represented by Haemonchus sp., Trichostrongylus sp., Oesophagostomum spp., Cooperia spp., , and Bunostomum sp.. These results reveal that Haemonchus sp. and Trichostrongylus sp., are the most dominant strongyles species in the digestive tract of small ruminants as reported by reported by Salifou (1996) in South Benin. This dominance would be explained by the fact that Haemonchus sp. is very prolific and can lay up to 10 000 eggs per day for several months (Raza et al., 2014). In addition, it acquires a faster resistance to synthetic antihelminthics compared to other strongyles species (Torres-Acosta et al., 2003). Trichostrongylus sp. is not significant but is the most constant parasite, as its presence is annual. These parasites could be associated with diarrhea mentioned by goat breeders in Benin (Idrissou et al., 2017; Challaton et al., 2022). A specific coprological study on goats presenting diarrhea will better evaluate the share of strongyles in the pathologies responsible for diarrhea in goats.
In the dry season, the proportion of Haemonchus sp. in the fecal cultures was decreased and was dominated by Trichostrongylus sp.. Unlike Trichostrongylus sp., the Haemonchus sp. population survives during the dry season partly as hypobiotic larvae (L4) in the mucosa of the abomasum (Salifou, 1996). This halt in larval growth during the dry season will result in a high larval burden (L4) and a low burden of adult Haemonchus sp. worms in the abomasum. This observation results in a drop in oviposition rate and low emission of eggs in the excrement. When conditions become favorable again in the rainy season, the hypobiotic L4 larvae will continue to develop into adults capable of laying eggs and consequently an increase in the number of eggs expelled in the feces. This finding proves the dominance of Haemonchus sp. and Trichostrongylus sp. larvae in fecal crops in the rainy and dry seasons, respectively. Haemonchus spp would thus preserve itself as a biological species in nature by using this strategy of increasing its prevalence on animals and inhibiting its development at the L4 larval stage, in particular, severe climatic periods of the year (Belem et al., 2005). Given the adverse effects of these two parasites on goats and the resistance to synthetic anthelmintics, special attention should be given to them in controlling gastrointestinal parasites of small ruminants.
Conclusion
This study shows that a wide range of gastrointestinal parasites infests goats reared under the traditional system in Benin. The coprological examinations revealed a strong presence of strongyles, Strongyloides spp., coccidian oocysts, and Moniezia spp., in order of importance, regardless of the Agricultural Development Poles, breed, sex, and age of the animals examined. The coproculture conducted revealed the presence of 5 genera of strongyles, namely: Haemonchus sp., Trichostrongylus sp., Oesophagostomum spp., Cooperia spp., and Bunostomum sp..The genera Haemonchus sp. and Trichostrongylus sp. were more frequent in all the Agricultural Development Poles. The importance of the economic losses (weight loss, reduction in the growth parameters, low milk production) caused by these gastrointestinal parasites should justify the implementation of a national program of animal deworming. The latter should be applied according to a schedule that considers the seasonal fluctuation of these parasites. Thus we suggest two treatments in PDA 1, PDA 2, and four PDA 4 and PDA 5: a periodic treatment of goats at the end of each dry and rainy season. Given the length of the wet season in PDA 1 and PDA 2, additional treatment in the middle of the rainy season would be desirable.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are grateful for the financial support of this work by the University of Abomey-Calavi through the ‘’Programme des Fonds Compétitifs de Recherche III (PFCRIII)’’. The authors also thank the farmers for accepting to take samples from their animals.
Authors’ contributions
Data collection in the field and laboratory analyses were done by PKC with the help of HAK. PKC processed and analyzed the data and interpreted the results. Then he wrote the first version of the manuscript. GCA and CKB supervised the field data collection and laboratory analysis. They also contributed to the critical revision of the manuscript. GGA supervised the static analyses and revised the manuscript. VPH contributed to the improvement of the scientific quality of the manuscript by proofreading the different versions. SMH-A made a general supervision of the work.
References
Alowanou GG, Adenilé AD, Akouèdegni GC, Bossou AC, Zinsou FT, Akakpo G-CA, Kifouly, HA, Rinaldi L, von Samson-Himmelstjerma G, Cringoli G (2021). A comparison of Mini-FLOTAC and McMaster techniques in detecting gastrointestinal parasites in West Africa Dwarf sheep and goats and crossbreed rabbits. J. Appl. Anim. Res. 49(1): 30–38. https://doi.org/10.1080/09712119.2021.1876703
Bosco A (2014). The coprological diagnosis of gastrointestinal nematode infections in small ruminants . (PhD Thesis, University Federico II of Naples, pp. 1–144)
Challaton KP, Boko KC, Akouedegni CG, Alowanou GG, Houndonougbo PV, Hounzangbé-Adoté MS (2022). Traditional goat rearing in Benin: health practices and constraints. Rev. Elev. Med. Vet. Pays Trop. 75(1): 9-17. https://doi.org/10.19182/remvt.36893
Cringoli G, Rinaldi L, Maurelli MP, Utzinger J (2010). FLOTAC : new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of
parasites in animals and humans. Nat. Protoc. 5: 503–515. https://doi.org/10.1038/nprot.2009.235
Dixit P, Rao MLV, Dixit AK, Shukla PC (2016). Prevalence of gastrointestinal parasites in goat kids in Jabalpur. Ruminant Science. 5(1): 39–42.
Dossa LH, Wollny C, Gauly M (2007). Smallholders’ perceptions of goat farming in southern Benin and opportunities for improvement. Trop Anim Health Prod. 39: 49–57. https://doi.org/10.1007/s11250-006-4440-2
FAOstat (2021). Statistiques de l’Organisation des Nations Unies pour l’Alimentation et l’Agriculture, http://www.fao.org/faostat/fr/#data/QA. Accessed 03 Oct 2021.
Gnanglè CP, Kakaï RG., Assogbadjo AE, Vodounnon S, Yabi JA, Sokpon N (2011). Tendances climatiques passées, modélisation, perceptions et adaptations locales au Bénin. Climatologie. 8: 27–40. https://doi.org/10.4267/climatologie.259
Idrissou N-D, Ahounou SG, Tougan U, Tamimou MI, Hounmanou YMG, Mensah GA, Karim IYA (2017). Morphometric and zootechnical characterization of dwarf goats in Northeastern Benin. Int. J. Agron. Agric. Res. 11(3): 26–42.
Khan MN, Sajid MS, Khan MK, Iqbal Z, Hussain A (2010). Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol. Res. 107(4): 787–794. https://doi.org/10.1007/s00436-010-1931-x
Nabi H, Saeed K, Shah SR, Rashid MI, Akbar H, Shehzad W (2014). Epidimiological study of gastrointestinal nematodes of goats in district swat, khyber pakhtunkhwa, Pakistan. Sci.Int.(Lahore). 26(1): 283–286.
Ntonifor HN, Shei SJ, Ndaleh NW, Mbunkur GN (2013). Epidemiological studies of gastrointestinal parasitic infections in ruminants in Jakiri, Bui Division, North West Region of Cameroon. J. Vet. Med. Anim. Healt. 5(12): 344–352. https://doi.org/10.5897/JVMAH2013.0209
Nwosu CO, Madu PP, Richards WS (2007). Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet. Parasitol. 144(1-2): 118–124. https://doi.org/10.1016/j.vetpar.2006.09.004
Paul BT, Biu AA, Ahmed GM, Mohammed A, Philip MH, Jairus Y (2016). Point prevalence and intensity of gastrointestinal parasite ova/oocyst and its association with Body Condition Score (BCS) of sheep and goats in Maiduguri, Nigeria. J. Adv. Parasitol. 3(3): 81–88. https://doi.org/10.14737/journal.jap/2016/3.3.81.88
Saidi M, Stear MJ, Elouissi A, Mokrani S, Belabid L (2020). Epidemiological study of goat’s gastrointestinal nematodes in the North West of Algeria. Trop Anim Health Prod. 52(4): 1787– 1793. https://doi.org/10.1007/s11250-019-02193-6
Schoenian S (2012). Periparturient Egg Rise-American Consortium for Small Ruminant Parasite Control. http://www.acsrpc.org/Resources/Topics/PPER.html
Sissay MM, Uggla A, Waller PJ (2007). Prevalence and seasonal incidence of nematode parasites and fluke infections of sheep and goats in eastern Ethiopia. Trop Anim Health Prod. 39(7): 521– 531. https://doi.org/10.1016/j.smallrumres.2009.06.013
Suarez VH, Martínez GM, Viñabal AE, Alfaro JR (2017). Epidemiology and effect of gastrointestinal nematodes on dairy goats in Argentina. Onderstepoort J. Vet. Res. 84(1): 1–5. https://doi.org/10.4102/ojvr.v84i1.1240.
Tariq KA, Chishti MZ, Ahmad F (2010). Gastro-intestinal nematode infections in goats relative to season, host sex and age from the Kashmir valley, India. J. Helminthol. 84(1): 93–97. https://doi.org/10.1017/S0022149X09990113
Terefe D, Demissie D, Beyene D, Haile S (2012). A prevalence study of internal parasites infecting Boer goats at Adami Tulu agricultural research center, Ethiopia. J. Vet. Med. Anim. Health. 4(4): 12–16. https://doi.org/10.5897/JVMAH11.046
Tidjani MA, Akponikpe PBI (2012). Évaluation des stratégies paysannes d’adaptation aux changements climatiques: cas de la production du maïs au Nord-Bénin. Afr. Crop Sci. J. 20: 425–441.
Torres-Acosta JFJ, Dzul-Canche U, Aguilar-Caballero AJ, Rodrıguez-Vivas RI (2003). Prevalence of benzimidazole resistant nematodes in sheep flocks in Yucatan, Mexico. Vet. Parasitol. 114(1): 33–42. https://doi.org/10.1016/S0304-4017(03)00076-1
Van Wyk JA, Mayhew E (2013). Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 80(1), Art. #539, 14 pages. https://doi.org/10.4102/ojvr.v80i1.539
Waller PJ (2006). From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 139(1-3): 1–14. https://doi.org/10.1016/j.vetpar.2006.02.036
Zeryehun T (2012). Helminthosis of sheep and goats in and around Haramaya, Southeastern Ethiopia. J. Vet. Med. Anim. Health. 4(3): 48–55. https://doi.org/10.5897/JVMAH12.0014
To share on other social networks, click on any share button. What are these?






