Effects of Polyphenols Supplemented Canola Meal Based Diet on Proximate Composition, Minerals Absorption and Hematology of Cyprinus carpio Fingerlings
Effects of Polyphenols Supplemented Canola Meal Based Diet on Proximate Composition, Minerals Absorption and Hematology of Cyprinus carpio Fingerlings
Syed Makhdoom Hussain1,*, Hina Gohar1, Muhammad Asrar1,
Muhammad Mudassar Shahzad2, Azhar Rasul1, Majid Hussain3,
Muhammad Zubair ul Hassan Arsalan1, Nisar Ahmad4 and Aqsa Sharif1
1Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad
2Department of Zoology, Division of Science and Technology, University of Education, Township Campus, Lahore
3Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore
4Department of Zoology, University of Education, DG Khan Campus, Dera Ghazi Khan
ABSTRACT
The research project was designed to check the effect of polyphenols supplemented canola meal-based diet on proximate composition, mineral absorption and hematology of Cyprinus carpio fingerlings. The diets were formulated in such a way that sufficient supply of all required nutrients were ensured for normal fish growth. Collection of feces from each tank was done twice a day. Impact of each treatment on the absorption of minerals, proximate analysis and hematology were determined using standard methods and formulae. Highest minerals absorption (Ca, Na, K, Fe, Cu, P, Mg and Al) was observed in the fish fed at 400mg/kg of polyphenols in canola meal based diets. Similarly, best hematological parameters (RBCs, WBCs, PLT, Hb, PCV, MCHC, MCH and MCV) as well as proximate composition (crude protein, crude fat, ash, moisture and carbohydrates) were noted in fish group fed on 400mg/kg of polyphenols in diet. Hence supplementation of polyphenols at 400mg/kg was found to be optimum for better hematology, minerals absorption and carcass composition of common carp.
Article Information
Received 14 February 2019
Revised 22 May 2020
Accepted 01 February 2021
Available online 05 May 2021
(early access)
Published 12 February 2022
Authors’ Contribution
SMH planned and supervised the research, provided research materials. HG conducted the trial and collected data. AR helped in statistical analysis. MH proffread the manuscript. HG prepared manuscript while MA and MMS helped her. MZHA and NA helped in feed analysis and compiling the results. AS helped in proximate analysis of fish.
Key words
Phenolic compounds, Blood parameters, Carcass composition, Minerals absorption, Common carp.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190214170258
* Corresponding author: drmakhdoom90@gmail.com
0030-9923/2022/0003-1071 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
One of the rapidly developing animal food-producing sectors is aquaculture, which accounts for almost fifty percent of the total food fish and serves replacement for wild fish was well as plants (FAO, 2010). However, the basic limitation to aquaculture industry is disease which imposes severe losses on farming facilities throughout the world (Kim et al., 2013b). Muscle of fish is a complex system; it offers an appropriate environment for the lipids to be oxidized quickly. This eventually results in off-odor as well as new flavors and restrictsits shelf-life. Fish muscle is prone to oxidation because of occurrence of high amounts of poly unsaturated fatty acids (PUFAs), iron, and heme from myoglobin and hemoglobin (Maqsood et al., 2012). According to El-Banna and Atallah (2009) body weight, body weight gain, livability, immunity of fish can be improved and the level of mortality can be reduced by inclusion of feed additives to diet of fish, they also play role in improvement of productive and economic efficiency of fish farms. Polyphenols enriched extracts of plants have been detected to be the safe supplements, as reserving them from natural sources is easy and lipid oxidation can be hindered effectively under their influence (Maqsood et al., 2014). Ubiquitously present throughout most tissues of plants are polyphenols, which play significant role in plant physiology. From their respective sources, polyphenols can be extracted and after that can be included to diet because of their antioxidant effects as well as coloring properties (Maqsood et al., 2013).
The fundamental challenge which faces the development and growth of aquaculture is feed (Gabriel et al., 2007). With expanding aquaculture, fish oil and fish meal have become more costly and infrequent. As a result, aqua feed producers are facing pressure for replacing them with suitable options (Pickova and Morkore, 2007). In fish feed, as a result of increasing cost and unforeseeable availability of fish meal, it is essential to replace it with less costly ingredients which are either plant or animal derived (Higgs et al., 1995; Mahboob, 2014). An appropriate protein substitution for fish meal is canola meal as it has relatively high (380 g/kg) content of protein (Yigit and Olmez, 2009). Canola meal, in comparison with soybean meal and fish meal, is reported to be more economical (Sajjadi and Carter, 2004; Hussain et al., 2015). It is generally used in aquaculture diets for species such as perch, bass, catfish, turbot, tilapia, carp, sea bream and shrimp with positive effects (Enami, 2011).
Cyprinus carpio is the most commonly cultured species of fish in the world. In ponds of Asia, Near and Far East, this fish is preferred for cultivation in ponds, alone or in combination with other fishes, due to its omnivorous habit, excellent growth rate, hardy nature, breeding in confined waters and easy adaptability to feeds (Khan et al., 2016). The present research was conducted to determine the effect of polyphenols in canola meal based diet on hematology, minerals absorption and carcass composition of C. carpio fingerlings.
Materials and methods
The experiment was carried out in Fish Nutrition Laboratory, Department of Zoology, Government College University, Faisalabad.
Fish and experimental conditions
Fingerlings of common carp were taken from the local fish seed hatchery and were acclimatized for two weeks. They were stocked, in specially designed water tanks that were V-shaped (water capacity 70 L), for two weeks and were fed on basal diet (Allan and Rowland, 1992). Parameters related to water quality were monitored using electrical conductivity meter (HANNA: HI. 8633), dissolve oxygen meter (Jenway 970) and pH meter (Jenway 3510). The water quality parameters ranges were noted; temperature 24.9–28.7°C, pH 7.4–8.6, electrical conductivity 1.30–1.52 dS/m and dissolved oxygen 5.8–7.3 mg/L. By means of capillary system, aeration was constantly provided to all the experimental tanks. Prior to initiation of trial, fingerlings were treated with saline solution (0.5 % NaCl) for killing all the pathogens if present (Rowland and Ingram, 1991).
Experimental design
Triplicate tanks, having 15 fingerlings each, were used for each treatment. Fingerlings were given feed at the rate of 5% live wet weight. Experimental trial continued for 70 days. Polyphenols supplemented canola meal-based diets were compared with control as well as with each other to determine parameters of carcass composition, minerals absorption and hematology using completely randomized design (CRD).
Feed ingredients and formulation of experimental diets
From a commercial feed mill, the ingredients of feed were taken. Before formulating experimental diet, the ingredients were analyzed (Table I) for chemical composition following AOAC (1995). They were finely ground and mixed for 10 min in an electric mixer followed by inclusion of fish oil. Water (10–15%) was added during mixing of ingredients (Lovell, 1989). Then through Lab Extruder (SYSLG30-IV Experimental Extruder), these ingredients were extruded to form floating pellets (3 mm). In the extruder, all canola meal based diets were treated equally to formulate seven CM-based test diets.
Polyphenols were obtained from Natural Product and Synthetic Chemistry Lab, Department of Applied Chemistry and Biochemistry Government College University, Faisalabad. Seven test diets were formed from the experimental diet and then were supplemented with graded levels (0, 100, 200, 300, 400, 500 and 600 mg/kg) of polyphenols (Robinson et al., 2002). Each diet was then dried and stored at the temperature of 4°C until use.
Table I.- Composition (%) of diet ingredients.
|
Ingredients |
Test diet-I (control diet) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
Test diet-VII |
|
Polyphenols (mg/kg) |
0 |
100 |
200 |
300 |
400 |
500 |
600 |
|
Canola meal |
55 |
55 |
55 |
55 |
55 |
55 |
55 |
|
Fish meal |
16 |
16 |
16 |
16 |
16 |
16 |
16 |
|
Wheat flour* |
11 |
10.9 |
10.8 |
10.7 |
10.6 |
10.5 |
10.4 |
|
Soybean meal |
8 |
8 |
8 |
8 |
8 |
8 |
|
|
Fish oil |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
|
Vitamin Premix |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Mineral mixture |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Ascorbic acid |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Chromic oxide |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
*Polyphenols were added at the expense of wheat flour.
Chemical analysis of feed, feces and carcass composition
After the research period, the feed ingredients, samples of experimental diets and feces were homogenized by using a motor and pestle. Four fish were taken from each tank and were sacrificed followed by drying at room temperature. These samples were then homogenized separately using a pestle and mortar, to be analyzed by using standard methods (AOAC, 1995). Oven-drying method was used for determining moisture in whole fish body at 105oC for 12 h. Crude protein was analyzed by (Nx6.25) Micro Kjeldahl`s (InKjel M behr Labor Technik GmbH D-40599 Dusseldorf) method and crude fat by petroleum ether using Soxhlet system (Soxhlet Extraction Heating Mantels, 250 ml 53868601). Contents of crude fiber were determined as loss on ignition of dried lipid-free residues after digestion with 1.25% sodium hydroxide and 1.25% H2SO4, whereas ash by ignition in electric furnace (Naberthern B170) at 650oC for 12 h to constant weight. Total carbohydrates (N-free extract) were calculated by difference, i.e. total carbohydrates (%) = 100- (CP% + EE% + CF% + Ash% + Moisture %).
Minerals absorption
Homogenization of the samples of experimental diets and feces was done by using standard methods (AOAC, 1995). Moisture was determined by oven-drying at 105oC for 12 h. Feed as well as samples of feces were digested in boiling nitric acid and perchloric acid mixture (ratio of 2:1). After appropriate dilution, mineral contents such as calcium (Ca), iron (Fe), magnesium (Mg), copper (Cu) and aluminum (Al) were determined using Atomic Absorption Spectrophotometer (Hitachi Polarized Atomic Absorption Spectrometer, Z-8200). Calibrated standards for mineral estimation were prepared from commercially available standards (AppliChem® Gmbh Ottoweg4, DE-64291 Darmstadt, Germany). Potassium (K) and Sodium (Na) were estimated through flame photometer (Jenway PFP-7, UK). Using ammonium molybdate as reagent, calorimetric determination of phosphorus (P) was done (UV/VIS spectrophotometer) at absorbance of 720nm through standard methods (AOAC, 1995). Contents of chromic oxide in the feed and feces were determined after oxidizing with molybdate reagent using a UV-VIS 2001 Spectrophotometer at 370nm absorbance (Divakaran et al., 2002).
Calculation of apparent absorption coefficient
Apparent absorption coefficients of minerals in test diets were calculated using standard formula (NRC, 1993).
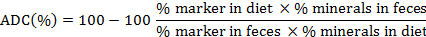
Hematological study
Clove oil (Sigma) was firstly dissolved in ethanol as it has poor solubility in water (Peake, 1998; Coyle et al., 2004) and then its concentrations of 60 mg/L were used to anesthetize fish fingerlings from each tank for 5 min. Blood was taken from each sample fish through caudal vein using heparinized syringe and after that blood samples were carried to the Molcare Lab, Department of Biochemistry, University of Agriculture, Faisalabad, Pakistan. Hematocrit was determined using micro-hematocrit technique using capillary tubes (Brown, 1980). Red blood cells (RBC) and white blood cells (WBC) were counted using a haemo-cytometer having approved Neubauer counting chamber (Blaxhall and Daisley, 1973). Estimation of concentration Hb (hemoglobin) was done using method explained by Wedemeyer and Yastuke (1977). The formulas stated below were used to calculate MCHC (mean corpuscular hemoglobin concentration); MCH (mean corpuscular hemoglobin) and MCV (mean cell volume):
MCHC = Hb / PCV × 100
MCV = PCV / RBC × 10
MCH = Hb / RBC × 10
Statistical analysis
Data of proximate composition, mineral absorption and hematological indices of test diets was subjected to one-way analysis of variance (Steel et al., 1996). By Tukey’s Honesty Significant Difference Test, the differences among means were compared and were considered significant at p<0.05 (Snedecor and Cochran, 1991). The Co-Stat computer software (Version 6.303, PMB 320, Monterey, CA, 93940 USA) was used for statistical analysis.
Results
Proximate composition
Significant differences (p<0.05) were observed among the fish carcass in terms of crude fat, crude protein, ash and moisture (Table III). According to the results, fish fed at 400 mg/kg level based diet had maximum contents of crude protein (62%) and crude fat (15%) as compared to fish fed on control diet (protein 54% and fat 9%). Highest amount of crude ash (9%) was found in test diet II (i.e., 100 mg/kg) whereas moisture (8%) was found to be highest in carcass of fish fed on control diet. However, lowest amount of crude ash (6%) was found in the fish fed at 400 mg/kg level based diet and lowest moisture (5%) was noted in group of fish which fed on test diet IV. On the basis of these results, it was found that fingerlings fed at 400 mg/kg level based diet showed highest amount of nutrients (protein, fat and gross energy) in carcass as compared to fish fed on control and other test diets.
Table II.- Chemical composition (%) of feed ingredients.
|
Ingredients |
Dry matter (%) |
Crude protein (%) |
Crude fat (%) |
Crude fiber (%) |
Ash (%) |
Carbohydrates (%) |
|
Fish meal |
91.63 |
48.15 |
7.16 |
1.07 |
25.73 |
17.89 |
|
Wheat flour |
92.45 |
10.10 |
2.35 |
2.65 |
2.08 |
82.82 |
|
Canola meal |
94.14 |
37.02 |
1.27 |
1.42 |
9.21 |
51.08 |
|
Soybean meal |
93.80 |
41.93 |
3.74 |
1.97 |
10.83 |
41.53 |
Table III.- Proximate composition (%) of C. carpio fed on polyphenols supplemented canola meal based diet.
|
Carcass parameters |
Polyphenols levels (mg/kg) |
Protein |
Fat |
Ash |
Moisture |
Carbohydrates |
|
Test diet –I |
0 |
53.62±0.65d |
8.86±0.59e |
9.29±0.14a |
7.49±0.37a |
19.45±0.84a |
|
Test diet –II |
100 |
55.27±0.86cd |
10.94±0.66d |
9.40±0.40a |
6.60±0.83a |
16.42±0.88b |
|
Test diet –III |
200 |
58.88±0.64b |
13.23±0.91ab |
8.75±0.13ab |
6.43±0.39ab |
11.43±0.83d |
|
Test diet –IV |
300 |
59 .60±0.95b |
14.04±0.48ab |
7.58±0.46c |
5.17±0.18c |
12.59±0.88c |
|
Test diet –V |
400 |
62.17±0.64a |
14.78±0.19bc |
6.43±0.35d |
5.30±0.18c |
10.07±0.32c |
|
Test diet –VI |
500 |
58.41±0.24b |
13.15±0.65bc |
7.82±0.75bc |
5.45±0.17bc |
13.98±1.01d |
|
Test diet –VII |
600 |
55.92±0.88c |
11.69±0.34ab |
8.83±0.16ab |
6.92±0.24a |
15.29±1.28c |
All values of Means within rows are different significantly (p<0.05). Data values are mean (Mean ± Standard deviation) of three replicate.
Table IV.- Percentage of minerals in test diets of C. carpio fingerlings fed on canola meal based diet supplemented with polyphenols.
|
Minerals / Polyphenols levels (mg/kg) |
Test diet –I (control diet) |
Test diet –II |
Test diet –III |
Test diet –IV |
Test diet –V |
Test diet –VI |
Test diet –VII |
|
0 |
100 |
200 |
300 |
400 |
500 |
600 |
|
|
Ca (%) in diets |
0.93± 0.05ab |
0.96± 0.08a |
0.79± 0.07abc |
0.79± 0.09abc |
0.69±0.03c |
0.96±0.07a |
0.74±0.10bc |
|
Na |
0.14± 0.02 a |
0.15± 0.01a |
0.14± 0.02 a |
0.14± 0.03a |
0.13±0.02a |
0.13±0.02a |
0.15±0.02a |
|
K |
0.017± 0.003a |
0.016± 0.001a |
0.018± 0.001a |
0.017± 0.001a |
0.0170±0.002a |
0.017±0.0009a |
0.018±0.001a |
|
Fe |
0.0543± 0.005a |
0.054± 0.004a |
0.054± 0.005a |
0.053± 0.007a |
0.0533±0.004a |
0.053±0.004a |
0.055±0.006a |
|
Cu |
0.006± 0.0004a |
0.006± 0.0006a |
0.006± 0.0004a |
0.006± 0.0004a |
0.006±0.0004a |
0.006±0.0004a |
0.006±0.0005a |
|
P |
2.11± 0.06a |
2.11± 0.05a |
2.12± 0.10a |
2.12± 0.04a |
2.11±0.09a |
2.11±0.06a |
2.10±0.04a |
|
Mg |
0.009± 0.0004a |
0.009± 0.0003a |
0.009± 0.0003a |
0.009± 0.0006a |
0.009±0.0006a |
0.009±0.0004a |
0.009±0.0005a |
|
Al |
0.0005± 0.00006a |
0.0005± 0.00007a |
0.0005± 0.00004a |
0.0005± 0.00006a |
0.0005± 0.00007a |
0.0005± 0.00005a |
0.0005± 0.00006a |
Data are means of three replicates. Ca, calcium; Na, sodium; K, potassium; Fe, iron; Cu, copper; P, phosphorus; Mg, magnesium; Al, aluminium.
Minerals absorption
The analyzed values of minerals in diet and feces have shown that there is equal composition of all the minerals in control diet as well as polyphenols supplemented diets (Table IV). It was noted that minerals such as Ca (71%), Fe (69%) and Al (64%) were found highly absorbed when the fingerlings were fed at test diet VI as compared to other test diets and control diet. Whereas, maximum absorption of K (74%) and P (76%) were found at 400 mg/kg level based diet and Cu (69%) as well as Mg (67%) were at 300 mg/kg level based diet. On the other hand all the minerals were found lowest when fingerlings were fed on control diet as compared to test diets except Cu that was lowest at test diet II as shown in Table VI.
Hematology
Values of WBCs (8×103mm-3) and RBCs (3×106mm-3) were noted to be highest in test diet V (400 mg/kg of polyphenols) whereas highest Hb (9 g/100ml) was noted at test diet IV (300 mg/kg of polyphenols). These value were significantly different (P>0.05) from fish group which consumed control diet, whereas values of WBCs and Hb were statistically similar with fish fed on test diet IV (Table VII). However, values of RBCs, WBCs and Hb were found to be lowest in the fish fed on control diet. On the other hand, PCV (25%) in test diet V and PLT (67%) in test diet VI were noted to be highest, and lowest values were noted in fish fed control diet. Highest values of MCV (188 fl) as well as MCHC (35%) were observed in fish fed on test diet V and MCH (57 pg) was reported to be highest in fish fed on test diet VI. These noted values significantly varied from the values noted in fish fed on control diet. The hematology of fish showed that 400 mg/kg level is optimum for monitoring stress response, healthy fish growth as well as measuring immune response of fish through WBCs count.
Table V.- Analyzed mineral compositions in feces of C. carpio fingerlings fed on canola meal based diet supplemented with polyphenols.
|
Minerals / Polyphenols levels (mg/kg) |
Test diet –I (control diet) |
Test diet –II |
Test diet –III |
Test diet –IV |
Test diet –V |
Test diet –VI |
Test diet –VII |
|
0 |
100 |
200 |
300 |
400 |
500 |
600 |
|
|
Ca |
0.51± 0.02a |
0.49± 0.03ab |
0.42± 0.04bc |
0.38± 0.04c |
0.27±0.02d |
0.29±0.02d |
0.39±0.02c |
|
Na |
0.07± 0.01ab |
0.07± 0.01a |
0.05± 0.01bc |
0.05± 0.01bc |
0.04±0.05c |
0.04±0.004c |
0.07±0.01ab |
|
K |
0.008± 0.001a |
0.007± 0.0005ab |
0.007± 0.0006ab |
0.006± 0.0005bc |
0.004±0.0005c |
0.007±0.0006ab |
0.008±0.0005a |
|
Fe |
0.030± 0.003a |
0.027± 0.002ab |
0.025± 0.002abc |
0.021± 0.002bcd |
0.020±0.001cd |
0.018±0.002d |
0.023±0.002bcd |
|
Cu |
0.003± 0.0002a |
0.003± 0.0003a |
0.002± 0.0002ab |
0.002± 0.0001c |
0.002±0.0002bc |
0.002±0.0002ab |
0.003±0.0004a |
|
P |
1.08± 0.05a |
0.94± 0.04b |
0.71± 0.02c |
0.67± 0.01c |
0.53±0.04d |
0.64±0.04c |
0.86±0.02b |
|
Mg |
0.005± 0.0003ab |
0.004± 0.0002abc |
0.003± 0.0002de |
0.003± 0.0003e |
0.003±0.0003cde |
0.004±0.0003bcd |
0.005±0.0007a |
|
Al |
0.0003± 0.00003a |
0.0002± 0.00003ab |
0.0002± 0.00002ab |
0.0002± 0.00003ab |
0.0002± 0.00003b |
0.0002± 0.00002b |
0.00025± 0.00003ab |
Means within same column having different superscripts are significantly different at p ˂ 0.05. Data are means of three replicate. Ca, calcium; Na, sodium; K, potassium; Fe, iron; Cu, copper; P, phosphorus; Mg, magnesium; Al, aluminium.
Table VI.- Minerals absorption of C. carpio fingerlings fed on canola meal based diet supplemented with polyphenols.
|
Minerals / Polyphenols levels (mg/kg) |
Test diet –I (control diet) |
Test diet –II |
Test diet –III |
Test diet –IV |
Test diet –V |
Test diet –VI |
Test diet –VII |
|
0 |
100 |
200 |
300 |
400 |
500 |
600 |
|
|
Ca |
48.54±0.92f |
52.40±0.58de |
50.77±0.25e |
54.71±0.65c |
63.44±0.89b |
71.34±0.49a |
52.97±0.79cd |
|
Na |
48.59±0.35a |
51.73±0.75a |
50.99±0.88a |
52.47±0.87a |
59.57±0.83a |
53.76±0.72a |
67.74±0.73a |
|
K |
55.62±0.90e |
57.78±0.91de |
61.60±0.88c |
66.68±0.91b |
73.64±0.79a |
61.49±0.92c |
58.55±0.96d |
|
Fe |
47.49±0.80f |
53.43±0.89e |
56.82±0.94d |
61.39±0.89c |
64.30±0.97b |
68.53±0.89a |
62.18±0.89bc |
|
Cu |
53.67±0.97d |
50.40±0.94e |
57.17±0.90c |
69.38±0.59a |
65.66±0.90b |
58.43±0.63c |
52.49±0.68de |
|
P |
52.51±0.43f |
58.39±0.19e |
68.46±1.06c |
70.26±0.53bc |
76.42±0.16a |
71.43±0.81b |
63.43±0.86d |
|
Mg |
51.75±1.30d |
53.79±1.45d |
64.31±0.97ab |
67.32±0.94a |
62.77±0.90b |
57.52±0.95c |
50.86±0.97d |
|
Al |
47.70±0.85d |
52.17±0.92c |
58.55±0.97b |
60.57±0.75b |
63.27±0.78a |
64.41±0.75a |
58.28±0.81b |
Means within same column having different superscripts are significantly different at p ˂ 0.05. Data are means of three replicates. Ca, calcium; Na, sodium; K, potassium; Fe, iron; Cu, copper; P, phosphorus; Mg, magnesium; Al, aluminium.
Table VII.- Hematological parameters of C. Carpio fingerlings fed different levels of canola meal based diet supplemented with polyphenols.
|
Experimental diets |
Polyphenols levels (mg/kg) |
WBCs (103mm-3) |
RBCs (106mm-3) |
PLT |
Hb (g/100ml) |
PCV (%) |
MCHC (%) |
MCH (pg) |
MCV (fl) |
|
Test diet-I (control) |
0 |
6.63±0.27b |
1.84± 0.40b |
54.42± 0.29e |
6.37±0.11d |
21.20± 0.50c |
24.68± 0.69e |
37.64± 0.09e |
90.26± 0.10g |
|
Test diet-II |
100 |
6.90±0.50b |
2.14± 0.28ab |
60.69± 0.33d |
6.57±0.10cd |
22.38± 0.10bc |
27.69± 0.31d |
38.61± 0.35e |
111.30± 0.90f |
|
Test diet-III |
200 |
7.35±0.20ab |
2.31± 0.14ab |
63.47± 0.44c |
7.35±0.18bc |
23.72± 0.65ab |
31.38± 0.18c |
41.70± 0.44d |
181.05± 0.48d |
|
Test diet-IV |
300 |
7.76±0.12a |
2.76± 0.32ab |
65.96± 0.13b |
8.47±0.08a |
24.51± 0.13a |
33.81± 0.22b |
49.97± 0.13c |
187.11± 0.16b |
|
Test diet-V |
400 |
7.85±0.08a |
2.98± 0.29a |
65.65± 1.00b |
8.45±0.15a |
24.91± 0.46a |
35.24± 0.46a |
52.10± 1.78b |
188.36± 0.39a |
|
Test diet-VI |
500 |
7.32±0.38ab |
2.26± 0.64ab |
67.34± 0.11a |
7.78±0.32ab |
23.91± 0.78a |
33.82± 0.71b |
56.84± 0.30a |
183.01± 0.27c |
|
Test diet-VII |
600 |
6.87±0.29b |
1.85± 0.16b |
66.66± 0.16ab |
7.89±0.32bcd |
23.66± 0.28ab |
33.12± 0.21b |
50.02± 0.21c |
173.69± 0.24e |
RBC, red blood cell; WBC, white blood cell; PLT, platelet; Hb, hemoglobin. Means within rows having different superscripts are significantly different at p< 0.05. Data are means of three replicates.
Discussion
Supplementation of polyphenols significantly influenced whole-body composition of fish in present study. The studies conducted by other researchers are in agreement with our results, who observed significant changes in whole-body crude protein (Nandeesha et al., 2001; Abdel-Tawwab and Ahmad, 2009; Promya and Chitmanat, 2011), crude lipid (Nandeesha et al., 1998, 2001; Abdel-Tawwab and Ahmad, 2009), ash (Nandeesha et al., 2001; Tongsiri et al., 2010) and moisture contents (Tongsiri et al., 2010; Promya and Chitmanat, 2011) of fish fed on feed with supplementation of spirulina (source of polyphenol). Significant increase in whole-body lipid was reported for Labeo rohita by spirulina intake (Nandeesha et al., 2001). Similarly, the results of study conducted by Wafaa et al. (2014) showed that crude protein was significantly (p<0.05) increased in groups of fish fed on green tea (GT), black seed and propolis extract (polyphenols sources), when compared to control group. Likewise, results of Abdel-Tawwab et al. (2010) are also in agreement with our findings, who confirmed that addition of GT extract in diet up to 0.5g and 1 g/kg diet increased content of protein and 0.5 g/kg diet significantly increased total lipids whereas, 1g/kg diet significantly decreased total lipids content. Supplementation of propolis, at the level of 50 g/kg, increased whole-body content of lipid and protein in juvenile upto peak values.
Cho et al. (2007) reported increase in total protein (TP) in flounder fed green tea polyphenols in diet. Using roselle calyx in the experimental diets, Mesallamy et al. (2016) observed a trend that with its increasing levels, crude protein of fish bodies increased whereas total lipid decreased significantly (p<0.05). However, contradictory results were also observed, Kim et al. (2013a) found that spirulina had no significant effect on whole-body composition of parrot fish, same results were reported in studies with silver sea bream, siberian sturgeon and red tilapia hybrid (El-Sayed, 1994; Palmegiano et al., 2005; Ungsethaphand et al., 2010). Kim et al. (2013a) used spirulina as replacement for fishmeal for parrot fish and did not find any significant differences in carcass or flesh protein content of common carp and mekong giant catfish (Nandeesha et al., 1998; Tongsiri et al., 2010) at any level of spirulina administration. Similarly, Zhai et al. (2013) reported that supplementation of polyphenols i.e. quercetin, lowered the crude lipid level in body of Nile Tilapia. No significant differences were noted in the whole body compositions of trout juveniles fed propolis for 10 weeks (Deng et al., 2011). Abdelwahab et al. (2012) observed that crude protein and crude ash contents of Asian sea-bass were not significantly affected by supplementation of 5 and 10g/kg black cumin seed to the diet. Likewise, results of study conducted by Amer (2016) depicted no significant difference in protein and ash content among treatments, but there was significant decrease in fat content between control group and other groups.
Abdel-Latif and Khalil (2014) reported non-significant differences among treatments for lipid content, moisture and ash, but the protein content in muscle of fish fed 1000 g/kg spirulina diet was the highest. Hwang et al. (2013) found that contents of crude protein and moisture in whole body, dorsal muscle as well as in liver were not significantly different between the control and test diet groups (p<0.05). Significantly lowered lipid and ash contents in whole body of the group fed on 500 g/kg level based diet than those of control diet group (p<0.05) were observed. Fallahpour et al. (2015), in their study found that polyphenols-rich marshmallow extract brought slight variations in body composition of fish as compared to controls. There were no significant changes in moisture levels in all groups. Compared to controls, lipid levels for the dried body were the lowest after feeding the fish marshmallow extract 50g/kg. The whole body ash reduced after administering marshmallow extract at 250 g/kg as compare to control groups. Katya et al. (2014) found that replacement of fish meal by fermented by-product of mushroom in the diets (p<0.05) did not significantly affect whole-body proximate composition of fish.
Our results have shown that 400 mg/kg is the optimum level of polyphenols which can increase the retention of minerals in body of C. carpio fingerlings fed polyphenols supplemented canola based diets. Since less work has been conducted on this parameter, therefore few relevant data was found. Contrary to our finding, Frejnagel and Wroblewska (2010) reported that they used various polyphenolic extracts in feed of a monogastric animal and conducted a comparative study. They reported that polyphenolic extracts reduced the concentrations and apparent absorption of some minerals as Zn and Cu. However, the concentrations of Ca, Fe, P and Mg in the femur ash were not affected. Differences encountered might be due to different specie used, source of polyphenols, conditions and concentration.
Hematological indices are indeed an important tool for judging the health status of fish (Abdel-Tawwab et al., 2010). Use of polyphenols aided in significantly higher Ht, Hb and RBC levels as compared to diet which lack polyphenols, i.e. control diet. Our findings are in tandem with the work of Mesallamy et al. (2016), who analyzed hematological parameters (Hb, Ht, and RBCs) for Nile tilapia fed on Hibiscus sabdariffa calyx, a potent source of polyphenols. They reported that there was a numerical increase in Hb, Ht and RBC, as the level of H. sabdariffa calyx increased in concentration. Similar results were found from the work of Olusola (2011), who found that the anti-oxidative potency of Roselle calyx extract, resulted in a gradual increase in Hb, Ht and RBC as the concentration increased. Another contradiction is witnessed in results of Hwang et al. (2013) who reported that Hb and Ht levels of group fed on control diet were found to be significantly higher than group fed on 5 % Green tea extract diet (p<0.05) in Sebastes schlegelii. Contradictions among our results and results of other researchers might be due to different source of polyphenols used, variation in species, their nutrient requirements, environmental conditions, varying levels and other such factors (Zhai et al., 2013).
Conclusion
Polyphenols, used directly or indirectly (i.e. through rich source), has significant effect on body composition of common carp as a result of increased mineral absorption, improved body composition and hematology of Common carp fingerlings. Supplementation of the polyphenols in canola meal based diet at the rate of 400 mg/kg showed maximum improvement in fingerlings of common carp.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Abdel-Latif, H.M. and Khalil, R.H., 2014. Evaluation of two phytobiotics, Spirulina platensis and Origanum vulgare extract on growth, serum antioxidant activities and resistance of Nile tilapia (Oreochromis niloticus) to pathogenic Vibrio alginolyticus. Int. J. Fish. aquat. Stud., 1: 250-255.
Abdel-Tawwab, M. and Ahmad, M.H., 2009. Live spirulina (Arthrospira platensis) as a growth and immunity promoter for nile tilapia, Oreochromis niloticus (L.), challenged with pathogenic Aeromonas hydrophila. Aquacul. Res., 40: 1037-1046. https://doi.org/10.1111/j.1365-2109.2009.02195.x
Abdel-Tawwab, M., Ahmad, M.H., Seden, M.E. and Sakr, S.F., 2010. Use of green tea, Camellia sinensis L. in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J. World Aquacul. Soc., 41: 203-213. https://doi.org/10.1111/j.1749-7345.2010.00360.x
Abdelwahab, A.M. and El-Bahr, S.M., 2012. Influence of black cumin seeds (Nigella sativa) and turmeric (Curcuma longa Linn.) mixture on performance and serum biochemistry of Asian sea bass, Lates calcarifer. World J. Fish Mar. Sci., 4: 496-503.
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidynus bidyanus). Austasia. Aquacul., 6: 39-40.
Amer, S.A., 2016. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex Nile tilapia (Oreochromis niloticus). Benha Vet. med. J., 30: 1-10. https://doi.org/10.21608/bvmj.2016.31332
AOAC, 1995. Official methods of analysis, 15th ed. Association of Official Analytical Chemists, Washington DC, USA, pp. 1094.
Blaxhall, P.C. and Daisley, K.W., 1973. Routine haematological methods for use with fish blood. J. Fish Biol., 6: 771-778. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Brown, B.A., 1980. Routine hematology procedures. In: Hematology: Principles and procedures. 3rd Ed, Lea & Febiger, Philadelphia.
Cho, S.H., Lee, S.M., Park, B.H., Ji, S.C., Lee, J., Bae, J. and Oh, S.Y., 2007. Effect of dietary inclusion of various sources of green tea on growth, body composition and blood chemistry of the juvenile olive flounder, Paralichthys olivaceus. Fish Physiol. Biochem., 33: 49-57. https://doi.org/10.1007/s10695-006-9116-3
Coyle, S.D., Durborow, R.M. and Tidwell, J.H., 2004. Anesthetics in aquaculture. Publication 3900, Southern Regional Aquaculture Center. Stoneville, Mississippi.
Deng, J., An, Q., Bi, B., Wang, Q., Kong, L., Tao L. and Zhang, X., 2011. Effect of ethanolic extract of propolis on growth performance and plasma biochemical parameters of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem., 37: 959-967. https://doi.org/10.1007/s10695-011-9493-0
Divakaran, S., Leonard, G.O. and Lan, P.F., 2002. Note on the methods for cetermination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
El-Banna, S.A. and Atallah, S.T., 2009. Study the role of feed additives in prevention of fish diseases incidence in Oreochromis niloticus and common carp fish and its economic importance. J. World Aquacul. Soc., 4: 121-140.
El-Sayed, A.F.M., 1994. Evaluation of soybean meal, spirulina meal and chicken offal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture, 127: 169-176. https://doi.org/10.1016/0044-8486(94)90423-5
Enami, H.R., 2011. A review of using canola/rapeseed meal in aquaculture feeding. J. Fish. aquat. Sci., 6: 22-36. https://doi.org/10.3923/jfas.2011.22.36
Fallahpour, F., Banaee, M. and Javadzade, N., 2015. The effects of hydro-alcohol extract of follower of marshmallow (Althaea officinalis L.) on some biochemical and hematological parameters in common carp (Cyprinus carpio L.). J. Herb. Drugs, 6: 73-83.
FAO, 2010. The state of world fisheries and aquaculture. Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations, Rome, Italy.
Frejnagel, S. and Wroblewska, M., 2010. Comparative effect of green tea, chokeberry and honeysuckle polyphenols on nutrients and mineral absorption and digestibility in rats. Annls. Nutr. Metab., 56: 163-169. https://doi.org/10.1159/000278747
Gabriel, U.U., Akinrotimi, O.A., Bekibele, D.O., Onunkwo, D.N. and Anyanwu, P.E., 2007. Locally produced fish feed: Potentials for aquaculture development in subsaharan Africa. Afr. J. agric. Res., 2: 287-295.
Higgs, D.A., Dosanjh, B.S., Prendergast, B.R.M., Hardy, R.W., Riley, W. and Deacon, G., 1995. Nutrition and utilization technology in aquaculture. Chapman and Hall Company, New York, USA, pp. 130-156.
Hussain S.M., Afzal, M., Nasir, S., Javid, A., Azmat, H., Shah, S.Z.H., Hussain, M., Mustafa, I. and Iqbal, M., 2015. Role of phytase supplementation in improving nutrient digestibility and growth performance for Labeo rohita fingerlings fed on canola meal-based diet. J. appl. Anim. Res., 45: 15–21. https://doi.org/10.1080/09712119.2015.1091331
Hwang, J.H., Lee, S.W., Rha, S.J., Yoon, H.S., Park, E.S., Han, K.H. and Kim, S.J., 2013. Dietary green tea extract improves growth performance, body composition, and stress recovery in the juvenile black rockfish, Sebastes schlegeli. Aquacul. Int., 21: 525-538. https://doi.org/10.1007/s10499-012-9586-5
Katya, K., Yun, Y.H., Park, G., Lee, J.Y., Yoo, G. and Bai, S.C., 2014. Evaluation of the efficacy of fermented by-product of mushroom, Pleurotus ostreatus, as a fish meal replacer in juvenile Amur catfish, Silurus asotus: Effects on growth, serological characteristics and immune responses. Asian-Australas. J. Anim. Sci., 27: 1478-1486. https://doi.org/10.5713/ajas.2014.14038
Khan, M.N., Shahzad, K., Chatta, A., Sohail, M., Piria, M. and Treer, T., 2016. A review of introduction of common carp Cyprinus carpio in Pakistan: Origin, purpose, impact and management. Croatian J. Fish., 74: 71-80. https://doi.org/10.1515/cjf-2016-0016
Kim, S., Rahimnejad, S., Kim, K. and Lee, K., 2013a. Partial replacement of fish meal with Spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus). Turkish J. Fish. aquat. Sci., 13: 197-204.
Kim, S., Rahimnejad, S., Kim, K., Lee, B. and Lee, K., 2013b. Effects of dietary supplementation of spirulina and quercetin on growth, innate immune responses, disease resistance against Edwardsiella tarda, and dietary antioxidant capacity in the juvenile olive flounder Paraliabrchthys olivaceus. J. Fish. aquat. Sci., 16: 7-14. https://doi.org/10.5657/FAS.2013.0007
Lovell, R.T., 1989. Fish nutrition and feeding. Van Nostrand-Reinhold Co., New York. https://doi.org/10.1007/978-1-4757-1174-5
Mahboob, S., 2014. Replacing fish meal with a blend of alternative plant proteins and its effect on the growth performance of Catla catla and Hypophthalmichthys molitrix. Pakistan J. Zool., 46: 747-752.
Maqsood, S., Benjakul, S. and Kamal-Eldin, A., 2012. Haemoglobin-mediated lipid oxidation in the fish muscle: A review. Trends Fd. Sci. Technol., 28: 33-43. https://doi.org/10.1016/j.tifs.2012.06.009
Maqsood, S., Benjakul, S. and Shahidi, F., 2013. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Fd. Sci. Nutr., 53: 162-179. https://doi.org/10.1080/10408398.2010.518775
Maqsood, S., Benjakul, S., Abushelaibi, A. and Alam, A., 2014. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compreh. Rev. Fd. Sci. Fd. Safe., 13: 1125-1140. https://doi.org/10.1111/1541-4337.12106
Mesallamy, A.M., Ahmad, M.H., Souleman, A.M., El Morsy, A.T. and El-Naby, A.S.A., 2016. Effects of roselle calyx (Hibiscus sabdariffa L.) supplemented diets on growth and disease (Aeromonas hydrophila) resistance in Nile tilapia (Oreochromis niloticus L.). Egypt. Pharmaceut. J., 15: 78-87. https://doi.org/10.4103/1687-4315.190403
Nandeesha, M.C., Gangadhar, B., Varghese, T.J. and Keshavanath, P., 1998. Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio L. Aquacul. Res., 29: 305-312. https://doi.org/10.1111/j.1365-2109.1998.tb01135.x
Nandeesha, M.C., Gangadhara, B., Manissery, J.K. and Venkataraman, L.V., 2001. Growth performance of two Indian major carps, catla (Catla catla) and rohu (Labeo rohita) fed diets containing different levels of Spirulina platensis. Bioresour. Technol., 80: 117-120. https://doi.org/10.1016/S0960-8524(01)00085-2
NRC, 1993. Nutrient requirements of fish. Committee on Animal Nutrition, Board on Agriculture, National Research Council, National Academy Press, Washington DC, USA.
Olusola, A.O., 2011. Evaluation of the antioxidant effects of Hibiscus sabdariffa calyx extracts on 2, 4-dinitrophenylhydrazine-induced oxidative damage in rabbits. Webmed Cent. Biochem., 2: WMC002283. https://doi.org/10.5923/j.ajb.20120202.01
Palmegiano, G.B., Agradi, E., Forneris, G., Gai, F., Gasco, L., Rigamonti, E., Sicuro, B. and Zoccarato, I., 2005. Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquacul. Res., 36: 188-195. https://doi.org/10.1111/j.1365-2109.2005.01209.x
Peake, S., 1998. Sodium bicarbonate and clove oil as potential anesthetics for nonsalmonid fishes. N. Am. J. Fish. Manage., 4: 919-924. https://doi.org/10.1577/1548-8675(1998)018<0919:SBACOA>2.0.CO;2
Pickova, J. and Morkore, T., 2007. Alternate oils in fish feeds. Eur. J. Lipid Sci. Technol., 109: 256-263. https://doi.org/10.1002/ejlt.200600222
Promya, J. and Chitmanat, C., 2011. The effects of Spirulina platensis and Cladophora algae on the growth performance, meat quality and immunity stimulating capacity of the African sharptooth catfish (Clarias gariepinus). Int. J. Agric. Biol., 13: 77-82.
Robinson, E.H., Li, M.H. and Manning, B.B., 2002. Comparison of microbial phytase and dicalcium phosphate or growth and bone mineralization of pond-raised channel catfish, Ictalurus punctatus. J. appl. Aquacul., 12: 81–88. https://doi.org/10.1300/J028v12n03_08
Rowland, S.J. and Ingram, B.A., 1991. Diseases of Australian native fishes. Fisheries Bulletin 4 NSW Fisheries, Sydney, NSW, Australia, pp. 21-23.
Sajjadi, M. and Carter, C.G., 2004. Effect of phytic acid and phytase on feed intake, growth, digestibility and trypsin activity in Atlantic salmon (Salmo salar, L.). Aquacult. Nutr., 10: 135-142. https://doi.org/10.1111/j.1365-2095.2003.00290.x
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods, 8th ed. Iowa State University Press, Americans, USA, pp. 503.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics. McGraw Hill International Book Co. Inc., New York, USA, pp. 336-352.
Tongsiri, S., Mang-Amphan, K. and Peerapornpisal, Y., 2010. Effect of replacing fishmeal with spirulina on growth, carcass composition and pigment of the Mekong giant catfish. Asian J. agric. Sci., 2: 106-110.
Ungsethaphand, T., Peerapornpisal, Y., Whangchai, N. and Sardsud, U., 2010. Effect of feeding Spirulina platensis on growth and carcass composition of hybrid red tilapia (Oreochromismossambicus × O. niloticus). MAEJO Int. J. Sci. Technol., 4: 331-336.
Wafaa, E., Doaa, I., El-Murr, A. and Rania, M., 2014. Effects of dietary inclusion of black cumin seeds, green tea and propolis extraction growth parameters, body composition and economic efficiency of Nile tilapia, Oreochromis niloticus. World J. Fish. Mar. Sci., 6: 447-452.
Wedemeyer, G.A. and Yastuke, W.T., 1977. Clinical methods for the assessment of the effects of environmental stress on fish health. U.S. Fish Wildl. Serv. Tech. Pap. No. 89.
Yigit, N.O. and Olmez, M., 2009. Canola meal as an alternative protein source in diets for fry of tilapia (Oreochromis niloticus). Israeli J. Aquacul. (Bamidgeh), 16: 35-41.
Zhai, S.W. and Liu, S.L., 2013. Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Italian J. Anim. Sci., 12: 523-527. https://doi.org/10.4081/ijas.2013.e85
To share on other social networks, click on any share button. What are these?










