Effects of Extraction Solvents and Drying Techniques on Antioxidant Content and Radical Scavenging Activity of Roselle (Hibiscus sabdariffa) Calyx
Effects of Extraction Solvents and Drying Techniques on Antioxidant Content and Radical Scavenging Activity of Roselle (Hibiscus sabdariffa) Calyx
Nur Anis Hashim1, Nor Hasima Mahmod1*, Abubakar Abdullahi Lema2, Lee-Hoon Ho3 and Mohammad Moneruzzaman Khandaker1
1School of Agricultural Science and Biotechnology, Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia; 2Department of Biological Sciences, College of Natural and Applied Science, Al-Qalam University Katsina, 2137, Katsina State, Nigeria; 3Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia.
Abstract | Hibiscus sabdariffa or roselle is well known for its high antioxidant content. This species is originated in West Africa and spread to Asia by the colonials in 1700s. The optimal conditions for the preparation of dried materials for further processing and suitable drying techniques while preserving their antioxidants have yet to be inferred. Thus, the present study aims to determine the content and activity of the antioxidants in fresh and dried roselle calyx. The random sampling technique was applied by collecting the calyx roselle from different plants. Calyx samples were labeled as fresh calyx (FC), air-dried (AD), oven-dried (OD), and freeze-dried (FD) according to treatments. Four extracting solvents, which were methanol, ethanol, aqueous, and hexane, were evaluated for their efficiency in extracting antioxidant components from the differently treated roselle calyx, and these extracts were used to determine antioxidant activities and contents by assessing DPPH scavenging activity, total phenolic content (TPC) and total flavonoid content (TFC). Next, the samples were subjected to qualitative phytochemical screenings. DPPH scavenging activity was the highest in an FC sample extracted by methanol with 81.697 mg/mL. The highest TPC was measured in an OD sample extracted by ethanol (172.04 ±0.43) and the most TFC was found in FC ethanol extract (22.9±0.03). Among the fresh calyx and three drying methods investigated, FC in ethanol produces the highest potential in antioxidant activity, followed by FD, AD and OD. Phytochemical screening tests found that the fresh and freeze-dried calyx possessed important antioxidative metabolites such as anthocyanins, tannins, saponins, glycosides, flavonoids and terpenoids. The findings demonstrated that fresh calyx retained the highest antioxidant capacity, thus providing insights into post-harvest processing of the calyx.
Received | February 22, 2024; Accepted | August 30, 2024; Published | October 09, 2024
*Correspondence | Nor Hasima Mahmod, School of Agricultural Science and Biotechnology, Faculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin, Besut Campus, 22200 Besut, Terengganu, Malaysia; Email: norhasima@unisza.edu.my
Citation | Hashim, N.A., N.H. Mahmod, A.A. Lema, LH. Ho and M.M. Khandaker. 2024. Effects of extraction solvents and drying techniques on antioxidant content and radical scavenging activity of roselle (Hibiscus sabdariffa) calyx. Sarhad Journal of Agriculture, 40(Special issue 1): 110-121.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.110.121
Keywords | DPPH roselle, Drying, Flavonoid content, Phenolic content
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Roselle (Hibiscus sabdariffa L.) is a Malvaceae (cotton or okra) family member and can be an annual or perennial shrub. It is a primary profit crop in China, Sudan, and Thailand, but it is considered an orphan crop in many tropical countries. Roselle is considered traditional medicine and has been used to treat sore throat problems and heal wounds (Singh et al., 2017). For instance, tea made from dried calyx is a standard beverage in Thailand and Sudan. Sudan is Africa’s leading roselle producer, with the flower and calyx having numerous uses in the food and beverage industry. The roselle calyx is commonly harvested as soon as it shows signs of maturity to maintain its quality (Tandoh et al., 2022). Roselle’s physical properties differ according to its moisture content. Size, weight, pH, anthocyanin concentrations, titratable acidity, soluble solids, organic acids, and sugars were used to evaluate the physicochemical properties of Malaysian roselle. Oxalic acids and succinic are the most predominant organic acids in roselle, with glucose being the most abundant carbohydrate (Aishah et al., 2013).
This study assessed the antioxidant content and activity in freshly harvested and dried roselle calyx, both quantitative and qualitatively. Numerous previous research studies have found that roselle calyx has a relatively high antioxidant activity and content. A combination of antioxidants may provide the most effective barrier against oxidative stress-related disorders (Huang et al., 2009). An antioxidant is a redox-active substance that lowers oxidative pressure by responding to a receptive oxidant. Enzymatic antioxidants are essential enzymes that are naturally generated, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). They generally protect from free radicals and harmful inflammatory reactions (Pisoschi and Pop, 2015). It is also high in catalytic properties and is involved in eliminating specific free radicals (Couttolenc et al., 2020). Non-enzymatic antioxidants are secondary metabolites that can exhibit the scavenging ability of oxidants.
In order to provide the best post-harvest storage at a minimum loss, the roselle industries need to determine a natural, efficient and safe method. In addition, the method must consider the storage period, too. At the moment, an adequate drying and storage system for roselle calyx has yet to be studied, causing the current roselle industries to face significant loss of production. Also, the high moisture content in roselle calyx leads to the rapid deterioration of roselle calyx (Gomaa and Nahed, 2016). The previous research states that roselle is a perishable and non-climatic product that is exposed to rapid physiological deterioration after harvest. In response to this problem, this study proposes a suitable drying condition for roselle calyx. This study will also provide good opportunities to commercialize Hibiscus sabdariffa in the future so that it can be widely used as one of the natural antioxidants. This study on antioxidant content and activities in roselle calyx have beneficial input to the roselle producer. This is because the difference in antioxidant content will influence the effect of drying methods of the calyx as well as suggest the best conditions for marketing it. Subsequently, by mitigating post-harvest losses, the roselle producer can position Malaysia as the leading producer of roselle calyx. Further details from scientific studies on the antioxidant content and activities of Hibiscus sabdariffa extracts will be presented, expanding its commercial value. This study aims to identify the antioxidant content and activity of fresh and dried roselle calyx and determine the effects of drying on antioxidant contents and activity in roselle calyx.
Materials and Methods
Sample collection and preparation
The calyx samples of Hibiscus sabdariffa UMKL-1 variety were collected from the Faculty of Bioresources and Food Industries farm (5.755048413750099, 102.627941810822). The calyx was washed to remove dirt and contaminants. The calyx samples were designated as fresh calyx samples (FC), air-dried samples (AD), oven-dried samples (OD), and freeze-dried samples (FD). The term “fresh calyx sample” (FC) refers to a sample in which the fresh calyx was processed immediately without undergoing drying. The Hibiscus sabdariffa air-dried sample (AD) was obtained by tapping the samples with a tissue after washing them and leaving them at room temperature (27–28°C) until completely dry. The oven-dried sample (OD) was prepared by keeping the calyces in an operational laboratory oven for three days. The temperature was kept at 60 °C until the weight was constant (Lema et al., 2022). The freeze-dried sample (FD) was prepared by placing the roselle calyces in the freezer at -80 oC for seven days.
Roselle extraction preparation
The roselle extraction was conducted using the method of Karimi and Moradi (2015). Ethanol, methanol, aqueous (polar solvents) and hexane (nonpolar solvent) were used as the extraction solvents. For the fresh calyx (FC) sample, the calyx was ground in the solvents by using a Waring blender with a ratio of 1:50 (sample: solvents). The calyx was ground to form powder, which then macerated at a 1:50 (sample: solvents) w/v ratio at room temperature. The mixture was sonicated for 10 minutes and paused for 5 minutes before being sonicated again for another 10 minutes. This process was repeated three times. Next, the mixture was filtered through Whatman Filter No. 1, and the filtrate was concentrated to dry in a rotary evaporator (Heidolph, Germany). The resulting crude extracts were kept at -20 °C before use. The percentage yield of the Hibiscus sabdariffa for each extraction was calculated using the formula:
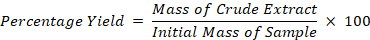
DPPH radical scavenging assay
The assay was carried out using a slightly modified method published by Clarke et al. (2013). In a 96-well microplate. Aliquots of 50 µL of each of the test samples and quercetin (positive control) serial dilutions (3.91, 7.81, 15.63, 31.25, 62.5, 125, 250, and 500 ppm) were added to separate well before adding 100 µL of 59 mg/L DPPH. A 30 minutes incubation followed this in the dark and absorbance measurement at 517 nm. The analysis was carried out in triplicates. The percentage inhibition was calculated from the formula.
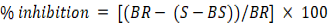
Where, BR= Absorbance of reagent blank, S= Absorbance of samples and BS= Absorbance of blank sample
Total phenolic content
The total phenolic content (TPC) was determined using the Folin-Ciocalteu technique (Miliauskas et al., 2004) and expressed as gallic acid equivalent (mg GAE/g). About 1 mL of gallic acid standard solution of different concentrations and the absorbance was measured at 765 nm to obtain a gallic acid standard curve. This was followed with 10 µL extracts and 100 µL of 10% Folin-Ciocalteu reagents (v/v). Next, 80 µL of Na2Co3 (7.5%) was added to the mixture and incubated at 45 °C for 40 minutes, and absorbance was measured again. Formula C = (c * V)/ m used. Where; C = Total Phenolic Content (mg/g), c = Concentration of gallic acid calculated from a standard curve (mg/mL), V = Volume of extracts (mL) and m = Weight of pure plant extract (g).
Total flavonoid content
The total flavonoid content (TFC) was determined by following a method described by Chang et al. (2002) and was expressed as quercetin equivalent (mg QE/g). About 1 mL serially diluted quercetin was added to separate wells of a 96-well plate and measured at 595 nm to establish a standard curve. Next, 25 µL of extracts and 10 µL of 5% NaNo3 were added before incubating the mixture for 6 minutes in the dark at room temperature. Then, 15 µL of AlCl3 was added and incubated for 5 minutes at room temperature. Next, 50 µL of 1 M NaOH was added, and the mixture was gently agitated for 1 minute. The absorbance was read at 595 nm by using an ELISA reader. C = (c * V) /g formula was used. Where; C = Total Flavonoid Content (mg/mL), c = Quercetin concentration from standard curve (mg/mL), V = Volume of extracts (mL) and m = Weight of extract (g).
Qualitative phytochemicals screening
The standard procedures of the phytochemical test was carried out as described by Iqbal et al. (2015).
Data analysis
All tests in the present study were conducted as triplicates. The data was analyzed by One-way ANOVA and Two-way ANOVA for single-factor and two-factor comparison, respectively, using SPSS version 25. Tukey post-hoc test was applied for multiple comparison analysis. The correlation analysis was conducted using Pearson’s correlation coefficient. Differences at p ≤ 0.05 were considered significant.
Results and Discussion
Extraction yields
Drying techniques are one of the main factors affecting extraction yield. The yield of sample calyx extracts resulting from different extraction solvents is summarized in Table 1. Overall, fresh calyx that did not undergo any drying process showed a higher percentage yield than the other sample. However, for the dried roselle calyx, freeze-dried showed the highest extraction yield. Based on Table 1, FD-EE produced the highest percentage. This is in agreement with a previous study by Phaechamud et al. (2012), which reported that freeze-dried extracts produce higher extraction yield than air-dried in their study in Sonneratia caseolaris flower.
Table 1: Percentage of extraction yield for fresh and dried roselle calyx.
|
Sample name |
Drying methods |
Extraction solvent |
Extraction yield (%) |
|
FC-ME |
Fresh calyx (FC) |
Methanol |
48.8 |
|
FC-EE |
Ethanol |
37.9 |
|
|
FC-AE |
Aqueous |
23.5 |
|
|
FC-HE |
Hexane |
17.32 |
|
|
OD-ME OD-EE |
Oven-dried (OD) |
Methanol |
30.4 |
|
Ethanol |
33.1 |
||
|
OD-AE |
Aqueous |
27.95 |
|
|
OD-HE |
Hexane |
15.04 |
|
|
AD-ME |
Air-dried (AD) |
Methanol |
29.98 |
|
AD-EE |
Ethanol |
27.09 |
|
|
AD-AE |
Aqueous |
23.32 |
|
|
AD-HE |
Hexane |
8.92 |
|
|
FD-ME |
Freeze-dried (FD) |
Methanol |
43.35 |
|
FD-EE |
Ethanol |
47.19 |
|
|
FD-AE |
Aqueous |
20.66 |
|
|
FD-HE |
Hexane |
11.18 |
According to a previous study, polar solvents such as ethanol and methanol were more efficient in producing high yields in plant material extracts such as leaves or petals. A more polar solvent was able to extract more secondary chemicals than a non-polar solvent, based on the data obtained. Polar substances that can dissolve include glycosides, sugar, amino acids (Hussein and El-Anssary, 2019), low and intermediate molecular weights and medium polarity phenolic compounds, anthocyanin, quacinoid, lacton, flavone, terpenoid, saponin, tannin, xantoxilin, phenone, and polyphenol (Widyawati et al., 2014). The present study also suggests that polar secondary metabolites are more abundant in H. sabdariffa calyx extracts than non-polar ones and organic solvents such as ethanol are superior in terms of recovering a larger extraction yield of antioxidant components from H. sabdariffa calyx extracts. Understanding that an ideal extraction yield may not necessarily result in increased antioxidant activity is important. Instead, it is possible that these solvents merely solubilize a wider variety of compounds, some of which may have negligible or no antioxidant activity. The method of drying the H. sabdariffa prior to extraction also affected the extraction yield. Fresh calyx had the highest extraction yield, while air-dried had the lowest.
Employing more advanced extraction techniques, such as microwave-assisted extraction (MAE) or ultrasound-assisted extraction (UAE), always increases cell wall disruption and surface contact between samples and solvents. This method improves extraction yield by lowering the internal and external mass transfer constraints.
Dhanani et al. (2017) reported, ultrasound changes the physical and chemical compositions of plant materials, breaking down the cell wall. This process aids in releasing compounds and improves the solvent’s ability to penetrate plant cells. According to Rasul (2018), ultrasound treatments are cost-effective, simple, and dependable, making them a viable alternative to traditional extraction methods.
DPPH free radical scavenging activity
The stable free radical 1, 1-diphenyl-2-picrylhydrazyl (DPPH) will decolorize when antioxidants are present. Proton radical scavenging is decreased by a protonated radical of DPPH that has the maximum absorbance at 517 nm. Thus, it is possible to evaluate how well natural antioxidants scavenge free radicals (Lalhminghlui and Jagetia, 2018). Theoretically, decolorization results from DPPH’s disappearance from absorption and stoichiometric electron uptake in the presence of a free radical scavenger (Adjimani and Asare, 2015). This study used the DPPH test to examine the potential for antioxidant activity utilizing different samples of H. sabdariffa calyx extract. The sample extracts’ DPPH radical scavenging activity was reported as an IC50 value.
Table 2 shows that at 0.5 mg/mL concentration, FC-ME or undried calyx, exhibited stronger DPPH scavenging action with more than 80% inhibition. Subsequently, FD calyx exhibits increased scavenging action among the dried samples. At only 9% concentration, the AD sample extracted with hexane exhibited the lowest percentage inhibition. This suggests that the non-polar extract contains fewer possible chemicals or secondary metabolites that contribute to this activity, which is complimentary because hexane extracts produced lower extraction yields. The percentage inhibition demonstrated a significant difference (p < 0.05) among all samples.
Table 2: Percentage inhibition of each sample.
|
Sample/ extraction solvents |
DPPH free radical scavenging activity |
|||
|
FC |
AD |
OD |
FD |
|
|
Inhibition (%) (0.5 mg/mL) |
||||
|
Methanol |
81.69 |
43.88 |
65.11 |
40.48 |
|
Ethanol |
63.85 |
54.85 |
25.01 |
72.19 |
|
Aqueous |
50.37 |
11.35 |
26.89 |
27.72 |
|
Hexane |
55.48 |
9.52 |
26.89 |
18.32 |
|
Quercetin |
88.87 |
|||
Among the samples tested, the fresh calyx with methanol extract did not undergo any drying method because it exhibited a low IC50 value (70 ug/mL), meaning that a minor concentration or amount is enough to indicate the high potential of antioxidant activity (Table 3). A previous study by Suryaningsih et al. (2021) reported that roselle calyx has a high potential for antioxidant activity. Moreover, many environmental factors might affect the breakdown and accretion of specialized metabolites, including heat, elevation, brightness, and water content (Li et al., 2020).
Among the dried roselle calyces, the freeze-dried extract by ethanol has the highest percentage, indicating a much lesser concentration of sample was needed to reach IC50. This is because the degradation of antioxidant compounds was low due to the cold environment. The roselle calyx that was freeze-dried possessed more significant antioxidative activity due to a higher yield of bioactive compounds when dried at -80 oC (Dadi et al., 2019).
A bioactive compound is a type of chemical present in minute quantities within plants and certain foods, including fruits, vegetables, nuts, oils, and whole grains. Bioactive substances function in the body in ways that benefit health. They are being researched for their prospect in preventing cancer, heart disease, and other disorders. Lycopene, resveratrol, lignin, tannins, and indoles are bioactive chemicals (Souza et al., 2020). Next, oven-dried samples extracted by ethanol had the highest IC50 (440 ug/mL), leading to the high concentration or amount of sample needed to indicate the high potential of antioxidants activity. This is because the high degradation of antioxidants compounds occurs in high temperatures, such as in laboratory ovens with temperatures above 40 oC (Hardinasinta et al., 2021).
Heat treatment has been associated with denaturation of enzymes, reduced antioxidative capacities and impairment of phytochemical compounds (Mukhtar et al., 2020). Also, the loss in antioxidant activity will lead to the loss of other bioactivities (Luzia and Jorge, 2014). Then, the air-dried sample required 400 ug/mL to reach IC50, which is much better than the oven-dried sample. Air drying, often conducted at lower temperatures, helps retain delicate antioxidants and phytochemicals susceptible to degradation under higher heat levels employed in oven drying. This gentler drying method is conducive to maintaining the overall antioxidant potential of the roselle calyx, resulting in enhanced DPPH scavenging activity. A previous study by Ahmed et al. (2016) reported that air-drying is a cheaper way to reduce moisture in herbs at moderate temperatures, which prevents the loss of active constituents.
Total phenolic content
The TPC was determined using the standard plot’s regression equation (Figure 1) and expressed as GAE. Plant phenolic compounds are critical as main antioxidants or free radical scavengers (Lin et al., 2016). The antioxidant activity of phenolic compounds is mostly related to their redox potential through neutralization of free radicals, stabilization of reactive oxygens, and decomposition of peroxides.
Table 3: IC50 value of samples and treatments. nd= not detected.
|
Sample/ solvents |
DPPH free radical scavenging activity |
|||
|
FC |
AD |
OD |
FD |
|
|
IC50 (µg/mL) |
||||
|
Methanol |
70 |
400 |
nd |
nd |
|
Ethanol |
80 |
nd |
440 |
320 |
|
Aqueous |
nd |
nd |
nd |
nd |
|
Hexane |
180 |
nd |
nd |
nd |
|
Quercetin |
25 |
|||
TPC is regarded as a quick and easy approach for determining total phenol in complex matrices such as leaves and petals. The TPC method was sensitive enough to estimate the total phenol in calyx samples (Aryal et al., 2019).
Overall, the FC contained the lowest phenolic content (Table 4, Figure 2) because the FC could not be the efficient extraction of the insoluble phenolic compounds. For FC, heat treatment that can dissolve the insoluble compound was not applied. The findings were parallel with Wang et al. (2021) in citrus, which reported that fresh citrus peel that was not exposed to heat contained a relatively low TPC. The OD samples had significantly (p ≤ 0.01) higher TPC than FC, AD samples, and FD of roselle calyces. The heat treatment applied in OD possibly assisted in the formation of phenolic glycans by breaking glycosidic bonds, which have higher affinity to the Folin–Ciocalteu reagent. Such findings were also reported in stinging nettle leaves, which reported that high-temperature dried leaves produced higher TPC content than fresh leaves (Garcìa et al., 2021).
The TPC has a significant difference (p ≤ 0.05) between the drying conditions and extraction solvents used with hexane being the least effective solvent and oven-drying being the most efficient method for recovering phenolic compounds. The recovery of polyphenols from plant materials depends greatly on the ability of the extraction solvent to solubilize phenolic compounds. This is similar to a study by Dirar et al. (2019), which found that the choice of solvent affected overall phenolics recovery. Other than that, the polarity of the solvent is critical in improving phenolics solubility (Muhamad et al., 2014). The results showed that a wide range of phenols might be dissolved more in the ethanol extracts.
Total flavonoid content
TFC was determined using the usual plot regression equation and reported as QE (Figure 3). Flavonoids, which have a benzo-y-pyrone structure, are the most common and widely dispersed phenolic chemicals (Kumar and Pandey, 2013). The TFC of calyx samples was determined using an AlCl3 method that was selective for flavones and flavonols. TFC can be measured in sample extracts via reaction with sodium nitrate, followed by the production of colored-aluminium complexes under alkaline conditions using AlCl3, which can be detected spectrophotometrically.
Table 4: Total phenolic content of various calyx extract.
|
Sample name |
Drying method |
Extraction solvent |
TPC (mg GAE/g) |
|
FC-ME |
Fresh calyx (FC) |
Methanol |
53.53 ± 1.25 a |
|
FC-EE |
Ethanol |
61.66 ± 1.12 a |
|
|
FC-AE |
Aqueous |
55.38 ± 2.27 a |
|
|
FC-HE |
Hexane |
48.23 ± 2.69 b |
|
|
OD-ME |
Oven-dried (OD) |
Methanol |
160.49 ± 1.53 a |
|
OD-EE |
Ethanol |
172.04 ± 0.43 a |
|
|
OD-AE |
Aqueous |
148.58 ± 1.47 a |
|
|
OD-HE |
Hexane |
95.311 ± 2.90 b |
|
|
AD-ME |
Air-dried (AD) |
Methanol |
82.80 ± 2.09 a |
|
AD-EE |
Ethanol |
87.09 ± 2.10 a |
|
|
AD-AE |
Aqueous |
82.27 ± 2.81 a |
|
|
AD-HE |
Hexane |
12.19 ± 0.22 b |
|
|
FD-ME |
Freeze-dried (FD) |
Methanol |
105.38 ± 2.52 a |
|
FD-EE |
Ethanol |
109.57 ± 1.19 a |
|
|
FD-AE |
Aqueous |
98.23 ± 1.11 a |
|
|
FD-HE |
Hexane |
3.24± 0.95 b |
Table 5 and Figure 4 shows the overall flavonoid content of the calyx extracts examined. There is statistically significant interaction between the solvents and drying methods used (p=0.000).
The results revealed that fresh calyx extracted with ethanol (FC-EE) has the highest source of flavonoids, with a total level of 22.9 mg QE/g whereas oven-dried calyx extracted with hexane samples had the lowest amount of flavonoid. Similar findings were reported by Kunyanga et al. (2011) which found that the ethanolic extract has the highest flavonoid content. This is associated with flavonoids requiring a less polar solvent or a higher proportion of ethanol, such as 100 % ethanol (Al-Rimawi et al., 2017). Flavonoids play a key function in free radical scavenging, and they are the phyto components that should be studied for numerous biological activities.
There was no significant difference between the type of drying conditions in extracting TFC in roselle calyx (p=0.160). However, it was shown that FC ethanolic extract has the richest source of flavonoids and is followed by FD, AD and OD. This is because the fresh calyx that was not dried did not receive the heat treatment that can degrade the phenolic compound. For the samples that undergo the drying process, FD had the highest TFC compared to the other drying methods consistent with Buitrago et al. (2019) which reported that the plant material that is fresh and stored in the freezer contains high amounts of flavonoid content. The results also showed that the OD sample has the lowest flavonoid content. Because many flavonoid compounds are heat sensitive. Previous study by Ioannou et al. (2020), flavonoid compounds easily degrade if exposed to high temperature.
Phytochemical tests
Phytochemical screening was performed on two best samples derived from the IC50 value of DPPH assay which were FCME and FDEE. The presence of chemical substituents in plant extracts are referred to as secondary metabolites, which hold various roles such as anticancer, antioxidant, antibacterial, and antifungal (Alabri et al., 2014). Most secondary metabolite components are commonly extracted from polar solvents. According to previous research by Hossain et al. (2013), the phytochemical constituents of plants is environmentally dependent. The environmental factors include vicinity, meteorological conditions, types of soil and seasons.
The phytochemical characteristics of fresh calyx methanol extract and freeze-dried ethanol extract tested were summarized in Table 6 and Figure 5. The results revealed the presence of most medically active compounds in both samples, with the exception of steroids. Anthocyanin can be found both in the fresh and freeze-dried roselle calyx. Anthocyanins are water-soluble pigments with color variations caused by differences in the acidity and alkalinity of the cell sap. Simple genetic alterations in species or varieties are linked to the formation of various anthocyanins (Mattioli et al., 2020).
Tannins were also found in both samples examined. Tannins are phenolics, and their variants are important free radical inhibitors (Formagio et al., 2014). Tannins have been linked to homeostatic functions, which aid in wound recovery and the improvement of irritated mucus membranes, as well as to inhibit the growth of unwanted pathogens (Hatil and Ahmed, 2015).
The samples were also found to contain saponins, a secondary metabolite known for its role in reducing inflammation (El-Aziz et al., 2019). Saponins are usually associated with bitterness and can generate foams and bind to cholesterol and hemolysis (Yadav and Agarwala, 2011). Saponins are essential in cough treatment by alleviating bronchial pain. According to Singh and Chaudhuri (2018), saponins have been shown to boost liver health, prevent diabetes and inhibit fungus growth. However, there is limited scientific evidence that saponin contributes to antioxidant activity.
According to numerous studies, glycosides are known to reduce blood pressure (Curfman, 2020). Cardiac glycosides impede the pumping of sodium or potassium ions, increasing the concentration of Na+ in the myocyte and contributing to an increase in the total amount of calcium ions available to the heart muscles. This serves to positively facilitate heart functions by minimizing chest congestion. Controlled consumption of cardiac glycosides aids in arresting cardiac arrhythmias and strengthens a weak heart, allowing the heart to work more efficiently (Osman et al., 2017).
Correlation of TPC, TFC and DPPH free radical scavenging activity
Pearson’s correlation analysis was employed to see whether flavonoids or phenolics possibly contributed to the overall antioxidative activity of the roselle calyx. There was a medium positive correlation between the TFC and DPPH radical scavenging activities (r = 0.531. p ≤ 0.05) and instead, a negligible correlation between TPC and DPPH radical scavenging activities (r= 0.051, p= 0.850) (Table 7). This suggests that the antioxidant action of roselle calyx extracts may be attributed to flavonoids rather than phenolics. This was predictable as anthocyanin, which is a type of flavonoid, is abundantly present in roselle, and it has been proposed as a post-harvest biomarker for roselle calyx (Lema et al., 2022). Phenolics could possibly be found higher in leaf extract or white roselles.
Table 5: Total flavonoid content of various calyx extract.
|
Sample name |
Drying method |
Solvent |
TFC (mg QE/g) |
|
FC-ME |
Fresh calyx (FC) |
Methanol |
20.9±0.06 b |
|
FC-EE |
Ethanol |
22.9±0.03 a |
|
|
FC-AE |
Aqueous |
20.6±0.05 b,c |
|
|
FC-HE |
Hexane |
20.0±0.00 c |
|
|
OD-ME |
Oven-dried (OD) |
Methanol |
20.6±0.06 b |
|
OD-EE |
Ethanol |
22.0±0.05 a |
|
|
OD-AE |
Aqueous |
20.5±0.05 b,c |
|
|
OD-HE |
Hexane |
20.0±0.00 c |
|
|
AD-ME |
Air-dried (AD) |
Methanol |
20.8±0.03 b |
|
AD-EE |
Ethanol |
22.4±0.07 a |
|
|
AD-AE |
Aqueous |
20.6±0.03 b,c |
|
|
AD-HE |
Hexane |
20.1±0.00 c |
|
|
FD-ME |
Freeze-dried (FD) |
Methanol |
20.7±0.03 b |
|
FD-EE |
Ethanol |
22.4±0.01 a |
|
|
FD-AE |
Aqueous |
20.4±0.04 b,c |
|
|
FD-HE |
Hexane |
20.0±0.00 c |
Table 6: Phytochemical test result. + indicates presence of phytochemicals, - indicates absence of phytochemicals.
|
Secondary metabolites |
Fresh Calyx (Methanol extract) |
Freeze-dried (Ethanol extract) |
|
+ |
+ |
|
|
Tannins |
+ |
+ |
|
Saponins |
+ |
+ |
|
Glycosides |
+ |
+ |
|
Flavonoids |
+ |
+ |
|
Terpenoids |
+ |
+ |
|
Steroids |
- |
- |
Table 7: Pearson’s correlation coefficient (r) between TPC, DPPH and TFC of roselle extracts.
|
TPC vs. DPPH |
TFC vs. DPPH |
|
|
r value |
0.051 |
0.531 |
|
p value |
0.850 |
0.034 |
Conclusions and Recommendations
In conclusion, the fresh calyx that was not exposed to heat treatment showed the highest percentage of inhibition, leading to the highest antioxidant content. The FC also had the most decadent amount of TFC, which was statistically insignificant. FD had a higher percentage of inhibition and TFC for the dried roselle calyx than AD and OD. In terms of extraction solvents, methanol is suggested as the best solvent to extract phenolic compounds, regardless of drying methods. As predicted, hexane was shown to be the least effective solvent for recovering both phenolics and flavonoids from roselle calyx, which was also explained by its low DPPH scavenging activity. In phytochemical screenings, anthocyanins, tannins, flavonoids, saponins, steroids, glycosides, and terpenoids were found. Based on this study, it is suggested that other methods or assays be used to assess the antioxidant activity of roselle, such as its antioxidative enzymatic activities. Further characterization of the compounds from the extracts is required by chromatography studies and nuclear magnetic resonance (NMR).
Acknowledgements
Authors are thankful to Universiti Sultan Zainal Abidin (UniSZA) for funding under LabMat 2020 grant (UniSZA/2020/LABMAT/01) and Centralized Laboratory Management Centre (CLMC) for technical assistance.
Novelty Statement
This study reports the different extraction solvents used combined with different drying techniques and their effects on antioxidant components of roselle calyx.
Authors’ Contribution
Nur Anis Hashim: Original draft writing of the manuscript.
Nor Hasima Mahmod: Designing of the experiments and revision of manuscript.
Abubakar Abdulahi Lema: Analysis and interpretation of statistical data.
Lee-Hoon Ho: Interpretation of phytochemical screening analysis.
Mohammad Moneruzzaman Khandaker: Formatting and revision of manuscripty.
Conflict of interest
The authors have declared no conflict of interest.
References
Adjimani, J.P. and P. Asare. 2015. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep., 2: 721-728. https://doi.org/10.1016/j.toxrep.2015.04.005
Ahmed, M., J. Pickova, T. Ahmad, M. Liaquat, A. Farid and M. Jahangir. 2016. Oxidation of lipids in foods. Sarhad J. Agric., 32(3): 230–238. https://doi.org/10.17582/journal.sja/2016.32.3.230.238
Aishah, B., M. Nursabrina, A. Noriham, A.R. Norizzah and H.M. Shahrimi. 2013. Anthocyanins from Hibiscus sabdariffa, Melastoma malabathricum and Ipomoea batatas and its color properties. Int. Food Res. J., 20(2): 827–834.
Alabri, T.H.A., A.H.S. Al-Musalami, M.A. Hossain, A.M. Weli and Q. Al-Riyami. 2014. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci., 26(3): 237–243. https://doi.org/10.1016/j.jksus.2013.07.002
Al-Rimawi, F., S. Abu-Lafi, J. Abbadi, A.A. Alamarneh, R.A. Sawahreh and I. Odeh. 2017. Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. Afr. J. Tradit. Complement. Altern., 14(2): 130-141. https://doi.org/10.21010/ajtcam.v14i2.14
Aryal, S., M.K. Baniya, K. Danekhu, P. Kunwar, R. Gurung and N. Koirala. 2019. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants, 8(4): 96. https://doi.org/10.3390/plants8040096
Buitrago, D., I. Buitrago-Villanueva, R. Barbosa-Cornelio and E. Coy-Barrera. 2019. Comparative examination of antioxidant capacity and fingerprinting of unfractionated extracts from different plant parts of quinoa (Chenopodium quinoa) grown under greenhouse conditions. Antioxidants, 8(8): 238. https://doi.org/10.3390/antiox8080238
Chang, C., M. Yang, H. Wen and J. Chern. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal., 10(3): 7802. https://doi.org/10.38212/2224-6614.2748
Clarke, G., K.N. Ting, C. Wiart and J. Fry. 2013. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity poten tial and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants, 2: 1-10. https://doi.org/10.3390/antiox2010001
Couttolenc, A., A. Díaz-Porras, C. Espinoza, M.E. Medina and A. Trigos. 2020. On the primary and secondary antioxidant activity from hydroxy-methylcoumarins: Experimental and theoretical studies. J. Phys. Org. Chem., 33(1): 1–10. https://doi.org/10.1002/poc.4025
Curfman, G., 2020. Digitalis glycosides for heart rate control in atrial fibrillation. J. Am. Med. Assoc., 324(24): 2508-2508. https://doi.org/10.1001/jama.2020.24578
Dadi, D.W., S.A. Emire, A.D. Hagos and J.B. Eun. 2019. Effect of ultrasound-assisted extraction of Moringa stenopetala leaves on bioactive compounds and their antioxidant activity. Food Technol. Biotechnol., 57(1): 77–86. https://doi.org/10.17113/ftb.57.01.19.5877
Dhanani, T., S. Shah, N.A. Gajbhiye and S. Kumar. 2017. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem., 10: S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015
Dirar, A.I., D.H.M. Alsaadi, M. Wada, M.A. Mohamed, T. Watanabe and H.P. Devkota. 2019. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot., 120: 261–267. https://doi.org/10.1016/j.sajb.2018.07.003
El-Aziz, M.M.A., A.S. Ashour and A.G. Melad. 2019. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res., 8(1): 282-288. https://doi.org/10.15406/jnmr.2019.07.00199
Formagio, A.S.N., C.R.F. Volobuff, M. Santiago, C.A.L. Cardoso, M.D.C. Vieira and Z.V. Pereira. 2014. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants, 3(4): 745-757. https://doi.org/10.3390/antiox3040745
Garcìa, L.M., C. Ceccanti, C. Negro, L. De Bellis, L. Incrocci, A. Pardossi and L. Guidi. 2021. Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. leaves. Horticulturae, 7(1): 10. https://doi.org/10.3390/horticulturae7010010
Gomaa, R.B.A. and M.R. Nahed. 2016. Post-harvest studies on reducing losses and maintaining quality of packaging Roselle calyxes. J. Sustain. Agric. Sci., 42(4): 68-86. https://doi.org/10.21608/jsas.2016.3027
Hardinasinta, G., M. Mursalim, J. Muhidong and S. Salengke. 2021. Degradation kinetics of anthocyanin, flavonoid, and total phenol in bignay (Antidesma bunius) fruit juice during ohmic heating. Food Sci. Technol., 4: e64020. https://doi.org/10.1590/fst.64020
Hatil, H., A.A.E. El-Kamali and A. Ahmed. 2015. Phytochemical screening, ethnoveterinary medicine, qualitative analysis, ethanolic, aqueous extracts. Adv. Life Sc., 5(2): 42-58.
Hossain, M.A., K.A.S. Al-Raqmi, Z.H. Al-Mijizy, A.M. Weli and Q. Al-Riyami. 2013. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed., 3(9): 705-710. https://doi.org/10.1016/S2221-1691(13)60142-2
Huang, W., Y. Cai, Y. Zhang, W. Huang and Y. Cai. 2009. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer, 62(1): 1-20. https://doi.org/10.1080/01635580903191585
Hussein, R.A. and A.A. El-Anssary. 2019. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med., 1(3): 11-30.
Ioannou, I., L. Chekir and M. Ghoul. 2020. Effect of heat treatment and light exposure on the antioxidant activity of flavonoids. Processes, 8(9): 1078. https://doi.org/10.3390/pr8091078
Iqbal, E., K.A. Salim and L.B.L. Lim. 2015. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci., 27(3): 224–232. https://doi.org/10.1016/j.jksus.2015.02.003
Karimi, A. and M.T. Moradi. 2015. Total phenolic compounds and in vitro antioxidant potential of crude methanol extract and the correspond fractions of Quercus brantii L. acorn. J. Herb. Med. Pharmacol., 4(1): 35–39.
Kumar, S. and A.K. Pandey. 2013. Chemistry and biological activities of flavonoids: An overview. Sci. World J., 2013: 1-16. https://doi.org/10.1155/2013/162750
Kunyanga, C.N., J.K. Imungi, M.W. Okoth, H.K. Biesalski and V. Vadivel. 2011. Flavonoid content in ethanolic extracts of selected raw and traditionally processed indigenous foods consumed by vulnerable groups of Kenya: Antioxidant and type II diabetes-related functional properties. Int. J. Food Sci. Nutr., 62(5): 465–473. https://doi.org/10.3109/09637486.2010.550273
Lalhminghlui, K. and G.C. Jagetia. 2018. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA, 4(2): FSO272. https://doi.org/10.4155/fsoa-2017-0086
Lema, A.A., N.H. Mahmod, M.M. Khandake and M.D. Abdulrahman. 2022. The role of anthocyanins in activating antioxidant enzymes during postharvest degradation of Hibiscus sabdariffa L. roselle calyx. Plant Omics, 15(1): 25-36.
Li, Y., D. Kong, Y. Fu, M.R. Sussman and H. Wu. 2020. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem., 148: 80–89. https://doi.org/10.1016/j.plaphy.2020.01.006
Lin, D., M. Xiao, J. Zhao, Z. Li, B. Xing, X. Li and S. Chen. 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules, 21(10): 1374. https://doi.org/10.3390/molecules21101374
Luzia, D.M.M. and N. Jorge. 2014. Study of antioxidant activity of non-conventional Brazilian fruits. J. Food Sci. Technol., 51(6): 1167–1172. https://doi.org/10.1007/s13197-011-0603-x
Mattioli, R., A. Francioso, L. Mosca and P. Silva. 2020. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules, 25(17): 3809. https://doi.org/10.3390/molecules25173809
Miliauskas, G., P.R. Venskutonis and T.A. Van Beek. 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem., 85(2): 231-237. https://doi.org/10.1016/j.foodchem.2003.05.007
Muhamad, N., S.A. Muhmed, M.M. Yusoff and J. Gimbun. 2014. Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. J. Food Eng., 4(2012): 255-260.
Mukhtar, A., S. Latif and J. Mueller. 2020. Effect of heat exposure on activity degradation of enzymes in mango varieties Sindri, SB Chaunsa, and Tommy Atkins during drying. Molecules, 25(22): 5396. https://doi.org/10.3390/molecules25225396
Osman, M.H., E. Farrag, M. Selim, M.S. Osman, A. Hasanine and A. Selim. 2017. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One, 12(6): e0178611. https://doi.org/10.1371/journal.pone.0178611
Phaechamud, T., K. Yodkhum, C. Limmatvapirat and P. Wetwitayaklung. 2012. Morphology, thermal and antioxidative properties of water extracts from Sonneratia caseolaris (L.) Engl. prepared with freeze drying and spray drying. CABI Dig. Lib., 3(1): 725-739.
Pisoschi, A.M. and A. Pop. 2015. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem., 97: 55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Rasul, M.G., 2018. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Comput. Sci. Res., 2(6): 5.
Singh, D. and P.K. Chaudhuri. 2018. Structural characteristics, bioavailability and cardioprotective potential of saponins. Integr. Med. Res., 7(1): 33-43. https://doi.org/10.1016/j.imr.2018.01.003
Singh, P., M. Khan and H. Hailemariam. 2017. Nutritional and health importance of Hibiscus sabdariffa: A review and indication for research needs. J. Nutr. Health Food Eng., 6(5): 125–128. https://doi.org/10.15406/jnhfe.2017.06.00212
Souza, A.V., J.M. Mello, V.F. Fávaro, V.F.S. Silva, D. Sartori and F.F. Putti. 2020. Bioactive compounds and antioxidant activity in tomatoes (Lycopersicon esculentum L.) cultivars in natura and after thermal processing. Res. Soc. Dev., 9(11): e44991110192. https://doi.org/10.33448/rsd-v9i11.10192
Suryaningsih, S., B. Muslim and M. Djali. 2021. The antioxidant activity of roselle and dragon fruit peel functional drink in free radical inhibition. J. Phys. Conf. Ser., 1836(1): 012069. https://doi.org/10.1088/1742-6596/1836/1/012069
Tandoh, P.K., B.K.B. Banful and I.A. Idun. 2022. Effects of harvesting times and germplasm accessions on the physical properties of roselle (Hibiscus sabdariffa L.) seeds. Adv. Agric., 2022(1): 8962737. https://doi.org/10.1155/2022/8962737
Wang, Y., X.J. Liu, J.B. Chen, J.P. Cao, X. Li and C.D. Sun. 2021. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr., 62(14): 3833-3854. https://doi.org/10.1080/10408398.2020.1870035
Widyawati, P.S., T.D.W. Budianta, F.A. Kusuma and E.L. Wijaya. 2014. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts. Int. J. Pharmacogn. Phytochem. Res., 6(4): 850-855.
Yadav, R.N.S. and M. Agarwala. 2011. Phytochemical analysis of some medicinal plants. J. Phytol., 3(12): 10-14.
To share on other social networks, click on any share button. What are these?







