Effects of Antibiotic and Sugar-Free Aloe Vera (Aloe barbadensis) Based Extender on Cryopreserved Sperm of Tilapia (Oreochromis sp.)
Effects of Antibiotic and Sugar-Free Aloe Vera (Aloe barbadensis) Based Extender on Cryopreserved Sperm of Tilapia (Oreochromis sp.)
Siew Ing Nguang, Nur Syafiqah Binti Zainal, Ahmad Mukhlis Bin Hafas, Hou Chew Ha, Connie Fay Komilus, and Asmad Kari*
School of Animal Science, Aquatic Science, and Environment, Faculty of Bioresources, and Food Industry, Universiti Sultan Zainal Abidin, Campus Besut 22200, Besut, Terengganu, Malaysia.
Abstract | Fish sperm cryopreservation is important for preserving sperm viability and genetic potential during freezing and thawing. Each fish species requires a specific extender because of diverse sperm characteristics. Tilapia (Oreochromis sp.) popular in Malaysia for its hardiness and rapid growth is an excellent choice for aquaculture. Aloe vera (Aloe barbadensis) (Av) shows promise in spermatogenesis, but its use as a cryoprotectant in semen extenders is still underexplored. This study investigates the effects of antibiotic- and sugar-free Av-based extenders on cryopreserved tilapia sperm. Semen samples were collected and divided into four treatment groups: Control (C, 0% Av), Treatment 1 (T1, 10% Av, antibiotic: sugar), Treatment 2 (T2, 10% Av, antibiotic: sugar-free), and Treatment 3 (T3, 10% Av, antibiotic-free: sugar) mixed with a Tris stabilizer. The samples were loaded into French straws and were subjected to an equilibration phase before being frozen and stored in liquid nitrogen. The straws were thawed after 48 hours to evaluate the effect of extender components. After cryopreservation, T1 showed significantly highest viability with 63.4±3.0%. The improved viability in T2 likely resulted from the antibiotics while the reduced viability in T3 may be due to their absence, with values of 45.7±1.9% and 36.6±1.8%, respectively. After 48 hours of storage, T1 exhibited decreased motility with most spermatozoa displaying strong vibration. In contrast, T2 and T3 experienced a greater decline in motility with a weaker vibration. Antibiotics in T2 likely contributed to the higher motility observed in this treatment. In summary, the absence of antibiotics and sugar negatively affected sperm viability and motility. The addition of antibiotic, sugar, and Av in the extender proved crucial for maintaining the viability and motility of cryopreserved tilapia sperm. These research findings enhance a better understanding of the potential of Av-based extenders in fish sperm cryopreservation that contribute to sustainable food security.
Received | February 22, 2024; Accepted | September 13, 2024; Published | October 14, 2024
*Correspondence | Asmad Kari, School of Animal Science, Aquatic Science, and Environment, Faculty of Bioresources, and Food Industry, Universiti Sultan Zainal Abidin, Campus Besut 22200, Besut, Terengganu, Malaysia; Email: [email protected]
Citation | Nguang, S.I., N.S.B. Zainal, A.M.B. Hafas, H.C. Ha, C.F. Komilus and K. Asmad. 2024. Effects of antibiotic and sugar-free aloe vera (Aloe barbadensis) based extender on cryopreserved sperm of tilapia (Oreochromis sp.). Sarhad Journal of Agriculture, 40(Special issue 1): 152-160.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.152.160
Keywords | Aloe vera, Antibiotic, Cryopreservation, Extender, Tilapia
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Tilapia (Oreochromis sp.) is a popular aquaculture species in Malaysia, ranking second in freshwater fish production after catfish (Mohamad et al., 2021). Among the species within the Cichlidae family, tilapia stands out because of its rapid growth rate, adaptability to varied environmental conditions, and consumer acceptability (Rahman et al., 2021). Since tilapia aquaculture is important in meeting the increasing demand for fish protein, successfully preserving sperm is crucial. This helps propagate superior fish genetics and addresses the challenges of transporting live animals.
Artificial fertilization is a unique technique in fish breeding, that involves the collection and controlled mixing of fish sperm and eggs, followed by incubation to generate fish larvae (Endoh et al., 2022; Niu et al., 2022). This process significantly improves breeding and accelerates the propagation of desirable genetic traits. The effectiveness of artificial fertilization depends on the quality and quantity of ejaculated fish sperm, as well as the ability to maintain sperm quality either in a short time or over extended periods. Cryopreservation, a widely used method plays an important role in preserving sperm by placing them at ultra-low temperatures using cryoprotectants to minimize damage caused by freezing (Ekici et al., 2022; Niu et al., 2022).
In fish sperm preservation, the quality of sperm is usually evaluated based on sperm viability, semen mass movements, and their ability to fertilize eggs (Gallego and Asturiano, 2018; Kowalski and Cejko, 2019). However, since different fish species show biological differences in their responses to cryoprotectants and freezing, it is essential to optimize the cryopreservation procedure for each specific species (Kommisrud et al., 2020). There are different types of semen extenders available on the market today for preserving fish sperm. However, many of these extenders contain a high percentage of chemicals that may be toxic to sperm cells (Bustani et al., 2021; Félix et al., 2021). Moreover, the most used extender ingredient, egg yolk, has some drawbacks. Even though the egg yolk is effective in protecting sperm cells from cold shock by dissolving lipids in the sperm membrane and binding to it (Mortazavi et al., 2020), it poses a risk of bacterial contamination due to its animal origin (Swelum et al., 2018). In addition, the granular nature of egg yolk particles could hinder the observation of sperm under the microscope and for biochemical tests (Pillet et al., 2011).
Researchers are exploring Aloe vera (Av) as a natural alternative to conventional semen extenders for fish sperm cryopreservation. Av has demonstrated cryoprotective properties similar to those of egg yolk, with positive effects on sperm motility, morphology, viability, and oxidative protection (Zareie et al., 2021). Its antibacterial and antioxidant qualities make it a promising candidate for maintaining sperm quality during and after cryopreservation. Despite its potential, there is still a significant lack of studies on Av-based extenders for fish sperm, which this research aims to address by investigating their effects on cryopreserved red tilapia sperm.
The current study investigates the feasibility of an Av-based extender for preserving tilapia sperm during cryopreservation. By eliminating the use of sugar and antibiotics from the extender formulation, the study seeks to assess whether Av alone can effectively maintain the quality of tilapia sperm after cryopreservation. Through this research, it is possible to contribute valuable insights into developing innovative plant-based alternatives for fish sperm cryopreservation, reduce the reliance on chemical-laden extenders, and advance sustainable aquaculture practices in the future.
Materials and Methods
The research was conducted at the Aquatic Laboratory I and Reproduction Laboratory, Faculty of Bioresources and Food Industry (FBIM), University of Sultan Zainal Abidin (UniSZA), Besut Campus. Before commencement, the ethical approval for this study was obtained from the UniSZA Animal Plant and Research Ethics Committee.
Sample collection and preparation
Fifteen healthy and mature male tilapias were purchased from a local fish farmer in the Besut area. The age range of the males was 6 months, 17.1±1.7 cm in total length (TL), and 275.3±15.4 g in body weight (BW). Upon arrival, the fish were gently transferred from plastic bags into a 700-L fiberglass fish tank in Aquatic Laboratory I. Pellets were fed to the tilapias twice at 9 am and 3 pm. The water temperature, dissolved oxygen, and pH ranges of the rearing tank were 25.0-29.5 oC, 4.8-6.4 mg/l, and 6.8-8.0, respectively.
Preparation of tris stabilizer
The Tris Stabilizer was prepared at the Reproduction Laboratory following the standard procedure of semen extender (Khan et al., 2016). The main ingredients and formulation are listed in Table 1. All the ingredients were diluted in distilled water to achieve a total volume of 100 ml. The pH of the solution was adjusted to 6.5 by adding NaOH or HCl to balance the pH level.
Table 1: The ingredients and formulation of Tris stabilizer.
|
Ingredients |
Formulation |
|
Tris base |
3.8 g |
|
Citric acid monohydrate |
2.1 g |
|
Streptomycin sulphate salt |
0.05 g |
|
Penicillin G potassium salt |
0.03 g |
Preparation of aloe vera-based extender (AvBX)
Aloe vera-based extender (AvBX) was prepared based on Yong et al. (2017). Fresh Av leaves were harvested and cleaned under running tap water. The leaves were dried using a clean, dry cloth after cleaning. The spines were carefully removed using a sharp knife. The outermost upper and lower layers were finely scraped away, revealing the inner gel core. This gel core was cut into small pieces and collected in a sterile Petri dish. Av gel was extracted by blending using a blender and filtered through a sieve. The final product was collected into a sterile beaker. Liquid Av was measured and divided into four treatment groups of extenders as follows: Control (C, 0% Av), Treatment 1 (T1, 10% Av, antibiotic: sugar), Treatment 2 (T2, 10% Av, antibiotics: sugar-free), and Treatment 3 (T3, 10% Av, antibiotics-free: sugar). The antibiotics were prepared with 0.3 g penicillin in 1000 ml and 0.5 g streptomycin in 1000 ml, and the sugar was 1% fructose in concentration. These components were mixed with the Tris stabilizer to create the respective extenders, enabling the investigation of the effect of sperm quality between sugar-free and antibiotic-free treatments.
Fish handling and semen collection
The fish were well-trained for handling around two weeks before sperm collection, following the protocol of Yong et al. (2017). To minimize stress, both eyes of the fish were covered with a piece of cloth immediately after removal from the tank. The genital area was gently cleaned with tissue to remove waste material, mucus, water, and blood cells to prevent semen contamination. A gentle abdominal massage was applied to extract sperm from the genital papilla. The collected sperm was immediately placed in a 2 ml Eppendorf tube using a 1000 μl micropipette. The collected semen samples were immediately macroscopically and microscopically to assess the quality of the initial sperm. Macroscopic analysis includes the evaluation of parameters such as semen colour and volume, while microscopic analysis includes assessments of semen concentration, motility, viability, and sperm morphology. This comprehensive evaluation was carried out immediately after semen collection to ensure an accurate and timely assessment of the initial characteristics of semen. Semen volume is an indicator of sperm concentration. Since fish semen is very small in amount, the volume was measured immediately using a 5 ml graduated microtube.
Semen dilution and cryopreservation
The collected sperm was diluted at a ratio 1: 9 of semen: Extender to preserve its quality (Bergiron et al., 2002; Bustani and Baiee, 2021). The diluted sperm was then placed in a French straw with around 0.25 ml and allowed to equilibrate at 4°C chiller for 30 minutes equilibration time. Subsequently, the straw was exposed to liquid nitrogen vapor for 10 minutes and then submerged in liquid nitrogen for storage. The French straws were stored in a goblet at -196°C for 48 hours. After the storage period, the French straws were thawed at room temperature, and the evaluation of sperm quality was conducted immediately.
Fish sperm concentration
The sperm concentration analysis was conducted using fresh semen extracted from tilapia. The protocol established by Yong et al. (2017) was followed to perform the analysis. Initially, the fresh semen was diluted with distilled water at a dilution factor of 1: 21, according to the method described by Pataki et al. (2022). Afterward, the diluted sample was placed on the grids of a haemocytometer and observed under 100x magnification using a compound microscope. To calculate the sperm concentration, Equation 1 was employed as follows (Pataki et al., 2022).
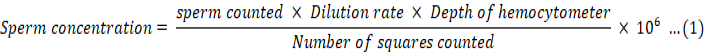
Fish sperm viability
According to Yong et al. (2017), the assessment of fish sperm viability used an Eosin-Nigrosin (EN) stain under a compound microscope at 400x magnification. Before observation, the sperm samples were preserved in a chiller for 48 hours. A minimum of 100 sperm were counted on each slide to ensure accuracy. To determine sperm viability, the live-dead ratio was calculated. Sperm with unstained membranes were classified as live, indicating intact membrane and viability. Conversely, sperm with stained membranes were categorized as dead, indicating non-viability. Equation 2 below was utilized to calculate sperm viability (Pataki et al., 2022):
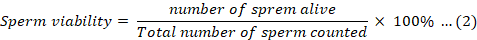
Fish sperm motility
Sperm motility is widely recognized as the most critical parameter for evaluating sperm quality since it directly reflects the inherent ability of spermatozoa to fertilize eggs. To assess semen mass motility also referred to as mass activity in spermatozoa or wave motion in the semen, the motility scoring system described by Betsy and Kumar (2014) (Table 2) was followed. Before observation, the semen samples were preserved in a chiller for 48 hours to maintain their integrity. The movement and activity of the sperm in the sample were then evaluated using this scoring system. The motility scoring system categorizes the level of sperm movement into different grades, providing valuable insights into the quality and functionality of the fish sperm. By employing this method, a comprehensive understanding of the motility of sperm was evaluated, a crucial aspect of assessing its potential for successful fertilization.
Thawing and sperm quality evaluation
The data obtained were reported in terms of means ± standard error. The effect of sperm quality among the four treatments of AvBXs (C, T1, T2, and T3) was compared using a one-way ANOVA with Minitab 17 statistical software.
Results and Discussion
Fish sperm concentration
The sperm concentration was determined using the concentration formula (WHO, 2010; Carvalho et al., 2021). The average fish sperm concentration obtained from three separate trials of pooled semen which are 1.6±1.5 x 109, 4.2±3.9 x 109, and 6.8±5.3 x 109 per ml in trial 1, trial 2, and trial 3, respectively (Table 3). The significant difference in sperm concentration among these trials indicates sample variability. Similar results were obtained in a study conducted by Bombardelli et al. (2010), Moraes et al. (2010), and Yong et al. (2017), where the sperm concentration was found to be 1.5 x 109, 2.6 x 109, and 8.2 x 109 per ml, respectively. The variation in the number of sperm cells in one ml could be due to individual factors such as the sizes and ages of fish (Luz et al., 2001; Bastardo et al., 2004; Costa et al., 2020). For instance, heavier tilapia (600-1000 g) exhibited a higher sperm concentration (2.44 × 109 sperm/mL) than lighter tilapia (250-500 g), which had a concentration of 1.66 × 109 sperm/mL (Paulino et al., 2016). This suggests that the physiological condition and size of the breeding males can directly affect the quantity of viable sperm produced, which is essential for successful fertilization.
Table 2: The new scoring system to evaluate the quality of fish spermatozoa (Betsy and Kumar, 2014).
|
Ingredients |
Formulation |
|
All spermatozoa (95 - 99%) are progressively motile with various flagella movements. |
10 |
|
Most spermatozoa (90 - 95%) are progressively motile, while others exhibit strong vibration with forward movement. |
9 |
|
Most spermatozoa (85 - 90%) are progressively motile, while others exhibit weak vibration with forward movement. |
8 |
|
Most spermatozoa (80 - 85%) exhibit strong vibration with forward movement, while others vibrate in loco. |
7 |
|
Most spermatozoa (75 - 80%) exhibit weak vibration with forward movement, while others vibrate in loco. |
6 |
|
All spermatozoa (90 - 95%) exhibit strong vibration in loco. |
5 |
|
All spermatozoa (90 - 95%) exhibit weak vibration in the loco. |
4 |
|
Most spermatozoa (85 - 90%) exhibit strong vibration in the loco while others oscillate. |
3 |
|
Most spermatozoa (85 - 90%) exhibit weak vibration in loco while others oscillate. |
2 |
|
Most spermatozoa (75 - 85%) oscillate while others vibrate. |
1 |
|
Most spermatozoa (60 - 75%) vibrate while others are immotile. |
0.75 |
|
All spermatozoa (90 - 95%) oscillate. |
0.5 |
|
Most spermatozoa oscillate while others are immotile. |
0.25 |
|
All spermatozoa immotile. |
0 |
Table 3: Sperm consistency, colour, and smelt of tilapia (n = 10).
|
Parameter |
Research results |
References |
|
Macroscopic |
||
|
Colour |
Milky white |
|
|
Odour |
Fishy odour |
Fishy and distinctive smell of sperm (Triastuti et al., 2018) |
|
pH |
7 |
7 (Triastuti et al., 2018) |
|
Consistency |
Viscous |
Medium thick (Triastuti et al., 2018; Chirwa et al., 2019) |
|
Microscopic |
||
|
Concentration (cells/ ml) |
1.6±1.5 x 109 to 6.8±5.3 x 109 |
0.9 x 109, 2.6 x 109, and 8.2 x 109 (Triastuti et al., 2018; Moraes et al., 2010; Yong et al., 2017) |
Table 4: The percentage of live sperm (mean ± standard deviation) in different treatments over time.
|
Time (Hour) |
The number of live sperm in different treatment |
|||
|
C (0% Av) |
T1 (10% Av, antibiotic: sugar) |
T2 (10% Av, antibiotic: sugar-free) |
T3 (10% Av, antibiotic free: sugar) |
|
|
0 |
72.6 ± 4.3a |
72.6 ± 2.5a |
72.6 ± 4.0a |
72.6 ± 4.2a |
|
48 |
27.1 ± 2.4d |
63.4 ± 3.0a |
45.7 ± 1.9b |
36.6 ± 1.8c |
a, b means with different superscripts within a column were significantly different (p<0.05).
Fish sperm viability
Fish sperm viability is a crucial indicator of sperm quality widely used in assessing reproductive health. It indicates the percentage of living sperm in each sample (Table 4). In the study by Eckel et al. (2017), the effects of treatments T2 and T3 on sperm viability over time were investigated, as shown in Figure 1A. At the start of the experiment (0 hours), there was no significant difference in sperm viability between T2 and T3. Both treatments exhibited a similar percentage of live sperm, with T2 at 72.6±4.0% and T3 at 72.6±4.2%. However, as the samples were subjected to cryopreservation for 48 hours, the sperm viability of T2 and T3 decreased significantly (p < 0.05). The percentage of live sperm for T2 decreased to 45.7±1.9%, while for T3, it dropped even further to 36.6±1.8%. This indicates that T2 showed a higher percentage of live sperm after 48 hours than T3.
The reasons for the increased sperm viability in T2 can be attributed to its sugar-free composition, combined with the presence of antibiotics. As observed by DeGraaf and Berlinsky (2004) in their study on Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) sperm, the addition of antibiotics to the sperm extender resulted in notable improvements in sperm viability, motility, and fertilization rates. On the other hand, T3’s decrease in sperm viability might be attributed to its lack of antibiotics, despite containing aloe vera, which possesses antimicrobial properties. The absence of antibiotics could render the sperm vulnerable to bacterial contamination, potentially leading to a decline in viability as noted in the study by Rahim-i et al. (2015). The study highlights the importance of both sugar-free formulations and the incorporation of antibiotics in sperm treatments to maintain sperm viability and improve reproductive success. By optimizing these factors, researchers and practitioners can enhance sperm quality, which holds significant implications for various reproductive processes (Mataveli et al., 2010).
Fish sperm motility
Sperm motility is a critical measure of the wave-like movement exhibited by spermatozoa in semen which plays an important role in assisted fish reproduction as highlighted by Kowalski and Cejko (2019). Evaluating sperm motility metrics such as sperm speed is of utmost importance in assessing the performance characteristics of fish sperm. A motility score table (Betsy and Kumar, 2014) is utilized to identify sperm motility. Table 5 presents the motility scores for treatments T2 and T3 over a specific time frame. At the beginning of the experiment (0 hours), there was no significant difference in sperm motility between T2 and T3, as both treatments exhibited a consistent motility score of 7±0. After 48 hours of storage, T2 and T3 still do not show any significant difference in motility scores, with values of 3±0 and 2±0, respectively. However, the decline in motility is evident in both treatments. For T2, the motility score decreases from 7±0 to 3±0 indicating that around 85-90% of spermatozoa exhibit strong vibration in loco, while the remaining spermatozoa oscillate.
Table 5: Motility score (score ± standard deviation) determined in different treatments over time.
|
Time (Hour) |
Motility score determined in different treatment (%) |
|||
|
C (0% Av) |
T1 (10% Av, antibiotic: sugar) |
T2 (10% Av, antibiotic: sugar-free) |
T3 (10% Av, antibiotic-free: sugar) |
|
|
0 |
7 ± 0a |
7 ± 0a |
7 ± 0a |
7 ± 0a |
|
48 |
0 ± 0a |
6 ± 0a |
3 ± 0a |
2 ± 0a |
a means with one superscript within a column was not significantly different.
Figure 1B further illustrates the impact of T2 and T3 on sperm motility after 48 hours of storage. T2 which contains antibiotics demonstrates a higher motility score (3±0) than T3 (score 2±0). This is likely because the antibiotics in T2 help reduce bacterial growth in the cells, prevent sperm cell death, and preserve motility (Gallego and Asturiano, 2018). On the other hand, T3 being antibiotic-free has experienced a decline in motility from the initial score of 7±0 to 2±0 after 48 hours. In this case, most spermatozoa (85-90%) exhibit weak vibration in loco, while others oscillate which may be attributed to the absence of protective measures against bacterial contamination. It is important to mention that no additional research has been done to evaluate sperm movement using the method described by Betsy and Kumar (2014). However, this study highlights how factors like antibiotics can improve sperm movement and reproductive success in fish.
The addition of antibiotics to the sperm extenders is a common practice in cryopreservation protocols to avoid bacterial contamination and maintain sperm quality during storage. However, antibiotics could have some detrimental effects on sperm viability and motility at certain concentrations. High concentrations of gentamicin, antibiotic solutions, and ampicillin significantly decreased sperm viability in refrigerated Nile tilapia sperm. The highest concentrations of each antibiotic tested impaired mitochondrial function in tilapia sperm (Segovia et al., 2000; Yong et al., 2017). To avoid the potential negative impacts of antibiotics, some studies have investigated natural alternatives like Av as an additive to sperm extenders. Av contains antioxidants that may protect sperm from oxidative stress during cryopreservation. The polysaccharides in Av maintain optimal osmotic pressure for sperm motility activation (Yong et al., 2017). These compounds can act as antioxidants, reducing oxidative stress during freezing and thawing processes. Av reduces reactive oxygen species and mitigates the detrimental effects of oxidative damage on sperm membranes, thereby preserving sperm functionality (Fakhrildin and Sodani, 2014; Ros-Santaella and Pintus, 2021).
Av has antimicrobial properties, potentially reducing bacterial contamination without the detrimental effects of antibiotics. Av as a natural additive in cryopreservation media potentially enhances sperm viability and motility post-thawing (Alavi et al., 2019). For instance, Av maintained sperm motility and viability in collared peccary semen and tilapia (Souza et al., 2016; Yong et al., 2017; Ros-Santaella and Pintus, 2021). In this study, Av-based extenders were proven to have higher sperm viability and mobility than those in the extenders that lack antibiotics and sugar. Moreover, Av extender without antibiotics and sugars is important. Antibiotics can sometimes lead to adverse effects on sperm motility and viability, while sugars may alter osmotic balance during cryopreservation (Salehi et al., 2018). By utilizing an antibiotic- and sugar-free formulation, the extender may provide a more natural environment for sperm preservation, possibly improving survival and fertility after thawing (Barbosa et al., 2020; Quispe et al., 2018).
The application of Av-based extenders in tilapia sperm cryopreservation could affect aquaculture. Improved cryopreservation techniques could enhance the efficiency of artificial insemination programs for better genetic management and conservation of tilapia stocks. This is related to the increasing pressures on fish populations due to overfishing and habitat loss. Besides, using natural products like aloe vera also supports sustainable aquaculture practices. As consumers become more concerned about the environmental impacts of food production, aquaculture products are more attractive in the market.
Conclusions and Recommendations
This study evaluated the effect of Av-based extenders on sperm quality during cryopreservation in tilapia. The results showed that the sperm concentration ranged from 1.6 billion to 6.8 billion sperm per ml, with significant variations observed among trials. Av-based extenders with antibiotics (T2) maintained higher sperm viability and motility after 48 hours of storage than sugar-based extenders without antibiotics (T3). The antibiotics in the extender appeared to play a crucial role in preserving sperm quality during cryopreservation. These research findings contribute to a better understanding of cryopreservation techniques in aquaculture and underscore the importance of optimizing extenders to enhance sperm quality and the success of breeding programs.
Acknowledgments
This research was financially supported by Internal Grant UniSZA (UniSZA/2023/DPU 2.0/26 | RD079). The authors would like to express their highest gratitude to all the science officers, staff hatchery, and undergraduate students of the faculty who participated in this project.
Novelty Statement
The addition of antibiotics, sugar, and the natural protectant Aloe vera to the extender showed significant benefits in preserving the viability and motility of cryopreserved tilapia sperm. This research underlines the potential of plant-based extenders as a sustainable and effective option for fish sperm cryopreservation.
Author’s Contribution
Siew Ing Nguang: Draft the manuscript, formatting, and supervise the experiment.
Nur Syafiqah Binti Zainal: Experimented and collected data.
Ahmad Mukhlis Bin Hafas: Experimented and collected data.
Hou Chew Ha: Review, formatting, and comments on the manuscript writing.
Connie Fay Komilus: Review, formatting, and comments on the manuscript writing.
Asmad Kari: Supervise and comment on the critical manuscript writing.
Conflict of interest
The authors have declared no conflict of interest.
References
Alavi, S.M.H., J. Cosson, O. Bondarenko and O. Linhart. 2019. Sperm motility in fishes: (III) diversity of regulatory signals from membrane to the axoneme. Theriogenology, 136: 143-165. https://doi.org/10.1016/j.theriogenology.2019.06.038
Barbosa, B.S., F.A. Santos, L.B. Macêdo, R.G. Izzo, D.P. Fernandes, E.A. Praxedes, A.R. Silva and M.B. Bezerra. 2020. Effect of supplementation of Aloe vera extracts in cold storage media and cryopreservation of domestic cat epididymal spermatozoa. Anim. Reprod., 17(1): e20190067. https://doi.org/10.21451/1984-3143-AR2019-0067
Bastardo, H., C. Guedez and M. Leon. 2004. Characteristics of the semen of rainbow trout of different ages, under cultivation conditions in Merida, Venezuela. Trop. Anim. Husb., 22(3): 277-288.
Bergiron, A., G. Vandenberg, D. Proulx and J.L. Bailey. 2002. Comparison of extenders, dilution ratios and theophylline addition on the function of cryopreserved walleye semen. Theriogenology, 57: 1061-1071. https://doi.org/10.1016/S0093-691X(01)00707-5
Betsy, C.J. and J.S.S. Kumar. 2014. New classification of motility score in fishes to determine the quality of spermatozoa. Int. J. Fish. Aquat. Stud., 1(4): 20-23.
Bombardelli, R.A., C. Hayashi, M.R.M. Natali, E.A. Sanches and P.A. Piana. 2010. Digestible energy levels on reproductive and zootechnical performance and lipid deposition in the hepatocytes of male Nile tilapia. Rev. Brasil. Zoot., 39(5): 941-949. https://doi.org/10.1590/S1516-35982010000500001
Bustani, G.S. and F.H. Baiee. 2021. Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World, 14(5): 1220-1233. https://doi.org/10.14202/vetworld.2021.1220-1233
Carvalho, F.M., C. Ramsey, C.B. Hanna, R.R. Valle, M. Nichi, M. Binelli, M.A.B.V. Guimarães and J.D. Hennebold. 2021. Cryopreservation and preparation of thawed spermatozoa from rhesus macaques (Macaca mulatta) for in vitro fertilization. J. Am. Assoc. Lab. Anim. Sci., 60(4): 396-406. https://doi.org/10.30802/AALAS-JAALAS-20-000028
Chirwa, E.R., A. Mtethiwa, W.L. Jere and D. Kassam. 2019. Effects of common carp and African catfish on plankton, periphyton, benthic macroinvertebrates in pond ecosystem. Aquat. Biol., 28: 91–100. https://doi.org/10.3354/ab00713
Costa, B.B., J.A. Povh, E.A. Sanches, L.N. Spica, R.B. Rodrigues, N.S. Teixeira, T.S., França, J.L. Benato, T.L.F. Machadoa, L.O. Brasileiro, R.Y.D. Kasai and D.P. Streita. 2022. Characterization of sperm quality in Brycon hilarii: How does morphology affect sperm movement? Theriogenol. Wild, 1(100007): 1-9. https://doi.org/10.1016/j.therwi.2022.100007
DeGraaf, J.D. and D.L. Berlinsky. 2004. Cryogenic and refrigerated storage of rainbow smelt Osmerus mordax Spermatozoa. J. World Aquacult. Soc., 35(2): 209–216. https://doi.org/10.1111/j.1749-7345.2004.tb01076.x
Eckel, B.A., R. Guo and K. Reinhardt. 2017. More pitfalls with sperm viability staining and a viability-based stress test to characterize sperm quality. Front. Ecol. Evol., 5(165): 1-14. http://doi.org/10.3389/fevo.2017.00165
Ekici, A., G. Yamaner and M.D. Demircan. 2022. Cryopreservation studies in aquaculture from past to present: Scientific techniques and quality controls for commercial applications. IntechOpen, 2022: 1-32. https://doi.org/10.5772/intechopen.108566
Endoh, M., R. Hazama, K. Kaya, Y. Futamura, S. Doi, I. Makinose, D. Pandey, O. Nishimiya, M. Havelka, T. Saito, R. Goto and T. Matsubara. 2022. Efficient artificial fertilization and ovulated egg preservation in Kawakawa Euthynnus affinis. J. Mar. Sci. Eng., 10(5): 599 (1-17). https://doi.org/10.3390/jmse10050599
Fakhrildin, M.B.M. and I.J. Sodani. 2014. Effect of Aloe vera extracts on in vitro human sperm parameters for asthenozoospermic Patients. Univ. Thi-Qar J. Sci., 5(1): 1-7.
Félix, F., C.C.V. Oliveira and E. Cabrita. 2021. Antioxidants in fish sperm and the potential role of melatonin. Antioxidants (Basel), 10(1): 36. https://doi.org/10.3390/antiox10010036
Gallego, V. and J.F. Asturiano. 2018. Fish sperm motility assessment as a tool for aquaculture research: A historical approach. Rev. Aquacult., pp. 1-28.
Khan, M.N.H., S. Shimazaki and S. Kawasaki. 2016. Coral sand solidification test through microbial calcium carbonate precipitation using Pararhodobacter sp. Int. J. Geomate, 11(26): 2665-2670.
Kommisrud, E., F.D. Myromslien, E.B. Stenseth, T.T. Zeremichael, N. Hofman, I. Grevle and J. Sunde. 2020. Viability, motility, ATP content and fertilizing potential of sperm from Atlantic salmon (Salmo salar L.) in milt stored before cryopreservation. Theriogenology, 151: 58-65. https://doi.org/10.1016/j.theriogenology.2020.04.008
Kowalski, R.K. and B.I. Cejko. 2019. Sperm quality in fish: Determinants and affecting factors. Theriogenology, 135: 94–108. https://doi.org/10.1016/j.theriogenology.2019.06.009
Luz, R.K., A.A. Ferreira and D.A. Reynalte-Tajate. 2001. Qualitative and quantitative evaluation of the semen of the pimelodid catfish (Steindachneridion scripta). Bull. Inst. Fish., 27(1): 39-42.
Mataveli, M., G.V. Moraes, D.P. Streit Junior, R.P. Ribeiro and E. Gasparino. 2010. Semen quality in Nile tilapia (Oreochromis niloticus), fed with diets containing different vitamin C levels. CABI Digital Library, 32(3): 345-349.
Mohamad, S.N., W.N. Noordin, M.N.F. Ismail and A. Hamzah. 2021. Red hybrid tilapia (Oreochromis spp.) broodstock development programme in Malaysia: Status, challenges, and prospects for future development. Asian Fish. Sci., 34(1): 73-81. https://doi.org/10.33997/j.afs.2021.34.1.008
Moraes, G.V. de, M. Mataveli, D.P. Streit Junior, R.P. Ribeiro and E. Gasparino. 2010. Evaluation of semen quality from Nile tilapia (Oreochromis niloticus) supplemented with different vitamin c concentrations B. Acta Sci. Anim. Sci., 32(3). https://doi.org/10.4025/actascianimsci.v32i3.7836
Mortazavi, S.H., M. Eslami and F. Farrokhi-Ardabili. 2020. Comparison of different carrier-compounds and varying concentrations of oleic acid on freezing tolerance of ram spermatozoa in tris-citric acid-egg yolk plasma semen diluent. Anim. Reprod. Sci., 219: 106533. https://doi.org/10.1016/j.anireprosci.2020.106533
Niu, J., X. Wang, P. Liu, H. Liu, R. Li, Z. Li, Y. He and J. Qi. 2022. Effects of cryopreservation on sperm with cryodiluent in viviparous black rockfish (Sebastes schlegelii). Int. J. Mol. Sci., 23(6): 3392 (1-14). https://doi.org/10.3390/ijms23063392
Pataki, B., A. Horváth, G. Mészáros, N. Kitanović, A. Ács, A. Hegyi, J. Molnár, B. Csorbai and B. Urbányi. 2022. Adjustment of common carp sperm concentration prior to cryopreservation: Does it matter? Aquacult. Rep., 24: 101109 (1-6). https://doi.org/10.1016/j.aqrep.2022.101109
Paulino, M.S., G.C. Veras, V.O. Felizardo, L.D. Solis-Murgas and R.T.F. Freitas. 2016. Assessment of gametes in Tilapia Oreochromis niloticus: Effects of body weight in a New Lineage. J. Fish. Sci., 10(2): 63-69.
Pillet, E., G. Duchamp, F. Batellier, V. Beaumal, M. Anton, S. Desherces, E. Schmitt and M. Magistrini. 2011. Egg yolk plasma can replace egg yolk in stallion freezing extenders. Theriogenology, 75(1): 105–114. https://doi.org/10.1016/j.theriogenology.2010.07.015
Quispe, C., M. Villalobos, J. Bórquez and M. Simirgiotis. 2018. Chemical composition and antioxidant activity of aloe vera from the pica oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem., 2018: 1-12. https://doi.org/10.1155/2018/6123850
Rahim-i, R., S. Hajirezaee, F. Shaluei and J.K. Katadj. 2015. Antibiotics, penicillin and sreptomyocin improve semen quality indices of endangered Caspian brown trout, Salmo trutta caspius (Kessler, 1870) during in vitro short-term storage. Aquacult. Res., 47(11): 3662-3666. https://doi.org/10.1111/are.12819
Rahman, M.L., M. Shahjahan and N. Ahmed. 2021. Tilapia farming in Bangladesh: Adaptation to climate change. Sustainability, 13(7657): 1-20. https://doi.org/10.3390/su13147657
Ros-Santaella, J.L. and E. Pintus. 2021. plant extracts as alternative additives for sperm preservation. Antioxidants, 10(5): 772. https://doi.org/10.3390/antiox10050772
Salehi, B., S. Albayrak, H. Antolak, D. Kręgiel, E. Pawlikowska, M. Sharifi-Rad, Y. Uprety, P.V.T. Fokou, Z. Yousef, Z.A. Zakaria, E.m. Varoni, F. Sharopov, N. Martins. M. Iriti and J. Sharifi-Rad. 2018. Aloe genus plants: From farm to food applications and phytopharmacotherapy. Int. J. Mol. Sci., 19(9): 2843. https://doi.org/10.3390/ijms19092843
Segovia, M., J.A. Jenkins, C. Paniagua-Chavez and T.R. Tiersch. 2000. Flow cytometric evaluation of antibiotic effects on viability and mitochondrial function of refrigerated spermatozoa of Nile tilapia. Theriogenology, 53(7): 1489-1499. https://doi.org/10.1016/S0093-691X(00)00291-0
Souza, A.L.P., G.L. Lima, G.C.X. Peixoto, A.M. Silva, M.F. Oliveira and A.R. Silva. 2016. Use of Aloe vera–based extender for chilling and freezing collared peccary (Pecari tajacu) semen. Theriogenology, 85(8): 1432–1438. https://doi.org/10.1016/j.theriogenology.2016.01.007
Swelum, A.A.A., I.M. Saadeldin, H. Ba-Awadh, M.G. Al-Mutary, A.F. Moumen, A.N. Alowaimer and H. Abdalla. 2019. Efficiency of commercial egg yolk-free and egg yolk-supplemented tris-based extenders for dromedary camel semen cryopreservation. Animals, 9(11): 999. https://doi.org/10.3390/ani9110999
Triastuti, J., D. Kintani, E.M. Luqman and D.Y. Pujiastuti. 2018. Effects of common carp and African catfish on plankton, periphyton, benthic macroinvertebrates in pond ecosystem. IOP Conf. Ser. Earth Environ. Sci., 137(012023): 1-7. https://doi.org/10.1088/1755-1315/137/1/012023
World Health Organization (WHO), 2010. Laboratory manual for the examination and processing of human semen fifth edition. Switzerland: WHO Press.
Yong, S.Y., S.I. Nguang and A. Kari. 2017. Comparison between the effect of egg yolk-based extender and Aloe vera (Aloe barbadensis) based extender on red tilapia (Oreochromis niloticus) sperm quality. J. Fundament. Appl. Sci., 9(2S): 813. https://doi.org/10.4314/jfas.v9i2s.50
Zareie, K., A. Farshad, J. Rostamzadeh, G. Azimi and F. Ariyan. 2021. Freezability of goat epididymal sperm using aloe vera extract and trehalose in diluents. Aust. J. Vet. Sci. Anim. Husband. 8(2): 1078.
To share on other social networks, click on any share button. What are these?






