Effect of Duckweed-Based Aquafeeds on Feed Digestibility and Growth Performance of Gibelion catla
Effect of Duckweed-Based Aquafeeds on Feed Digestibility and Growth Performance of Gibelion catla
Javairia Shafi*, Kashifa Naghma Waheed, Zahid Sharif Mirza and Shaista Razaq
Fisheries Research and Training Institute, Manawan, Lahore, Pakistan
ABSTRACT
The present study was conducted to find viable and economic ingredients for fish feed by evaluating the effect of partial fish meal replacement with duckweed (Lemna minor) in Gibelion catla feed. Four different isonitrogenous feeds (with 0, 10, 20 and 30% replacement of fish meal with) were prepared and fed to juvenile catla for 60 days at the rate of 5% fish biomass. No significant difference (P>0.05) in fish growth parameters and feed efficiency was observed among the treatments. There was no significant difference in apparent digestibility coefficient (ADC) of dry matter among treatments. Feed ADC (protein) with duckweed at 30% inclusion level was significantly lower (P<0.05) compared to the other treatments. There was no significant difference in muscle composition of fish reared under the four treatments. Results of the present study showed that duckweed can be used to replace up to 20% of fish meal in feed of catla without any negative effect on fish growth or feed digestibility.
Article Information
Received 22 July 2022
Revised 18 August 2022
Accepted 20 September 2022
Available online 14 January 2023
(early access)
Published 29 March 2024
Authors’ Contribution
JS planned the study, conducted the experiments, prepared and revised the manuscript. KNW supervised the research project. ZSM helped in data interpretation and statistical analysis. SR helped in experimental work.
Key words
Apparent digestibility coefficient, Duckweed, Fish meal, Isonitrogenous feeds
DOI: https://dx.doi.org/10.17582/journal.pjz/20220722090715
* Corresponding author: javairiamalik@gmail.com
0030-9923/2024/0003-1109 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Major challenges faced by aquaculture, one of the world’s fast-growing food sector, are feed cost and nutritional requirements of fish (Hixson, 2014; WorldBank, 2013). At present, fish oil and fish meal are mainly utilized in aqua feeds due to their nutritional composition and their ability to enhance feed palatability (De Silva et al., 2011). As these ingredients are derived from the world’s declining capture fisheries resources, sustainable development of aquaculture necessitates to investigate low cost and viable ingredients for formulation and production of economically feasible aqua feeds. At present, most of the aquaculture production in Punjab, Pakistan, is based on major carps and Chinese carps cultured in either extensive or semi-intensive culture systems. Due to low feed inputs, aquaculture fish production in Pakistan is remarkably lower than other Asian countries (FAO, 2020). However, there is a growing trend towards use of commercially available fish feed for carp’s culture among the aquaculturists (Shafi et al., 2021). Fish meal or soybean meal are the two main ingredients used in commercial fish feeds as major protein source. Due to their high cost, research should be conducted to find viable and economic ingredients for fish feed that can provide optimum fish growth and production.
The prime objective of fish feed formulation is to prepare a diet that can provide maximum fish production with minimum possible cost (Hardy, 2022). Feed cost is one of the major challenges faced by sustainable development of aquaculture. Use of fish meal in feed not only increases its cost but also raises concerns about viability of feed ingredients due to continuous decline in capture fisheries. Therefore, current practice in fish feed manufacturing industry is the use of low-cost plant-based ingredients as replacement of fish meal (Egerton et al., 2020; Figueiredo-Silva, 2014; Jalili et al., 2013). Soybean meal, with its high-quality protein and balanced amino acid profile, is currently used as a protein source in fish feed on commercial scale (Jannathulla et al., 2019). Other oilseed meals including cottonseed meal, canola meal, rapeseed meal also have shown the potential to replace fish meal in aquafeeds (Collins et al., 2013).
Duckweed belonging to the family Lemnaceae is world’s smallest angiosperm comprising of four genera; Lemna, Spirodela, Wolffiella, and Wolffia. Duckweed is among the world’s fast-growing plants which can double its biomass in 2-3 days under suitable environmental conditions (Ali et al., 2016). It can, therefore, be grown abundantly at lesser cost than alternative plant sources. Due to the high (20-42%) crude protein content combined with a favourable array of amino acids, duckweed present a potential plant-based ingredient for incorporation in aqua feeds (Mwale and Gwaze, 2013). Nevertheless, duckweed has been investigated for its potential as feed ingredient for other major and Chinese carps, there is no published report of its use in feed of Gibelion catla to the best of our knowledge. As fish species from the same family may show marked differences in food preference (Rahman et al., 2006; Ronald, 2022), the feeding behaviour of Gibelion catla towards duckweed cannot be predicted according to the earlier investigation based on food selection by carps other than catla. Present study was, therefore, conducted to evaluate the effect of partial fish meal replacement with duckweed in the feed of Gibelion catla on its growth, body composition and feed digestibility.
MATERIALS AND METHODS
Study site
The experiment was conducted at Fisheries Research and Training Institute (FRTI), Lahore, Pakistan (31.589435° N, 74.465944° E) from June to October 2020. Duckweed was cultured in two fiberglass tanks placed at a sunny open place. Fish culture experiment was carried out in indoor glass aquaria in Chemistry Laboratory of FR and TI using four treatments.
Culture of duckweed
Fresh duckweed (Lemna minor) was collected from a nearby freshwater body and transferred to FRTI, Lahore where it was cultured into two fiberglass tanks each of dimensions 0.89 x 0.58 x 0.60 m. Prior to stocking of duckweed, the fiberglass tanks were filled with fresh water up to 0.45 m and supplied with 100 g urea and 50 g single super phosphate (SSP). Each tank was stocked with 1.0 kg duckweed (wet weight) and left for 15 days for culture. Duckweed was collected from the tanks on daily basis for 30 days. Water temperature, pH, electrical conductivity and dissolved oxygen were regularly monitored using standard methods (APHA, 2017).
Preparation of aquafeeds
A control feed (T1) was prepared following Abbas et al. (2008) with slight modifications. Prior to feed formulation, crude protein content of all the ingredients was determined. The formulation of the four experimental feeds and their proximate composition is shown in Table I. Duckweed collected from culture tanks was washed with fresh water and air dried in shade for 15 days. Fish meal, canola meal, corn gluten and duckweed were ground into fine powder. All feeds were formulated to have final crude protein content of 30.60- 30.70%. T1 was prepared using fish meal and canola meal as major protein sources. Feeds T2, T3 and T4 were prepared replacing 10%, 20% and 30% of fish meal in T1 with duckweed. Due to unequal crude protein content in fish meal (41.41%) and duckweed (21.51%), appropriate quantities of duckweed to balance the protein content in formulated feed were used. Arrowroot starch was used as a filler in all the feeds. Proximate composition of prepared feeds was determined using standard methods (AOAC, 2012).
Table I. Formulation and proximate composition of experimental fish feeds.
|
Ingredient |
Experimental feed |
|||
|
T1 |
T2 |
T3 |
T4 |
|
|
Fish meal (%) |
30 |
27 |
24 |
21 |
|
Duckweed (%) |
--- |
6.10 |
12.3 |
18.4 |
|
Canola meal (%) |
30 |
30.0 |
30.0 |
30 |
|
Corn gluten (%) |
10 |
10.0 |
10.0 |
10 |
|
Rice polish (%) |
8 |
8.0 |
8.0 |
8 |
|
Vitamin and minerals (%) |
1.5 |
1.5 |
1.5 |
1.5 |
|
Filler (%) |
20.5 |
17.4 |
14.2 |
11.1 |
|
Total |
100 |
100 |
100 |
100 |
|
Proximate composition |
||||
|
Moisture (%) |
11.95 |
11.23 |
11.29 |
11.32 |
|
Ash* (%) |
12.49 |
13.39 |
13.03 |
13.34 |
|
Crude protein* (%) |
30.62 |
30.63 |
30.64 |
30.67 |
|
Crude fat* (%) |
3.24 |
3.13 |
3.07 |
3.24 |
*: Crude protein, ash and crude fat content are reported on dry basis.
Fish culture experiment
Juveniles of Gibelion catla (average weight: 2.5 g ± 0.4 g) were procured from Central Fish Seed Hatchery, Lahore, Pakistan and acclimatized in glass aquaria for 15 days at FR and TI, Lahore. Aerators were installed in the aquaria used for fish acclimatization and subsequent culture to maintain the water dissolved oxygen within optimum range. During acclimatization period, the fish were fed with a control diet (crude protein content: 30.60%) at 5% of their body weight daily. To initiate the experiment, fish weight and length was recorded and 15 juveniles were stocked in each aquarium following Li et al. (2021). There were four experimental treatments with reference to different feed compositions (Table I) and each treatment was run in duplicate. In T1, control feed was supplied to fish. In T2, T3 and T4, fish was fed with feed prepared by 10%, 20% and 30% replacements of fish meal with duckweed. Fish in each aquarium was fed at 2.5% of their body weight twice a day at 10:00 and 16:00 (GMT + 5.0). Fish culture experiment was conducted for 60 days. About half of water in aquaria was exchanged with fresh water after every 48 h. Water temperature was maintained at 25°C ± 0.3°C using aquaria heaters and other Physico-Chemical parameters of water (dissolved oxygen, pH, electrical conductivity, total alkalinity, total hardness and chloride contents) were analyzed on weekly basis using standard methods (APHA, 2017).
Five specimens of fish were randomly captured from each aquarium fortnightly for
growth monitoring in terms of body weight and total length. Feed was adjusted fortnightly according to the increase in fish biomass in each aquarium throughout the culture period. At the end of the experiment, all fish from each aquarium were harvested for recording of fish growth parameters and muscle’s composition.
Analytical procedures
Acid insoluble ash (AIA) was used as the marker to determine apparent digestibility coefficient (ADC) of dry matter and protein. To determine acid insoluble ash, fish faeces from each aquarium were collected daily for last 30 days of culture period and stored in a cool and dry place. At the end of the experiment, collected faeces were used to determine AIA following Van Keulen and Young (1977). A 5.0-g sample of faeces from each replicate of each treatment was dried in an oven at 135 ºC for 2 h, reweighed and subjected to ashing overnight at 450 ºC. Resulting ash was transferred to a 500 ml beaker and mixed with 2N HCl. The mixture was boiled on a hot plate for about 5 min and filtered. Ash and filter paper were washed free of acid using hot distilled water. Filter paper with ash was transferred to a pre-weighed crucible, subjected to ashing overnight at 450 ºC and weighed. ADC of dry matter and protein was calculated through appropriate formulae following Atkinson et al. (1984).
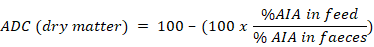
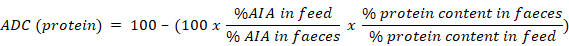
At the end of the experiment, proximate composition of fish muscles from each replicate was determined following standard methods of AOAC (2012).
Statistical analysis
Data on fish growth was subjected to one-way analysis of variance followed by least significant difference (LSD) test to detect any significant difference among four feed treatments at P<0.05. Data on physico-chemical parameters of water, apparent digestibility coefficient of feed and muscle composition of fish was also statistically analysed using ANOVA and LSD to find any significant difference among four treatments. Prior to analysis, normality and homogeneity of variance of data was assessed through relevant statistical tests. All statistical analysis was carried out using SPSS version 16.0.
RESULTS
Water quality parameters monitored during the 60-day culture period in the glass aquaria are presented in Table II. Water pH, dissolved oxygen and electrical conductivity were found to be in the range of 8.24 – 8.55, 4.00 – 4.47 mgL-1 and 724.0 – 732.0 µScm-1, respectively. Total alkalinity and total hardness ranged from 478.0 - 487.0 mgL-1 and 266.0 – 286.0 mgL-1, respectively. No significant differences were observed in water quality parameters among treatments (P>0.05).
Table II. Water quality parameters during fish culture (Mean ± Standard Deviation).
|
Parameter |
Optimum range* |
Treatment |
|||
|
T1 |
T2 |
T3 |
T4 |
||
|
pH |
6.5 – 9.0 |
8.55 ± 0.01 |
8.24 ± 0.06 |
8.46 ± 0.03 |
8.36 ± 0.14 |
|
Dissolved oxygen (mgL-1) |
>5.0 |
4.33 ± 0.04 |
4.00 ± 0.23 |
4.47 ± 0.08 |
4.24 ± 0.39 |
|
Carbon dioxide (mgL-1) |
<15 |
ND* |
ND |
ND |
ND |
|
Temperature (°C) |
22.0–30.0 |
25.30 ± 0.07 |
25.20 ± 0.14 |
25.25 ± 0.21 |
25.25 ± 0.07 |
|
Electrical conductivity (µScm-1) |
< 1000 |
724.0 ± 1.41 |
730.5 ± 2.12 |
732.0 ± 2.83 |
730.0 ± 4.24 |
|
Total alkalinity (mgL-1) |
>20 |
478.0 ± 2.83 |
481.5 ± 3.53 |
487.0 ± 2.83 |
487.0 ± 2.83 |
|
Total hardness (mgL-1) |
>20 |
286.0 ± 8.49 |
271.0 ± 5.66 |
266.0 ± 5.66 |
280.0 ± 5.66 |
Means that are in a row are statistically non-significant (P > 0.05). *: (Boyd and Tucker, 2012). **, Not Detected.
Table III. Fish growth and feed utilization parameters (Mean ± Standard Deviation).
|
Parameters |
Treatment |
|||
|
T1 |
T2 |
T3 |
T4 |
|
|
Fish growth |
||||
|
Initial weight (g) |
2.39 ± 0.11 |
2.50 ± 0.02 |
2.44 ± 0.04 |
2.5 0± 0.06 |
|
Final weight (g) |
3.69 ± 0.42 |
4.67 ± 0.07 |
4.31 ± 0.45 |
3.88 ± 0.23 |
|
Weight gain (g) |
1.30 ± 0.31 |
2.16 ± 0.05 |
1.87 ± 0.40 |
1.39 ± 0.16 |
|
SGR |
1.45 ± 1.03 |
1.53 ± 0.70 |
2.44 ± 2.10 |
1.43 ± 0.99 |
|
Increase in weight (%) |
54.09 ± 10.26 |
86.24 ± 1.28 |
76.55 ± 15.23 |
55.48 ± 5.20 |
|
Feed utilization |
||||
|
Feed conversion ratio (FCR) |
7.12 ± 2.04 |
3.47 ± 0.91 |
4.48 ± 0.80 |
5.56 ± 0.74 |
|
Condition factor |
1.04 ± 0.12 |
1.22 ± 0.30 |
1.08 ± 0.03 |
1.08 ± 0.17 |
|
Survival rate (%) |
98.5 ± 2.12 |
99.0 ± 1.41 |
98.5 ± 2.12 |
99.0 ± 0 |
Means that are in a same row are statistically non-significant (P>0.05).
Table IV. Apparent digestibility coefficient (ADC) of the four feeds and composition of fish muscles (Mean ± Standard Deviation).
|
Parameters |
Treatment |
|||
|
T1 |
T2 |
T3 |
T4 |
|
|
Feed digestibility coefficient |
||||
|
ADC dry matter (%) |
51.97 ± 5.75 a |
59.29 ± 5.59 a |
50.79 ± 1.54 a |
42.28 ± 6.71 a |
|
ADC protein (%) |
78.54 ± 1.21a |
82.05 ± 3.53a |
79.99 ± 0.59a |
68.95 ± 5.06b |
|
Fish muscles composition |
||||
|
Moisture (%) |
82.52 ± 1.55 a |
81.68 ± 0.30 a |
81.67 ± 1.40 a |
83.64 ± 1.47 a |
|
Ash (%) |
1.59 ± 0.21 a |
1.73 ± 0.170 a |
1.75± 0.48 a |
1.66 5 ± 0.03 a |
|
Crude protein (%) |
14.15 ± 0.46 a |
14.85 ± 0.160 a |
16.12 ± 1.1 a |
14.54 ± 3.20 a |
Means that do not share a letter in a same row are statistically significant (P<0.05).
Growth parameters of fish are presented in Table III. There were no significant differences in live fish weight gain among treatments. Highest live mean weight gain was found in T2 (2.16 g ± 0.05 g) followed by T3 (1.87 g ± 0.40 g). Also, mean feed conversion ratios (FCRs) were lower (3.47 and 4.48), respectively in T2 and T3, compared to those (5.56 and 7.12) of T1 and T4 respectively. Highest (59.29%) specific growth rate (SGR)was found in T3, followed by T2, T1 and T4.
Feed ADC for dry matter was highest in T2 (59.29% ± 5.59%) and it ranged from 42.28 to 51.97% in the rest of the treatments (Table IV). Protein apparent digestibility coefficient of feed ranged from 78.54 to 82.05% in T1-T3 while it was 68.95% in T4. Feed ADC (protein) was found to be significantly lower (P<0.05) in T4 compared to the other three treatments. Crude protein contents of fish muscles ranged from 14.15 to 16.12%. There was no significant difference (P>0.05) in muscle composition of fish among the four treatments (Table IV).
DISCUSSION
Duckweed is reported to contain 20-35% crude protein and 4 - 7% crude fat on dry weight basis. Amino acid profile of duckweed is also comparable to that of other plant-based protein and it ensures the supply of several essential amino acids (Appenroth et al., 2017). In the present study, duckweed was found to contain 21.51% crude protein and 5.24% crude fat, which is in agreement with earlier investigations which focussed on nutritional constituents of different varieties of duckweed. Ash content (18.24%) of duckweed was also close to that of fish meal (25.04) and it provides a variety of macro and micronutrients (Appenroth et al., 2017, 2018).
One of the major difficulties with the use of plant-based protein sources is presence of anti-nutritional factors (Adeyemo and Onilude, 2013; Samtiya et al., 2020). Duckweed, however, is reported to have low content of anti-nutritional factors compared to other plant crops (Hu et al., 2022). In the present study, partial replacement (up to 30%) of fish meal in feed of catla juveniles did not cause any significant change in fish growth performance, muscle composition and feed digestibility. It is noteworthy that catla fed with feed in which duckweed replaced 10 and 20% of fish meal showed higher percent (86.24 and 76.55%) increase in weight gain compared to those fed with feed without duckweed (54.09%). Percent weight gain (55.84%) was also low in fish fed with feed containing duckweed at 30% inclusion level. Lowest FCR was observed for feed containing duckweed at 10% inclusion level (3.47±0.91) where it was 50% lower than that of feed with no duckweed (7.12±2.04).
The results of this study are in agreement with earlier studies of Goswami et al. (2020), Yılmaz et al. (2004) and Fasakin et al., (1991) who studied the effect of duckweed inclusion on fish growth. Goswami et al. (2020) reported that partial replacement of fish meal with duckweed resulted in improved weight gain and SGR of Labeo rohita fingerlings. Authors also reported low FCR for duckweed-based feeds, indicated efficient utilization of feed. Yılmaz et al. (2004) observed that replacement of up to 20% commercial feed’s protein with duckweed did not significantly affect fish growth and FCR when the feed was used for nursing common carp fry. Fasakin et al. (1999) used duckweed to replace fish meal protein in feed of tilapia. They reported no significant difference in tilapia growth and feed utilization fed with feed containing duckweed up to 20% inclusion level (P>0.05). However, higher duckweed inclusion (>20%) resulted in reduced growth.
ADC of dry matter for feed with duckweed at 10% inclusion level was highest among the four experimental feeds. For feed with duckweed at 20% inclusion level, ADC of dry matter was comparable to that of the control. Feeds with no duckweed and with duckweed at 10 and 20% inclusion levels showed comparable ADCs (protein). El-Shafai et al. (2004) however, reported significantly lower (P<0.05) ADC (dry matter) and ADC (protein) of feeds in which duckweed was used to replace 20 and 40% dry matter of fish meal and other plant-based protein sources compared to the control feed. They also found significantly lower SGR for duckweed containing feeds (20 and 40% inclusion levels) compared to the control diet. Noor et al. (2000) also reported significantly reduced ADC (protein) of feed containing 17.07 to 59.24% of duckweed as replacement of fishmeal (P<0.05). In the present study, no significant effect of fish meal replacement with duckweed was observed on fish muscle composition (P > 0.05). Proximate composition of fish muscle was in agreement with reported nutritional composition of Gibelion catla (Hussain et al., 2018).
A cost-benefit analysis for the formulated feeds is shown in Table V. Estimated cost of formulated feed with 20% fishmeal replacement is 10.6% lower compared to fish meal-based control feed. Moreover, percent weight gain of catla using the former was 41.52% higher compared to control feed.
Table V. Cost benefit analysis for formulated feeds.
|
Type of formulated feed |
Estimated cost (USD)* |
Percent fish weight gain (g) |
|
|
1 kg |
50 kg |
||
|
Fish meal (30%) based feed |
1.09 |
54.67 |
54.09** |
|
Feed with 10% fish meal replacement |
1.04 |
51.77 |
86.23 |
|
Feed with 20% fish meal replacement |
0.98 |
48.85 |
76.55 |
|
Feed with 30% fish meal replacement |
0.92 |
45.95 |
55.48 |
1 USD= 235.26 PKR, **, During 60 days culture period using 210 g feed.
Conclusion
Conclusively, duckweed can be economically used to replace up to 20% fish meal in the diet of Gibelion catla without any negative effect on fish growth and feed digestibility. In future, efficacy of duckweed- based formulated feeds in polyculture of major as well as Chinese carps should be investigated for sustainable development of aquaculture in Pakistan. Duckweed (Lemna minor) can be used to replace up to 20% fish meal in Gibelion catla diet without any negative impact on fish growth. Partial replacement of fish meal with duckweed did not cause any significant change in apparent digestibility of feed dry matter and muscle composition of experimental fish. Estimated cost of feed formulated with 20% fishmeal replacement is 10.6% lower than fish meal-based control feed.
Acknowledgement
We thank our colleagues at Fisheries Research & Training Institute, Lahore, Pakistan for their assistance and support to conduct this study.
Funding information
The study didn’t receive any funding.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbas, S., Ahmed, I., Hafeez-Ur-Rehman, M., and Mateen, A., 2008. Replacement of fish meal by canola meal in diets for major carps in fertilized ponds. Pak. Vet. J., 28: 111-114.
Adeyemo, S., and Onilude, A., 2013. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Fd. J., 31: 84-90. https://doi.org/10.1016/S0189-7241(15)30080-1
Ali, Z., Waheed, H., Kazi, A.G., Hayat, A. and Ahmad, M., 2016. Duckweed: An efficient hyperaccumulator of heavy metals in water bodies. In: Plant metal interaction, Elsevier. pp. 411-429. https://doi.org/10.1016/B978-0-12-803158-2.00016-3
AOAC, 2012. Official methods of analysis of AOAC International (18th ed.). AOAC International, Gaithersburg, Md.
APHA, 2017. Standard methods for the examination of water and waster water (23rd ed.). American Public Health Association, American Water Works Association and Water Pollution Control Federation, New York.
Appenroth, K.J., Sree, K.S., Bog, M., Ecker, J., Seeliger, C., Böhm, V., Lorkowski, S., Sommer, K., Vetter, W. and Tolzin-Banasch, K., 2018. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem., 6: 483.https://doi.org/10.3389/fchem.2018.00483
Appenroth, K.J., Sree, K.S., Böhm, V., Hammann, S., Vetter, W., Leiterer, M. and Jahreis, G., 2017. Nutritional value of duckweeds (Lemnaceae) as human food. Fd. Chem., 217: 266-273. https://doi.org/10.1016/j.foodchem.2016.08.116
Atkinson, J., Hilton, J. and Slinger, S., 1984. Evaluation of acid-insoluble ash as an indicator of feed digestibility in rainbow trout (Salmo gairdneri). Can. J. Fish. aquat. Sci., 41: 1384-1386. https://doi.org/10.1139/f84-170
Boyd, C.E. and Tucker, C.S., 2012. Pond aquaculture water quality management. Springer Science and Business Media.
Collins, S.A., Øverland, M., Skrede, A. and Drew, M.D., 2013. Effect of plant protein sources on growth rate in salmonids: Meta-analysis of dietary inclusion of soybean, pea and canola/rapeseed meals and protein concentrates. Aquaculture, 400: 85-100. https://doi.org/10.1016/j.aquaculture.2013.03.006
De Silva, S.S., Francis, D.S. and Tacon, A.G., 2011. Fish oils in aquaculture in retrospect. In: Fish oil replacement and alternative lipid sources in aquaculture feeds (eds. G.M. Turchini, W.K. Ng, and D.R. Tocher). Boca Raton. 551 p, CRC Press. pp. 1-20. https://doi.org/10.1201/9781439808634-c1
Egerton, S., Wan, A., Murphy, K., Collins, F., Ahern, G., Sugrue, I., Busca, K., Egan, F., Muller, N. and Whooley, J., 2020. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep., 10: 1-16. https://doi.org/10.1038/s41598-020-60325-7
El-Shafai, S.A., El-Gohary, F.A., Verreth, J.A., Schrama, J.W. and Gijzen, H.J., 2004. Apparent digestibility coefficient of duckweed (Lemna minor), fresh and dry for Nile tilapia (Oreochromis niloticus L.). Aquacult. Res., 35: 574-586. https://doi.org/10.1111/j.1365-2109.2004.01055.x
FAO, 2020. The state of world fisheries and aquaculture Sustainability in action. Retrieved from Rome. http://www.fao.org/3/a-i5555e.pdf
Fasakin, E., Balogun, A. and Fasuru, B., 1999. Use of duckweed, Spirodela polyrrhiza L. Schleiden, as a protein feedstuff in practical diets for tilapia, Oreochromis niloticus L. Aquacult. Res., 30: 313-318. https://doi.org/10.1046/j.1365-2109.1999.00318.x
Figueiredo-Silva, C., Lemme, A., Sangsue, D. and Kiriratnikom, S., 2014. Effect of DL-methionine supplementation on the success of almost total replacement of fish meal with soybean meal in diets for hybrid tilapia (Oreochromis niloticus×Oreochromis mossambicus). Aquacult. Nutr., 21: 234-241. https://doi.org/10.1111/anu.12150
Goswami, R.K., Shrivastav, A.K., Sharma, J.G., Tocher, D.R. and Chakrabarti, R., 2020. Growth and digestive enzyme activities of rohu Labeo rohita fed diets containing macrophytes and almond oil cake. Anim. Feed Sci. Technol., 263: 114456. https://doi.org/10.1016/j.anifeedsci.2020.114456
Hardy, R.W., 2022. Fish nutrition (3rd ed.). Academic Press, London.
Hixson, S.M., 2014. Fish nutrition and current issues in aquaculture: The balance in providing safe and nutritious seafood, in an environmentally sustainable manner. J. Aquacult. Res. Dev., 5: 1. https://doi.org/10.4172/2155-9546.1000234
Hu, Z., Fang, Y., Yi, Z., Tian, X., Li, J., Jin, Y., He, K., Liu, P., Du, A. and Huang, Y., 2022. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT-Fd. Sci. Technol., 153: 112477. https://doi.org/10.1016/j.lwt.2021.112477
Hussain, B., Sultana, T., Sultana, S., Ahmed, Z. and Mahboob, S., 2018. Study on impact of habitat degradation on proximate composition and amino acid profile of Indian major carps from different habitats. Saudi J. biol. Sci., 25: 755-759. https://doi.org/10.1016/j.sjbs.2018.02.004
Jalili, R., Tukmechi, A., Agh, N., Noori, F. and Ghasemi, A., 2013. Replacement of dietary fish meal with plant sources in rainbow trout (Oncorhynchus mykiss) effect on growth performance, immune responses, blood indices and disease resistance. Iran J Fish Sci., 12: 577-591.
Jannathulla, R., Rajaram, V., Kalanjiam, R., Ambasankar, K., Muralidhar, M. and Dayal, J.S., 2019. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquacult. Res., 50: 3493-3506. https://doi.org/10.1111/are.14324
Li, X., Zheng, S., Ma, X., Cheng, K. and Wu, G., 2021. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids, 53: 33-47. https://doi.org/10.1007/s00726-020-02920-6
Mwale, M., and Gwaze, F.R., 2013. Characteristics of duckweed and its potential as feed source for chickens reared for meat production: A review. Sci. Res. Essays, 8: 689-697.
Noor, J., Hossain, M., Bari, M. and Azimuddin, K., 2000. Effects of duckweed (Lemna minor) as dietary fishmeal substitute for silver barb (Barbodes gonionotus Bleeker). Bangladesh J. Fish., 4: 35-42.
Rahman, M., Verdegem, M., Nagelkerke, L., Wahab, M., Milstein, A. and Verreth, J., 2006. Growth, production and food preference of rohu Labeo rohita (H.) in monoculture and in polyculture with common carp Cyprinus carpio (L.) under fed and non-fed ponds. Aquaculture, 257: 359-372. https://doi.org/10.1016/j.aquaculture.2006.03.020
Ronald W, H., 2022. Fish nutrition (4th ed.). Academic Press, London, United Kingdom.
Samtiya, M., Aluko, R.E. and Dhewa, T., 2020. Plant food anti-nutritional factors and their reduction strategies: An overview. Fd. Prod. Process. Nutr., 2: 1-14. https://doi.org/10.1186/s43014-020-0020-5
Shafi, J., Waheed, K.N., Mirza, Z.S. and Zafarullah, M., 2021. Variation in bottom soil quality with increasing pond age in freshwater aquaculture. Turk. J. Fish. aquat. Sci., 22: TRJFAS18305. https://doi.org/10.4194/TRJFAS18305
Van Keulen, J., and Young, B., 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci., 44: 282-287. https://doi.org/10.2527/jas1977.442282x
WorldBank, 2013. Fish to 2030 Prospects for fisheries and aquaculture. Agriculture and Environmental Services Discussion Paper 03. Retrieved from http://www.fao.org/docrep/019/i3640e/i3640e.pdf
Yılmaz, E., Akyurt, İ. and Günal, G., 2004. Use of duckweed, Lemna minor, as a protein feedstuff in practical diets for common carp, Cyprinus carpio, fry. Turk. J. Fish. aquat. Sci., 4: 105-109.
To share on other social networks, click on any share button. What are these?










