Effect of Biochar, Farmyard Manure and Poultry Manure on Zn Adsorption in Calcareous Alkaline Soil
Research Article
Effect of Biochar, Farmyard Manure and Poultry Manure on Zn Adsorption in Calcareous Alkaline Soil
Abida Saleem*, Sajida Parveen and Muhammad Jamal Khan
Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | The effect of Farm Yard Manure (FYM), Press Mud (PM), and Biochar (BC) applied to a fresh soil was evaluated on Zn adsorption potential in calcareous alkaline soils [Piedmont alluvium, silty clay loam, Ustochrept]. The Zn adsorption capacity was determined by adding 50 ml of 0, 5, 10, 20, 40, 60, 120, 240 and 360 mg Zn L-1 (initially Zn applied, IZnA) to 5 g soil with absence (non-treated) or to soil pretreated with FYM, PM or BC at rate of 10 g kg-1. The suspension was shaken on horizontal shaker for 20 h continuously to assess the equilibrium Zn concentration (EZnC). The difference between the IZnA and EZnC was assumed to be adsorbed. Results showed that FYM, PM or BC yielded lower EZnC and higher Zn adsorption (x/m) at higher IZnA but at lower IZnA< 20 mg Zn L-1 the reverse was observed. The data in non-treated soils were fit to both Langmuir and Freundlich but in treated soils they were fit only when adsorption values for initial IZnA were disregarded. The adsorption, as indicated by b and K values was higher for BC followed by FYM but MCB and 1/n value that reflects the buffering capacity was higher for FYM than BC or PM. The lower adsorption of Zn at lower IZnA below 20 mg Zn L-1 and higher for higher IZnA for amended soil over non-treated soil suggesting its contrasting effect at different IZnA levels.
Received | May 24, 2016; Accepted | August 03, 2016; Published | November 03, 2016
Correspondence | Abida Saleem, Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: abda_saleem@yahoo.com
Citation | Abida Saleem, A., S. Parveen and M.J. Khan. 2016. Effect of biochar, farmyard manure and poultry manure on Zn adsorption in calcareous alkaline soil. Sarhad Journal of Agriculture, 32(4): 354-363.
DOI | http://dx.doi.org/10.17582/journal.sja/2016.32.4.354.363
Keywords | Zinc, Zn adsorption, Biochar, Isotherm model, Calcareous soil
Introduction
Zinc content in soil ranges from 10 to 300 mg kg-1 with an average value of 50 mg kg-1 (Kiekens, 1995); however, as compared to its total amount available Zn concentration in soil solution is very low. Values greater than 0.9 mg kg-1 AB-DTPA ext. Zn are regarded as optimum levels for agronomical crops (Soltanpour, 1985). The soil zinc exists in different forms i.e. in soil solution, in exchangeable form, bound with soil organic matter, Fe, Al and Mn oxide in primary or secondary minerals (Viets, 1962; Catlett et al., 2002). The immediate source for plant uptake is soil solution Zn2+ which is determined by different reactions like complexation, adsorption and precipitation of metals in soils. Adsorption process is very important in determining zinc (Zn) availability and fate in soil. Several adsorption isotherms have been employed to relate the Zn concentration in solution to that retained by the solid phase (Xie and Mackenzie, 1991).
Soil properties like pH (Zahedifar et al., 2010; Pérez-Novo et al., 2011), clay type, (Dahiya et al., 2005), clay content (Dahiya et al., 2005), organic matter (Perez-Novo et al., 2008), total carbonate and active carbonate content (Al-Kaysi, 2000; Al-Tamimi, 2006), amount of zinc applied and zinc carrier (Obrador et al., 2003) and sulfur contents (Alloway, 2009) have strong influence on adsorption-desorption process and regulating the Zn solubility and fractionation in soils. Low redox potential (Johnson-Beebout et al. 2009), soil solution composition (Wang and Harrell, 2005; Zhao et al., 2010), ionic strength (Shuman, 1986) and complex formation with inorganic ligands in soil solution (Mattigod and Sposito, 1977) are the other factors which affect Zn solubility. Zinc solubility is also controlled by phosphate-Fe oxides interactions (Wang and Harrell, 2005). The deficiency is more common in calcareous and salt affected soils (Khoshgoftarmanesh et al., 2006) where elevated lime and pH levels are reportedly responsible for high Zn adsorption (Karimian and Moafpouryan, 1999).
The organic fraction of soil could influence these reactions through chelation and associated soil physico-chemical characteristics that lead to either decrease or increase in availability of Zn to plants. Organic matter content increased the Zn sorption (Vega et al., 2007) resulting in Zn deficiency due to formation of insoluble Zn- organic complexes (Andriano, 2001). It is a common practice to add organic matter amendments, such as compost, fertilizers and wastes for immobilization of heavy metals and amelioration of contaminated soils (Clemente et al. 2005). The effect of organic matter amendments on heavy metal bioavailability depends on the nature of the organic matter, their microbial degradability, salt content and effects on soil pH and redox potential, as well as on the particular soil type and metals concerned (Walker et al. 2003). But on the other hand, this organic matter especially the soluble C fraction acting as chelating agent which decreases the Zn adsorption and increases formation of soluble organic-Zn complexes and thus increases the availability to plants (Shuman, 1999). The increase in crop yields could be attributed to improvement in soil physical, chemical and biological activities promoting soil-plant system. The increase in nutrition especially of micronutrients is usually believed as result of decomposition of initially applied amendments. In such situation the improvement in the specific nutrient would depend on its concentration in the substrate and its rate of decomposition. However, the chemical characteristics of the adding material and changes brought about in the soil properties are site specific.
In low concentration of micronutrients organic amendments are argued to enhance the nutrient availability to plant but on the other hand these amendments are advised for phyto-stabilization of contaminated soils (Khan et al., 2012). At low concentration, the amendments are believed to reduce the bonding energy and thus the adsorption of Zn could be decreased but they also increase the CEC of soil (Bohn et al., 2001) that can increase the adsorption of Zn at higher levels. These changes in soil can change with the organic amendments, and as such this study was initiated to evaluate the effect of BC, FYM and PM on Zn adsorption capacity of calcareous soil to assess their role in Zn nutrition of calcareous soil.
Materials and Methods
The effect of organic amendments including FYM, PM and BC on Zn adsorption potential of strongly calcareous (16.6 % CaCO3) alkaline (pH 8.36) soils of Peshawar series [Piedmont alluvium, silty clay loam, Ustochrept] was evaluated in the laboratory of Soil and Environmental Sciences, The University of Agriculture Peshawar during 2016. The soil under study was silty clay loam (clay 11 %) and low in organic matter (0.68 %) and AB-DTPA extractable Zn (0.8 mg kg-1). Fresh soil sample was collected from Research Farm of the university and was air dried and sieved through 2 mm sieve. The soil was first added with FYM, PM and BC each at 20 t ha-1 (10 g kg-1 soil) before addition of Zn solution. The stock solution of Zn was prepared from dissolving elemental Zn in HCl solution at the rate of 1000 mg L-1 whereas the working solutions of 0, 5, 10, 20, 40, 60, 120, 240 and 360 mg Zn L-1 were prepared in 0.01 M CaCl2 solution.
The Zn adsorption isotherms were determined by the procedure of Fox and Kamprath (1970) which has been used by several researchers including Solis and Torrent (1989), Hussain et al. (2007) and Manzoor (2013) for P adsorption. In this study, 50 ml solution containing 0, 10, 20, 40, 60, 120, 240 and 360 mg Zn L-1 (initially applied Zn, IZnA) was applied to 5 g soil in 250 mL open mouthed conical flasks. The soil plus respective Zn solutions taken in duplicate were shaken on horizontal shaker for 20 h continuously. The suspensions were filtration through Whattman No. 42. The Zn in the supernatants was determined using atomic absorption spectrophotometry after diluting the sample to get machine readings in the range of 0.1 to 2 mg L-1. This concentration was represented as equilibrium Zn concentration (EZnC). The difference between the IZnA and EZnC was assumed to be the adsorbed Zn mg kg-1 denoted by x/m.
Table 1: Effect of organic amendments on Zn adsorption characteristics in strongly calcareous alkaline soil of Peshawar series
|
Initial Zn applied (IZnC) |
EZnC |
Zn adsorbed |
Xad |
Kd |
EZnC/X/m |
||
|
mg/L |
mg/kg |
mg/L |
mg/L |
mg/kg |
% |
||
|
--------------------------------------------- Zn alone --------------------------------------------- |
|||||||
| 5 |
50 |
0.56 |
4.44 |
44.38 |
88.75 |
78.89 |
0.013 |
|
10 |
100 |
1.23 |
8.78 |
87.75 |
87.75 |
71.63 |
0.014 |
|
20 |
200 |
2.95 |
17.05 |
170.50 |
85.25 |
57.80 |
0.017 |
|
40 |
400 |
8.35 |
31.65 |
316.50 |
79.13 |
37.90 |
0.026 |
|
80 |
800 |
30.58 |
49.43 |
494.25 |
61.78 |
16.17 |
0.062 |
|
120 |
1200 |
61.40 |
58.60 |
586.00 |
48.83 |
9.54 |
0.105 |
|
240 |
2400 |
149.23 |
90.78 |
907.75 |
37.82 |
6.08 |
0.164 |
|
360 |
3600 |
245.50 |
114.50 |
1145.00 |
31.81 |
4.66 |
0.214 |
|
----------------------------------------------- Zn + PM ----------------------------------------------- |
|||||||
| 5 |
50 |
1.80 |
3.20 |
32.03 |
64.05 |
17.82 |
0.056 |
|
10 |
100 |
3.13 |
6.88 |
68.75 |
68.75 |
22.00 |
0.045 |
|
20 |
200 |
3.70 |
16.30 |
163.00 |
81.50 |
44.05 |
0.023 |
|
40 |
400 |
7.58 |
32.43 |
324.25 |
81.06 |
42.81 |
0.023 |
|
80 |
800 |
26.60 |
53.40 |
534.00 |
66.75 |
20.08 |
0.050 |
|
120 |
1200 |
50.61 |
69.39 |
693.90 |
57.83 |
13.71 |
0.073 |
|
240 |
2400 |
132.43 |
107.57 |
1075.68 |
44.82 |
8.12 |
0.123 |
|
360 |
3600 |
234.30 |
125.70 |
1257.00 |
34.92 |
5.36 |
0.186 |
|
----------------------------------------------- Zn + FYM ----------------------------------------------- |
|||||||
| 5 |
50 |
1.68 |
3.33 |
33.25 |
66.50 |
19.85 |
0.050 |
|
10 |
100 |
2.48 |
7.53 |
75.25 |
75.25 |
30.40 |
0.033 |
|
20 |
200 |
2.65 |
17.35 |
173.50 |
86.75 |
65.47 |
0.015 |
|
40 |
400 |
4.35 |
35.65 |
356.50 |
89.13 |
81.95 |
0.012 |
|
80 |
800 |
23.83 |
56.18 |
561.75 |
70.22 |
23.58 |
0.042 |
|
120 |
1200 |
48.75 |
71.25 |
712.50 |
59.38 |
14.62 |
0.068 |
|
240 |
2400 |
123.73 |
116.28 |
1162.75 |
48.45 |
9.40 |
0.106 |
|
360 |
3600 |
219.68 |
140.33 |
1403.25 |
38.98 |
6.39 |
0.157 |
|
----------------------------------------------- Zn + BC----------------------------------------------- |
|||||||
| 5 |
50 |
2.53 |
2.48 |
24.75 |
49.50 |
9.80 |
0.102 |
|
10 |
100 |
3.26 |
6.74 |
67.38 |
67.38 |
20.65 |
0.048 |
|
20 |
200 |
3.40 |
16.60 |
166.00 |
83.00 |
48.82 |
0.020 |
|
40 |
400 |
4.96 |
35.04 |
350.43 |
87.61 |
70.69 |
0.014 |
|
80 |
800 |
20.18 |
59.83 |
598.25 |
74.78 |
29.65 |
0.034 |
|
120 |
1200 |
49.80 |
70.20 |
702.00 |
58.50 |
14.10 |
0.071 |
|
240 |
2400 |
116.69 |
123.31 |
1233.10 |
51.38 |
10.57 |
0.095 |
|
360 |
3600 |
202.73 |
157.28 |
1572.75 |
43.69 |
7.76 |
0.129 |
Xad: Ratio of adsorption in percent with applied Zn level; Kd: ratio of adsorbed Zn with equilibrium [Zn]
Results and Discussion
Zn Adsorption as Influenced by Organic Amendments
The Zn adsorption (x/m) and equilibrium Zn (EZnC) increased with increase in applied Zn levels (IZnA) in both treated and untreated soils but with different pattern. Treating the soil with organic amendments the Zn adsorption was comparatively higher over respective non-treated soils at all corresponding applied Zn levels (IZnA) and as well as equilibrium Zn (EZnC) when IZnA was higher than 20 mg Zn L-1 (Table 1). Among the amendments, BC was more effective in promoting adsorption of Zn followed by FYM and then PM (Figure 1). With application of BC, the Zn adsorption ranged from 2.48 to 157.28 mgL-1 (equivalent to 24.75 to 1572 mg kg-1 at IZnA levels from 0-360 mg L-1, which was significantly higher than the range of 4.44 to 114.50 mg L-1 recorded in case of non-treated soil or FYM and PM.
This higher adsorption of Zn in amended soils resulted in significantly lower EZnC (mg L-1) at each IZnC level than those received alone Zn in case when the IZnA levels were higher than 20 mg L-1. Figure 1 and 2 both revealed that Zn adsorption at any IZnA or EZnC levels were higher for treated soils than non-treated and the BC superseded following FYM and then PM. The higher adsorption and lower EZnC revealed that amendment application increased the Zn adsorption over alone Zn application.
The Zn adsorbed (x/m) expressed in percent of IZnA (Xad) and ratio of x/m to EZnC denoted as distribution coefficient (Kd) decreased with increased in IZnA. However, in non-treated both Xad and Kd decreased with each increment of IZnA from 5 to 360 mg Zn L-1 while in treated soil they first increased with initial lower IZnA and then decreased. As such in all amended soils the maximum Xad and Kd were observed at IZnA 40 mg L-1 which is totally different from non-amendment soil (Zn alone) where the maximum Xad and Kd were recorded at lower IZA i.e. 5 mg Zn L-1 It is an established criteria that high Xad and Kd values indicate more efficient removal of adsorbate (here Zn) from the soil solutions by soils (Hussain et al., 2007). The higher Xad and Kd in alone Zn treated soil at lower IZnA <40 mg Zn L-1) indicated its comparatively higher affinity and more adsorption of Zn than treated soils. But amended soils showed higher higher Xad and Kd than non-amended soil at higher IZnA especially beyond >40 mg Zn L-1. This contrasting pattern of Xad and Kd at low and higher IZnA indicate that organic amendments reduces the adsorption of Zn at low IZnA and increases the adsorption at higher IZnA. In other words the fresh applied organic amendments can facilitate Zn availability at low Zn levels and can play role in phytostabilization (Adsorption) at higher Zn levels (contaminated soil). Among amendments, BC had comparatively lower Xad and Kd in initial lower IZnA and higher at higher IZnA suggesting its stronger influences in controlling the Zn adsorption than PM and FYM.
Addition of organic amendments reduce the adsorption potential of Zn (Jalali and Jalali, 2011) and P (Hussain et al., 2006; Yusran, 2010). The same has been reported by many researcher that application of organic amendments increase the availability of nutrients especially at lowconcentration. But on the other hand, the same organic amendments are recommended for phytostabilization of heavy metals in the contaminated soils. This reflects that organic amendments play a dual role, at low soil Zn they can increase the Zn in solution but at higher Zn they can increase the adsorption. The same observations were recorded in our study.
Adsorption Isotherm Equation
The adsorption and equilibrium Zn concentrations data received from all treated soils were plotted in Langmuir and Freundlich Isotherm Models. As discussed below Langmuir model was best fit to alone Zn at all IZnA but became fit to amended soil when data for initial IZA was disregarded. Similarly, Freundlich model was fit to non-amended soil at all IZnA but to organically amended soil only when values at initial IZnA were disregarded.
Langmuir Adsorption Isotherm Equation
The adsorption isotherm models of Langmuir and Freundlich were applied to investigate Zn adsorption in the soil by comparing the bonding strength, maximum Zn adsorption and buffering capacity of the soil. The classical Langmuir adsorption equation and its linear model, expressed by the following formulas were used:

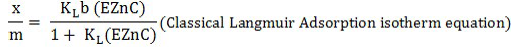
Whereas in the formula, x/m is the amount of Zn adsorbed (mg Zn kg-1), ’KL’ is bonding energy constant, ‘b’ is Langmuir adsorption maximum (mg Zn kg-1), EZnC is the concentration of Zn in soil solution at equilibrium (mg L-1) ‘KLb’ is also known as maximum buffering capacity of the soil system. By plotting the EZnC/(x/m) against EZnC gives the Langmuir Adsorption Model that produced curvilinear linear form in both organically amended and non-amended soils.
However, for amended soil the initial values of EZnC/(x/m) were disregarded as they did not fit in the graph (Figure 3). Sanyal et al. (1993), Hussain et al. (2006) and Hussain et al. (2007) also observed curvilinear Langmuir plots. Manzoor (2013) also reported the curvilinear Langmuir plot in the same soil for P adsorption. The curvilinear behavior suggested that originally the soil had varying bonding energy for Zn adsorption and the adsorption capacity of soil decreased with each additional increment of IZnA. Gunary (1970) stated that the curvilinear relationship mean that the soil would adsorb a small amount of adsorbate (here the Zn) less firmly and so on. However, Syers et al. (1973) were of the opinion that that two or more population of sites in soils having different affinity for adsorabte (here the Zn) might be the reason for curvature.
The assumption of constant energy of adsorption, adsorption on specific sites and monolayer adsorption on which the simple Langmuir adsorption isotherm equation relies (Bohn et al., 1985) were fully (100%) satisfied in all treated soils. The r2 values were comparatively higher enough to be relied. For example the alone Zn, Zn + FYM, Zn + PM, Zn + BC produced the r2 values of 0.95, 0.96, 0.98, 0.95, respectively, however, due to curvilinear nature, the modified Langmuir model was used for all soil (Figure 4).
The bonding energy, maximum adsorption and buffering capacity were calculated based on simple Langmuir adsorption isotherm model where the initial IZnA values were disregarded. Zn buffering capacity is the potential of soil to resist changes in Zn soil solution concentration, when Zn added or removed from the soil (Ozanne, 1980; Jalali, 2007). The soil with high buffering capacity can release Zn to the soil solution slowly and reversible retention of Zn by colloids of soil during soil reaction (Barrow and Shaw, 1975). As compared to non-treated soil, the amended soils produced higher adsorption maximum, buffering capacity and bonding energies. The higher adsorption maximum and KL values closely determine the strength of attachment with which Zn is bonded to soil particles (Bahl et al., 1986). Among the amendments, the higher absorption maximum of BC indicated its higher potential of Zn adsorption over the FYM and PM. However, the buffering capacity did not support its supremacy over FYM and PM. The different KL also indicated that sites for Zn adsorption for the given amendments were different (Javid, 1999) but surely higher than non-treated soils.
Table 2: Comparative Equilibrium parameters of the Langmuir adsorption isotherm equation for the given amendments in calcareous soil
|
Parameter |
Zn alone |
Zn+PM |
Zn+FYM |
Zn + BC |
|
Intercept |
0.0391 | 0.0291 | 0.0246 | 0.0312 |
|
Slope |
0.0008 | 0.0007 | 0.0006 | 0.0005 |
|
Square r |
0.96 | 0.98 | 0.963 | 0.957 |
|
Adsorption maximum (b) |
1250 mg kg-1 |
1428 | 1667 | 2000 |
|
Bonding Energy (KL) |
0.020 L mg-1 |
0.0240 | 0.0244 | 0.016 |
|
Buffering Capacity (MBC) |
25.58 mg P Kg-1 |
34.36 | 40.65 |
32.05 |
Because curvilinearity in all treated soils, the adsorption seems to have formed multisite and multilayer adsorption as expressed by the following modified Langmuir Model was applied as given by Bohn et al. (1985).
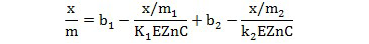
Where the subscripts 1 and 2 refer to the region (or mechanism) 1 and 2 respectively.
The graph yielded two curve lines for each treated soil; one at low equilibrium [Zn] (EZnC) and the other at higher EZnC. The region I representing the first straight portion may be associated to adsorption while at high EZnC precipitation may be responsible for the second straight line in region II (Lin et al., 1983).
In contrast to sorption, the EZnC/(x/m) was lower for organically amended soil than non-treated soils (alone Zn) indicating higher adsorption in amendment soils. Among the treatment, the BC had the lowest EZnC/x/m than FYM and PM treated soil revealing higher adsorption potential of BC.
Freundlich Adsorption Isotherm Equation
The Freundlich equation implies that the energy of the adsorption on a uniform surface is independent of the surface coverage and that it decrease logarithmically as the fraction of the covered surface increases. The decrease in energy of adsorption with the increase in surface coverage is due to surface heterogeneity (Bohn et al., 1985). It is usually used in the condition where the Langmuir equation fails (Bohn et al., 1985). The equation is expressed by the following formula for Zn:
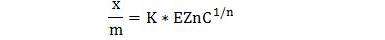
Where K and n are empirical constants, x/m is the adsorption and the EZnC is the equilibrium of Zn.
The linear model of the equation is:
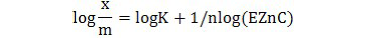
The data were plotted according to the above linear model in Figure 5 which produced linear relationships with r2values of 0.97 to 0.98 over all treated and non-treated soils suggesting that both Langmuir and Freundlich models were equally best fit for Zn adsorption in the present scenario.
Values of K representing the adsorption coefficient of soil were 136.65 in case of alone Zn (non-treated soil) which increased to 143.25, 195.89 and 176.21 with application PM, FYM and BC indicating increase in adsorption with amendment application (Table 3).
Table 3: Comparative equilibrium parameters of the Freudlichadsorption isotherm equation for the given treatments of alone Zn and with amendments
|
Parameters |
Zn alone |
Zn + PM |
Zn + FYM |
Zn alone |
|
Intercept |
2.136 | 2.156 | 2.292 | 2.246 |
|
Slope |
0.377 | 0.403 | 0.357 | 0.399 |
|
Square r |
0.98 | 0.99 | 0.97 | 0.97 |
|
Adsorption maximum (K) |
136.65 | 143.25 | 195.89 | 176.21 |
|
N value |
2.65 | 2.48 | 2.80 |
2.50 |
Similarly, the value of N representing inverse of the slope of graph were higher for amended soil suggesting slower increase in adsorption with IZA than non-treated soil where sharp increase in adsorption were observed. Among treatments, FYM had higher K followed by BC and PM suggestion more adsorption by FYM in the present study. Since, the lower n or higher 1/n value indicates more heterogeneity (Gregory et al. 2005) suggesting that application of organic amendments increased the heterogeneity of soil. Javid (1999) reported that K is the adsorbed specie that would sustain its concentration in equilibrium solution. Cole et al. (1953) and Holford and Mattingly (1976) suggested that at low concentration adsorption is expected while at higher concentration the dominant mechanism is precipitation.
Table 4: NPK and Zn concentrations of organic amendments
| Nutrients | Unit | FYM | PM | Biochar |
| N | % | 2.13 | 1.83 | 0.63 |
| P | % | 1.23 | 1.54 | 0.35 |
| K | % | 2.86 | 1.69 | 2.37 |
| DTPA Zn |
mg kg-1 |
72.4 | 154 | 21.36 |
| DTPA Cu |
mg kg-1 |
26.0 | 49 | 15.0 |
| DTPA Fe |
mg kg-1 |
296 | 557 |
218 |
Yongku et al. (2010) reported that dissolved organic matter (DOM) obtained from humus soil (DOM H), rice straw (DOMR), and pig manure (DOMP) reduced maximum Hg (mercury) adsorption capacity up to 40% over control in order of DOM H (250.00 mg kg-1) < DOMR (303.03 mg kg-1) < DOMP (322.58 mg kg-1) < control (416.67 mg kg-1) and increased its desorption from the soil. Tani et al. (2010) reported that Andisols usually fix large amounts of phosphate on surface-reactive sites but with addition of water-soluble organic matter (WSOM) reduced the binding strength of phosphate and possibly induced subsequent phosphate desorption and recommended the combine use of manure and inorganic phosphate fertilizer. The same trend was observed in our study at low IZnC especially below < 20 mg Zn L-1 but at higher concentration the adsorption of Zn increased with organic amendment application. This increase over control may be associated to increase in the charges sites resulting in higher ion exchange capacity. The increase in CEC with organic matter is an established fact (Bohn et al., 2001). The higher CEC would have resulted in higher adsorption of Zn in our study.
Acknowledgement
The financial support of HEC as Indigenous Ph.D study is highly acknowledged. The help of Dr. Dost Muhammad, Assistant Professor, Department of SES, the University of Agriculture, Peshawar in planning and data analysis of this paper is highly acknowledged.
Conflict of Interest
There is no conflict of interest.
Authors’ Contribution
Abida Saleem was student of PhD and worked on research study. Dr. Sajida Perveen was major supervisor of the research work who guided and supervised at all stages during research. Prof. Dr. Jamal Khan Khatak helped and guided in different aspects during work .
References
Adriano, D.C. 2001. Trace Element in Terrestrial Enviornments; Biogeohemistry, Bioavailability and Risk of metals. 2nd Edn, Spinger-Verlag, NewYork, pp. 626-668. http://dx.doi.org/10.1007/978-0-387-21510-5
Al-Kaysi, S.C. 2000. Effects of physical and chemical properties of carbonate minerals in some Iraqi soils in zinc fixation: Properties of carbonate minerals. Iraqi J. Agric. Sci. 30:53-72. (in Arabic).
Alloway, B.J. 2009. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health. 31:537–548. http://dx.doi.org/10.1007/s10653-009-9255-4
Al-Tamimi, R.A. 2006. Zinc sorption by some Torrifluvents of sub-Saharian region south of Libya. Emirates J. Agric. Sci. 18:1-10
Bahl, G.S., N.T. Singh and A.C. Vig. 1986. Phosphate uptake by maize and wheat in relation to P adsorption characteristic of soil. J. Indian. Soc. Soil. Sci. 34:791-798.
Barrow, N.J. and T.C. Shaw. 1975. The slow reaction between soil and anions: 2. Effect of time and temperature on the decrease in phosphate concentration in the soil solution. J. Soil Sci. 119:167- 177. http://dx.doi.org/10.1097/00010694-197502000-00010
Bohn, H.L., B.L. McNeal and G.A. O’Gonnor. 1985. Soil chemistry. Wiley Interscience, New York.
Bohn, H.L., B.L. McNeal and G.A. O’Gonnor. 2001. Soil Chemistry. 3rd Ed. John Wiley and Sons, Inc. New York.
Catlett, K.M., D.M. Heil, W.L. Lindsay and M.H. Ebinger. 2002. Soil chemical properties controlling zinc activity in 18 Colorado soils. Soil Sci. Soc. Am. J. 66:1182-1189. http://dx.doi.org/10.2136/sssaj2002.1182
Clemente, R., D.J. Waljker, and M.P. Bernal. 2005. Uptake of heavy metals and as by Brassica Juncea grown in a contamination soil in Arnalcollar (Spain): The effect of soil amendments. Environ. Pollut. 136:46-58. http://dx.doi.org/10.1016/j.envpol.2005.02.019
Cole, C.V., S.R. Olsen and C.O. Scott. 1953. The nature of phosphate sorption by calcium carbonates. J. Soil Sci. Soc. Am. Proc. 17:352-356. http://dx.doi.org/10.2136/sssaj1953.03615995001700040013x
Dahiya, S., A.V. Shanwal and H.A.G. Hedge. 2005. Studies on the sorption and desorption characteristics of Zn(II) on the surface soils of nuclear power plants sites in India using a radiotracer technique. Chemosphere. 60:1253-1261. http://dx.doi.org/10.1016/j.chemosphere.2005.01.089
Fox, R.L., and E.J. Kamprath. 1970. Phosphate sorption isotherm for the evaluating the phosphate requirement of soils. Soil Sci. Soc. Am. Proc. 34:902-907. http://dx.doi.org/10.2136/sssaj1970.03615995003400060025x
Gregory, T., L.K. Chelsey and K.D. Shimizu. 2005. A critical examination of the use Hamadan, westren Iran. J. Environ. Geol. 53:365-374.
Gunary, D. 1970. A new adsorption isotherm for phosphate in soil. J. Soil Sci. 21:72-77. http://dx.doi.org/10.1111/j.1365-2389.1970.tb01153.x
Holford, I.C.R., and G.E.G. Mattingly. 1976. Phosphate adsorption and plant availability of phosphate. J. Plant Soil. 44:377-389. http://dx.doi.org/10.1007/BF00015889
Hussain, A., A. Ghafoor, and G. Murtaza. 2006. Use of model for phosphorus adsorption of some sodic soil of Punjab. Int. J. Agric. Biol. 8:242-248.
Hussain, A., G. Murtaza and A. Ghafoor. 2007. Derermination internal and external P requirement of wheat on calcareous soils by adsorption isotherm. Pakistan J. Agric. Sci. 44:103-109.
Jalali, M. 2007. Phosphorus status and sorption characteristics of some calcareous soils of Hamadan, western Iran. J. Environ. Geol. 53:365-374. http://dx.doi.org/10.1007/s00254-007-0652-7
Jalali, M., and A. Jalali. 2011. Competitive adsorption of trace elements in calcareous soils as affected by sewage sludge, poultry manure, and municipal waste compost. J. Environ. Earth. Sci. 63:731-739. http://dx.doi.org/10.1007/s12665-010-0742-9
Javid, S. 1999. Residual effect of phosphate fertilizer measured using the Olsen method in Pakistani soils. Ph.D. Diss. Uni. Reading, UK.
Johnson‐Beebout, S.E., J.G. Lauren and J.M. Duxbury. 2009. Immobilization of zinc fertilizer in flooded soils monitored by adapted DTPA soil test. Commun. Soil Sci. Plant Anal. 40:1842– 1861. http://dx.doi.org/10.1080/00103620902896738
Karimian, N., and G. R. Moafpourian. 1999. Zinc adsorption characteristics of selected soil of Iran and their relationship with soil properties. Commun. Soil Sci. Plant Anal. 30: 1722-1731. http://dx.doi.org/10.1080/00103629909370325
Khan, N. I., A.U. Malik, F. Umer and M.I. Bodla. 2012. Effect of tillage and farmyard manure on physical properties of soil. Int. Res. J. Plant Sci. 1(4):75-82.
Khoshgoftarmanesh, A.H., H. Shariatmadari, N. Karimian, M. Kalbasi and M.R. Khajehpour. 2006. Zinc efficiency of wheat cultivars grown on a saline calcareous soil. J. Plant Nut. 27: 1953–1962. http://dx.doi.org/10.1081/PLN-200030068
Kiekens, L. 1995. Zinc. In B.J. Alloway (ed.) Heavy Metals in Soils. Chapman & Hall, London. pp. 284–303. http://dx.doi.org/10.1007/978-94-011-1344-1_13
Lin, C., W. J. Busscher, and L. A. Douglas. 1983. Multifactor kinetics of phosphate reactions with minerals in acidic soils: I Modeling and simulation. Soil Sci. Soc. Am. J. 47:1097-1103. http://dx.doi.org/10.2136/sssaj1983.03615995004700060008x
Lindsay, W.L. 1974. Role of chelation in micronutrient availability. In E.W. Carson (ed.) The Plant Root and Its Environment. Univ. of Virginia Press, Charlottesville.
Manzoor, A. 2013. Critical soil solution phosphorus concentrations essential for plant growth in calcareous soil series. PhD thesis, SES department, The University of Agriculture Peshawar, Pakistan.
Mattigod, S.V., and G. Sposito. 1977. Estimated association constants for some complexes of trace metals with inorganic ligands. Soil Sci. Soc. Am. J. 41: 1092- 1097. http://dx.doi.org/10.2136/sssaj1977.03615995004100060015x
Obrador. A., J. Novillo, and M. Alvarez. 2003. Mobility and availability to plants of two zinc sources applied to calcareous soil. Soil Sci. Soc. Am. J. 67: 564-572. http://dx.doi.org/10.2136/sssaj2003.5640
Ozanne, P. G. 1980. Phosphate nutrition of plants- A general treatise. In The Role of phosphorus in agriculture. American society of Agronomy. Crop Science Society of America. Soil Science Society of America, Madison, WI. pp. 559-589.
Pérez-Novo, C., D. Fernández-Calviño, A. BermúdezCouso, J.E. López-Periago, and M. Arias-Estévez. 2011. Phosphorus effect on Zn adsorption-desorption kinetics in acid soils. Chemosphere. 83: 1028-1034. http://dx.doi.org/10.1016/j.chemosphere.2011.01.064
Perez-Novo, C., M. Pateiro-Moure, F. Osorio, and J. C. Novoa-Munoz . 2008. Influence of organic matter removal and competitive and non-competitive adsorption of copper and zinc in acid soils. J. Colloid Interface Sci. 322: 33- 40. http://dx.doi.org/10.1016/j.jcis.2008.03.002
Sanyal. S. K., S. K. De-Datta, and P.Y. Chan. 1993. Phosphate sorption-desorption behavior of some acidic soils of south and south-east Asia soil. Sci. Soc. Am. J. 57:937-945. http://dx.doi.org/10.2136/sssaj1993.03615995005700040011x
Shuman, L.M. 1986. Effect of ionic strength and anions on Zn sorption by two soils. Soil Sci. Soc. Am. J. 50:1438-1442. http://dx.doi.org/10.2136/sssaj1986.03615995005000060012x
Shuman, L.M. 1999. Organic waste amendments effect on zinc fractions of two soils. J. Environ. Qual. 28: 1442–1447. http://dx.doi.org/10.2134/jeq1999.00472425002800050008x
Solis, P., and J. Torrent. 1989. Phosphate sorption of calcareous vertisols and inceptisols of Spain. Soil Sci. Soc. Am. J. 53:456-459. http://dx.doi.org/10.2136/sssaj1989.03615995005300020026x
Soltanpour, P.N. 1985. Use of ammonium bicarbonate- DTPA soil test to evaluate elemental availability and toxicity. Commun. Soil Sci. Plant Anal. 16(3):323-338. http://dx.doi.org/10.1080/00103628509367607
Syers, J.K., M.G. Browman, G.W. Smillic and R.B. Corey. 1973. Phosphate sorption by soil evaluated by the Langmuir adsorption equation. Soil Sci. Soc. Am. Proc. 37:358-363. http://dx.doi.org/10.2136/sssaj1973.03615995003700030015x
Tani, M.A., K.T. Kato and M. Koik. 2010. Effect of organic ligands on phosphate adsorption and availability in Andisols of eastern Hokkaido, Japan. 19th World Congress of Soil Science, Soil Solutions for a Changing World 52 1 – 6 August 2010, Brisbane, Australia. Published on DVD.
Vega, F.A., E.F. Covelo, J.J. Vazguez and L. Andrade. 2007. Influence of mineral and organic components on copper, lead, and zinc sorption by acid soils.J Environ Sci Health A Tox Hazard Subst. Environ Eng. 14:2167-73. http://dx.doi.org/10.1080/10934520701629682
Viets, F.G. 1962. Chemistry and availability of micronutrients in soils. J. Agri. Food Chem. 10:174-178. http://dx.doi.org/10.1021/jf60121a004
Walker, D.J., R. Clemente, A. Roig and M.P. Bernal. 2003. The effect of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ. Pollut. 22:303-312. http://dx.doi.org/10.1016/S0269-7491(02)00287-7
Wang, J.J., and D.L. Harrel. 2005. Effect of ammonium, potassium and sodium cations and phosphate, Nitrate, and chloride anions on Zn sorption and lability in selected acid and calcareous soils. Soil Sci. Soc. Am. J. 69:1036-1046. http://dx.doi.org/10.2136/sssaj2004.0148
Wang, Z., P. Yin, R. Qu, H. Chen, C. Wang and S. Ren. 2013. Adsorption kinetics, thermodynamics and isotherm of Hg (II) from aqueous solutions using buckwheat hulls from Jiaodong of China. Food Chem. 136:1508–1514. http://dx.doi.org/10.1016/j.foodchem.2012.09.090
Xie, R.J., and A.F. Mackenzie. 1991. Effects of autoclaving on surface properties and sorption of phosphate and zinc in phosphate-treated soils. Soil Sci. 152(4):250-258. http://dx.doi.org/10.1097/00010694-199110000-00003
Yongku, Y., L.Li, and W. Dingyong. 2010. Effect of dissolved organic matter on adsorption and desorption of mercury by soils. J. Environ. Sci. 20:1097–1102.
Yusran, F.H. 2010. The Relationship between phosphate adsorption and soil organic carbon from organic matter addition. J. Trop. Soil. 15(1):1-10.
Zahedifar, M., N. Karimian and J. Yasrebi. 2010. Zinc desorption of calcareous soils as influenced by applied zinc and phosphorus and described by eight kinetic models. Comm. Soil Sci. Plant Anal. 41:897-907. http://dx.doi.org/10.1080/00103621003592408
Zhao, K., and H.M. Selim. 2010. Adsorption-desorption kinetics of Zn in soils: influence of phosphate. Soil Sci. 175:145-153. http://dx.doi.org/10.1097/SS.0b013e3181dd51a0
To share on other social networks, click on any share button. What are these?








