Effect of Sodium Chloride Stress on the Adaptation of Zea mays Seedlings at the Expense of Growth
Research Article
Effect of Sodium Chloride Stress on the Adaptation of Zea mays Seedlings at the Expense of Growth
Muhammad Junaid Yousaf 1, Farhad Ali2*and Fawad Ali2
1Abdul Wali Khan University, Mardan, Pakistan; 2Bacha Khan University, Charsadda, Pakistan.
Abstract | Zea mays cultivar; Hycorn 984, is considered a moderate salt-tolerant plant cultivar that shows varying effects and adaptations to different concentrations of salt stress (i.e. NaCl) to which it is exposed. When three days old seedlings of maize were subjected to aerial salt stress of concentrations; 0.2%, 0.6% 1.2%, and 2% after a week in soil, the seedlings revealed the antagonistic effects which increased with elevated concentrations of salt stress. The experiment was kept for 5 weeks and the chlorophyll content, relative water contents (RWC), leaf water loss (WL), and Electrolytic leakage (EL) were measured, the seedlings showed adaptation to the salt stress at the expense of growth rate as high seedling death occurred at 1.2% and 2% treated pots. However, the high growth observed in control pots had low chlorophyll content and high electrolytic leakage, similar to the plants aerially treated with 2% NaCl while plants treated with 0.2%. 0.6% and 1.2% salt concentrations had high chlorophyll content and low electrolytic leakage, receptively. Seedlings exposed to a 2% salt stress had the highest RWC and low WL, followed by 1.2% and 0.6% while 0.2% and control plants performed similarly. In conclusion, few seedlings survived under salt stress with stunted growth, however, exhibited higher stability as compared to plants grown in control conditions. From the current study results, it is recommended that Hycorn 984 be cultivated in saline field conditions due to its relative adaptability to saline conditions, which may result in economic production compared to open-pollinated maize varieties or other maize varieties released for normal field conditions.
Received | April 02, 2021; Accepted | September 30, 2021; Published | December 09, 2021
*Correspondence | Farhad Ali, Bacha Khan University, Charsadda, Pakistan; Email: [email protected]
Citation | Yousaf, M.J., F. Ali and F. Ali. 2022. Effect of sodium chloride stress on the adaptation of Zea mays seedlings at the expense of growth. Sarhad Journal of Agriculture, 38(1): 249-259.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.249.259
Keywords | Maize, Salt stress, Adaptation, Growth rate, Physiological alteration
Introduction
Salinization is the loading of salts that dissolves in water in the soil column to a level, that drastically affects crop physiology and crop yield, hence economic losses to the farmers and the nation (Shiraz et al., 2020). Soil salinity is the most important problem amongst many faced in the flooded areas because it adversely affects the profitability of crops all over the world. In some areas, this is because of low precipitation and high transpiration which unsettle the soil yield potential (Qadir et al., 2017). Moreover, the adverse effect of salinity has been exacerbated by humans activity (Iqra et al., 2020). With the persistent rise in population, particularly in the under-developed nations of the world, and the corresponding decrease in new agribusiness handles, the need for sustainable agricultural yields is intense (Kumar et al., 2021).
A high concentration of complex inorganic salts present in the soil deteriorates the nutrients the plants rely upon when compared to the optimal salt concentration (Kumar et al., 2020). High salinity causes both hyperosmotic and ionic stress, which causes modification in plant normal physiological activities including water, photosynthesis, respiration, and ion homeostasis (Arif et al., 2020). Salinity also influences the morphology, as well as alters the assimilation systems of plants (Islam et al., 2019). This degree of change relies upon cultivars, stress span, and severity. Most of the plans endure salinity stress to varying levels and survive, with lower yield with increasing salinity stress (Farooq et al., 2015). Plant breeders and Agronomists have devised different strategies to tackle salinity stress effectively (Himabindu et al., 2016).
One of the essential techniques to cope with salinity stress is to produce tolerant genotypes that may support a reasonable yield under saline soil conditions and/or treating plants with compatible solutes (Zulfiqar et al., 2021). This approach includes understanding the response of plants at various developmental phases under saline conditions (Che-Othman et al., 2020). These additionally give pieces of reproducible information to plant breeders for genetic enhancement where agronomists get higher production due to enhanced salt tolerance. Screening for salinity tolerance in the field is challenging due to spatial heterogeneity of soil physico-substance properties and regular changes in abiotic factors, especially precipitation.
Maize (Zea mays L.) is one of the essential crops in Pakistan, which fills three principle needs i.e. sustenance and corn oil for human utilization, forage for domesticated animals and poultry, and crude material for agro-based industries. The normal yield of maize in Pakistan is low when compared to other developing areas of the world. Nonetheless, major factors contributing to low yield domestically are not just linked to cultivars available to the farmers but also insufficient irrigation due to water scarcity, imbalanced macro and micronutrients, and abiotic stresses (Shahid et al., 2020). The stresses to which maize crop is exposed in Pakistan are for the most part calcareous in nature. Salt tolerant plants embrace numerous techniques that range from morpho-anatomical to physiological and biochemical. Tolerant plants modify osmotically by the combination of extreme water dissolvable good osmotica (e.g. glycine betaine, free proline, and low atomic weight sugars) and control turgidity (Ait-El-Mokhtar et al., 2020). Among these, free proline improves salt-actuated oxidative harm to plants, and both reducing and non-reducing sugars add to turgor support under salt or water stress. Amongst the mineral supplements, K assumes a specific part for the translocation of nitrates to the root and shoot, expanded fast N-digestion, and support for water potential in adding to the plant survival under ecological stress conditions (Huang et al., 2017). Realizing the significance of maize crop, improving the nourishing significance of potassium, it is required to develop saline land with little farm utilization and to elucidate levels of potassium application on biochemical qualities (oil contents, dissolvable sugars, protein, proline, and rough fiber) under salinity stress.
After wheat and rice, maize is the third most significant field crop and is cultivated throughout the world under varied natural conditions. Maize is highly polymorphic with high genetic diversity and being open-pollinated, salinity resistance may exist in maize (Deinlein et al., 2014) and screened material can be used as cultivars in salinity-hit regions. We analyzed the response of the maize seedlings to the salinity stress as several maize varieties show a degree of adaptation to salt stress.
Materials and Methods
Plant material
The experiment was conducted in Abdul Wali Khan University Mardan on 7, 14, 21, 28, and 35-days old seedlings of Zea mays L. (Hycorn 984) for which the seeds were collected from the Agriculture Department (Mardan Extension), Khyber Pakhtunkhwa, Pakistan.
Preparing seed for sowing
Intact seeds, of similar size and color, free from wrinkles, were first soaked in tap water for enough imbibition for 2 hours then sterilized in 70% ethanol solution, and finally rinsed 4 times with sterilized distilled water. Ten seeds were sown in a sterilized Petri plate containing distilled water.
After 4 days, three same size seedlings were shifted to moderately wet soil pots. Initially, pots were kept in a close box for two days to keep enough moisture for the developing seedlings. After two days, the pots were shifted to the sunlight which turned the leaves coated in coleoptile into green. Seedlings were irrigated with distilled water on the 6th and 7th day of germination in each pot. Out of 50 pots, 10 pots were kept aside from the treatment in the next phase. The seedlings were grown in midsummers with a temperature of on average 29°C of the day and of an average 16°C of the night while the relative humidity was 52 to 67%.
Treatment
On the 8th day, 40 pots were sprayed with NaCl solution in a concentration of 0.2%. On the 9th day, 30 out of 40 pots were treated with 0.4% while the remaining 10 pots were kept at 0.2%. Similarly, the next day, 20 pots were treated with 0.6% (i.e. 0.2%+0.4%+0.6%) while the remaining 20 pots were kept on 0.2% and 0.6% (i.e. 0.2%+0.4%) of NaCl; 10 pots each while, 10 pots were sprayed with 0.8% (i.e. 0.2%+0.4%+0.6%+0.8%) concentration while the remaining were kept on their old concentration.
Growth measurements
To analyze the adaptation of maize seedlings to the applied salinity stress, seedlings were allowed to grow for 7 days to record the response of seedlings to salt stress. At every weekend up to 4 weeks, 10 pots with two replicate pots of the salt concentration 0.2%, 0.6%. 1.2%, 2%, and control were used for data collection. To collect the seedlings without causing any harm, the soil in the pots was first dissolved in water. The calculated data contained the mean, sum, and standard deviation of the following;
- Shoot length
- Root length
- Fresh weight
- Dry weight
- Leaf area
On the 35th day, a chemical analysis of the remaining 10 pots was carried out. The chemical analysis included:
Electrolytic leakage: Electrolytic leakage was measured as earlier described by Campos et al. (2003). Leaf (2 gm) from every plant of every pot was washed with deionized water and then placed in 15 ml of falcon tube containing 15 ml of deionized water. The samples were incubated for 2 hours at 25 °C. The electrolytic conductivity was determined for the samples as L1. Samples were then autoclaved at 120 oC for 20 minutes and L2 was measured after equilibrium was established at 25 °C. The final read was obtained using the formula:
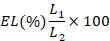
Relative water content: It was measured by the method of Mata and Lamattina (2001). Fresh leaves (2 gm) from every concentration were taken and allowed to rehydrate for two hours in water. The turgor weight was measured, and the leaf sample was pre-heated in the oven for 2 days at 35°C. The final reading was obtained using the formula:
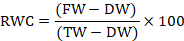
Where;
FW = leaf fresh weight, DW = leaf dry weight & TW = leaf turgid weight
Leaf water loss: Fresh leaf (2 gm) sample for each concentration was taken and kept for 2 hours at 30 °C. After 2 hours the samples were weighed again, and the final data were analyzed using the formula; W1 is the initial weight taken before treatment and W2 is the final weight after treatment:
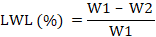
Chlorophyll content: Chlorophyll content was measured for every plant leaf at different salinity stress levels for Chlorophyll on the LI-600 - Porometer/Fluorometer (Meteo-Tech, Israel), and the data were noted.
Statistical analyses
Variation in the data was measured using Duncan’s test and ANOVA through SPSS software for windows 16.0 (SPSS Inc., Chicago, IL, United States), and tables based on the mean and standard deviation of noted data on excel were drawn to analyze the difference in growth measurements.
Results and Discussion
Our current study focuses on measuring the effect of salinity on the growth and development of maize variety; Hycorn 984. From the first week of the experiment, the seedlings showed adaptation to varying concentrations of NaCl, i.e. 0.2%, 0.6%, 1.2%, and 2%. There was a high growth rate in control plants while there was a very low growth rate at 2% salinity stress (Figure 1 and 3) where many seedlings could not survive. Regarding the shoot length in contrast to the root length, shoot length was widely affected by the increasing stress of NaCl, where root length was high at a moderate concentration of the salt stress. This concluded that aerial application of the salt had triggered the growth of the root. Plants driving for water due to salinity stress have an elongated and dense root system (Rahnama et al., 2019). Therefore, we can deduce that at a moderate concentration of salt, the maize plant enhanced its root system at the expense of the shoot system (Ma et al., 2021). In most plant species grown under salinity, Na+ appears to reach a toxic concentration before Cl− does, hence most studies have concentrated on Na+ exclusion and the control of Na+ transport within the plant. Therefore, another essential mechanism of tolerance involves the ability to reduce the ionic stress to which the plant is exposed by minimizing the amount of Na+ that accumulates in the cytosol of cells, particularly those in the transpiring leaves. This process, as well as tissue tolerance, involves up and down-regulation of the expression of specific ion channels and transporters, allowing the control of Na+ transport throughout the plant.
The Na+ exclusion from leaves is associated with salt tolerance in cereal crops including rice, durum wheat, bread wheat, and barley. Exclusion of Na+ from the leaves is due to low net Na+ uptake by cells in the root cortex and the tight control of net loading of xylem by parenchyma cells in the stele, Na+ exclusion by roots ensures that Na+ does not accumulate up to toxic concentrations within the leaf blades. A failure in Na+ exclusion manifests its toxic effect after days or weeks, depending on the species, and causes premature death of older leaves. This is also referred to as the hormonal interaction of the plant under salt stress because the root and shoot system are highly co-related with hormonal signaling in the plant. The data showed similar results, however, interestingly the chlorophyll content increased with the increasing salt concentration as the seedlings adapted to the salt stress. Similar results were reported by (Bogoutdinova et al., 2020), meanwhile, there is an opposite aspect obtained from the experiment that few surviving seedlings of maize had low electrolytic leakage and high-water content whereas salinity promotes the electrolytic leakage as concluded by Abdelaal et al., (2020). This low electrolyte leakage and retention of high water contents may be an adaptive edge of the maize cultivar (Hycorn 984) to the salinity stress that has induced survival instincts of the seedlings at the expense of vegetative growth.
Taking into consideration root and shoot length in response to the applied concentration of NaCl stress, the average shoot length appeared to be high in control as compared to treated plants (Table 2 and 5), but the root growth appeared to be high at 1.2% and 0.6% salinity stress, however, it was comparatively low in control. The relative water content also appeared like the chlorophyll content in the seedlings. Salinity stress besides ionic imbalance also induces drought stress. To cope with this latent drought stress introduced by salinity, the genetically capable plant adapts to this situation through root proliferation; an adaptive edge of salt-tolerant cultivars. This high proliferation of plant roots in response to salinity stress helps the plant to cope with the stress albeit low vegetative growth and ultimately reproductive growth i.e. yield, nonetheless surviving another day for the survival of the species.
The water content increased with the increasing accumulation of Na ion concentration in maize seedlings which decreases water potential (Voronin et al., 2021), therefore, more water is supplied to the leaves. At the same time, it decreased the dry weight difference when compared to the control grown plant. Our studies suggest that at higher salinity stress lower will be the plant dry weight as the priority of the plant is now shifted to retain the water contents to survive the stress. In high salinity, there is a problem of assimilation of nutrients in plants and low flow in the phloem. Moreover, maize-controlled grown plants also show high water loss from the leaves when kept for two hours at room temperature as compared to treated plants. This indicated that the formation of compatible solutes occurs, such as glycine betaine and proline betaine which incorporated water molecules with themselves (Stasiulewicz et al., 2021). In summary, it is concluded that maize growth is highly affected by the level of salinity stress to which it is exposed, whereby a plant is compelled to utilize/waste its energy in the production of secondary metabolites to cope with the stress rather than utilize its energy on growth and development for higher yield.
Zea mays (Hycorn 984) treated with NaCl in concentrations of 0.2%, 0.6%, 1.2% and 2% after
Table 1: Hycorn 984 plant adaptability to salt stress.
|
Parameters |
Control |
0.2% |
0.6% |
1.2% |
2% |
|
Chlorophyll Content |
14.6d± 0.3 |
15.9d± 0.6 |
20c± 0.4 |
25.6b± 1.2 |
12a± 0.1 |
|
Relative Water Content |
0.026a± 0.01 |
0.04b± 0.01 |
0.05c± 0.02 |
0.08d± 0.05 |
0.1e± 0.03 |
|
Total Water Loss |
0a± 0 |
0.16b± 0.09 |
0.19b±0.03 |
0.2c± 0.01 |
0.25d± 0.1 |
|
Electrolytic Leakage |
78%a ± 3 |
71%d ± 8 |
66%c ± 4 |
61%e ± 7 |
82%b ± 4 |
7. 14, 18, 28, 35 day showed varying degree of adaptability to the stress (Table 1). the data were obtained from the analyses of these plants on the 36th day of germination. Data shown are the mean and standard deviation of the triplicate taken for the experiment whereas the means were compared on SPSS software to analyze the differences, expressed in alphabetic letters assigned to each mean. The chlorophyll content was high in 1.2% salt-treated plants as compared to control-grown plants. The relative water content was high with increasing salt concentration. Electrolytic leakage was high in control plants as compared to 1.2%, indicating that salt stress did not harm the cell shape whereas, in 2%, the electrolytic leakage was high amongst all.
The Figure 1 shows the pots with varying concentration of NaCl (0.2% 0.6%, 1.2%, 2%) in distilled water in which a) is for control and b), c), d), e), are for 0.2% 0.6%, 1.2% and 2% NaCl concentration, respectively. Plants in pots show the response to salt treatment which was given a week ago. The salt stress has not only reduced the growth rate but also the number of plants in the pot.
Figure 2 represents a reduction in growth rate and the number of plants per pot with increasing concentration. The figure is taken after 3 weeks of the treatment of salinity stress in concentrations of 0.2%, 0.6%, 1.2%, 2%, respectively.
Figure 3 shows the comparison of the shoot growth rate of the plant exposed to stress with a solution of NaCl having a concentration of 0.2%, 0.6%, 1.2%, and 2%, respectively with the control grown plant. The result clearly shows that with increasing salt, there is a reduction in the growth rate of the shoot. The figure also shows the number of plants surviving in the pot as there are 3/3 plants in control and are of equal length which means that under normal conditions, all plants respond normally while under 0.2% salinity stress, one plant died while the other two plants were alive and equal in length and leaf number. The rest showed irregular growth among their replicates as in 1.2% and 2% concentration of NaCl, only 1:1 plants are left while the other died due to high salinity. Under 0.6% salinity stress, treated plants appeared in transition as the two plants are different in lengths and leaf numbers. Therefore, it is evident that salt affect plant variedly even among the same genotype, where the potent plant among the same germplasm survives while others may not.
Figure 4 shows the comparison of the root growth rate of the plant stressed with the solution of NaCl having a concentration of 0.2%, 0.6%, 1.2%, and 2%
Table 2: Hycorn 984 plant response to 7 days salt stress.
|
Solution Concentration |
Root Length |
Shoot Length |
Fresh Weight (Root) |
Dry Weight (Root) |
Fresh Weight (Stem) |
Dry Weight (Stem) |
Fresh Weight (Leaf) |
Dry Weight (Leaf) |
Leaf area (cm3) |
|
Control (dil.H20) |
15e± 0.6 |
12d± 1.1 |
2.5d± 0.1 |
1.1d± 0.1 |
3.5e± 0.1 |
2a± 0.2 |
3.3d± 0.1 |
1.7c± 0.2 |
7.9a± 0.8 |
|
0.2% |
12d± 1 |
9c± 1 |
2c± 0.2 |
0.8d± 0.1 |
2.8d± 0.1 |
1a± 0.2 |
2.6d± 0.1 |
1.0c± 0.2 |
5.6ab± 0.1 |
|
0.6% |
7c± 0.2 |
7b± 0.2 |
1b± 0.1 |
0.3c± 0.1 |
2.0c± 0.2 |
0.6b± 0.1 |
1.8c± 0.2 |
0.1c± 0.1 |
3.5b± 0.6 |
|
1.2% |
4b± 0 |
5ab± 0.9 |
0.7a± 0.1 |
0.05b± 0.01 |
1.1b± 0.1 |
0.1c± 0 |
0.9b± 0.1 |
0.01b± 0.01 |
2.2c± 0.1 |
|
2% |
2a± 0.1 |
3a± 1 |
0.3a± 0.1 |
0.01a± 0 |
0.7a± 0.2 |
0.03d± 0 |
0.5a± 0.2 |
0.01a± 0.01 |
1d± 0.6 |
|
LSD0.05 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
(P<) |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
with the control grown plant. The root health is optimal in 0.6% and 1.2% as compared to 2% and 0.2% treated plant roots. 0.2% treated plant has a necrotic root with poor health which means that maize seedlings on this particular concentration cannot endure salinity for root health. However, the root growth is low in all stress conditions. Interestingly the results of the current study reveal an interesting trend among salinity-stressed plants, where the plants exposed to mild stress of 0.2% and high stress of 2% performed poorly when compared to the medium stressed plants exposed to salinity stress of 0.6% and 1.2%, respectively. Poor root growth at mild salinity stress of 0.2% may be due to the reason that the stress was incapable of generating survival response of the plant as evident from reasonable growth of the plant (Figure 1 and 3). However, at high physiological stress of 2%, the plant seems to be overwhelmed by the stress resulting in both poor vegetative as well as root growth nonetheless still surviving the adverse growth conditions due to adaptability of the plant to salinity stress (Figure 1 and 4).
To evaluate the root and shoot length of the treated maize plants mean and standard deviation of the data of seedlings of Zea mays (Hycorn 984) treated with NaCl solution in concentrations of 0.2%, 0.6%, 1.2%, and 2% were compared with SPSS to observe differences (Table 2). The 3 days old seedlings of the same size were sown in soil and the plants were treated aerially with the concentrations of salt in an amount of 0.5 mL intermittently for 7 days and on the 8th day, the harvest was taken. The root length and shoot length were observed to have reduced at varying levels of the salt concentration. Similarly, the fresh and dry weight of the root, shoot, and leaf were also low at high concentrations of salt. However, the leaf area was relatively closer at higher concentrations as in 1.2% and 2%.
To observe the root and shoot length of the maize plants exposed to different concentrations of intermittently applied aerial salinity stress in an amount of 0.5 mL for 14 days and on the 15th day, the harvest was taken (Table 3). The root length and shoot length were observed to have decreased at varying levels of the salt concentration. Similarly, the fresh weight and dry weight of the root, shoot, and leaf area of the maize seedlings were also low at high salt concentrations.
The root length and shoot length of the plants were measured which were treated aerially with different concentrations of salt in the amount of 0.5 mL intermittently for the 21st day, and on the 22nd day, the harvest was taken (Table 4). The root length and shoot length were observed to show a decreasing trend at varying levels of the salt concentration. Similarly, the fresh weight and dry weight of the root, shoot, and leaf area of the maize seedlings were also low at a high concentration of salt.
When 0.5 mL of different concentrations of salt stress was applied intermittently for 28th days, and on the 29th day, the harvest was taken to evaluate the fresh and dry weight of the root, shoot, and leaf area as well
Table 3: Hycorn 984 plant response to 14 days salt stress.
|
Solution Concentration |
Root Length |
Shoot Length |
Fresh Weight (Root) |
Dry Weight (Root) |
Fresh Weight (Stem) |
Dry Weight (Stem) |
Fresh Weight (Leaf) |
Dry Weight (Leaf) |
Leaf area (cm3) |
|
Control (dil.H20) |
17c± 0.7 |
16d± 0.2 |
4.4c± 0.2 |
2.3d± 0.1 |
5a± 0.1 |
3a± 1.5 |
4.9d± 0.1 |
2.7e± 0.1 |
10d± 0.1 |
|
0.2% |
12c± 1 |
13d± 0.5 |
3b± 0.1 |
1.7c± 0.1 |
4b± 0.2 |
2b± 1.6 |
4.2c± 0.2 |
1.9d± 0.1 |
7d± 0.6 |
|
0.6% |
10b± 2 |
10c± 2 |
2b± 0.1 |
0.5b± 0.1 |
3.6c± 0.1 |
1.7c± 1 |
3.4b± 0.1 |
1.5c± 0.1 |
3c± 0.6 |
|
1.2% |
4b± 0.1 |
5b± 0.4 |
1.8a± 0.1 |
0.2a± 0.007 |
2.5d± 0.2 |
1d± 1 |
2.3a± 0.2 |
0.9b± 0.07 |
1.9b± 0.8 |
|
2% |
3a± 0.4 |
3.2a± 1 |
1a± 0.1 |
0.1a± 0.007 |
1.7e± 0.2 |
0.1e± 1.1 |
1.5a± 0.2 |
0.07a± 0.01 |
1.2a± 0.2 |
|
LSD0.05 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
(P<) |
0.001 |
0.01 |
0.001 |
0.001 |
0.001 |
0.001 |
0.0001 |
0.0001 |
0.001 |
Table 4: Hycorn 984 plant response to 21 days salt stress.
|
Solution Concentration |
Root Length |
Shoot Length |
Fresh Weight (Root) |
Dry Weight (Root) |
Fresh Weight (Stem) |
Dry Weight (Stem) |
Fresh Weight (Leaf) |
Dry Weight (Leaf) |
Leaf area (cm3) |
|
Control (dil.H20) |
27d± 1 |
25d± 0.6 |
5d± 0.2 |
3.5e± 0.1 |
6d± 0.4 |
3e±2 |
6.2a± 0.4 |
3.1e± 0.2 |
15e± 0.1 |
|
0.2% |
10d± 1 |
22d± 0.2 |
4c± 0.2 |
2d± 0.4 |
5c± 0.2 |
2d±2 |
5.1ab± 0.2 |
2.2d± 0.3 |
11d± 1 |
|
0.6% |
11c± 0.9 |
10c± 0.9 |
3.8c± 0.1 |
1.7c± 0.1 |
4c± 0.2 |
1c±2 |
4b± 0.2 |
1.5c± 0.1 |
7.2c± 0.6 |
|
1.2% |
9b± 1 |
6b± 1.3 |
3b± 0 |
0.9b± 0.1 |
3.6b± 0.2 |
0.6b±2 |
3.5bc± 0.2 |
0.63b± 0.07 |
6.8b± 0.3 |
|
2% |
5a± 0.3 |
5.1a± 0 |
1a± 0.5 |
0.08a± 0.02 |
2a± 0.8 |
0.08a±1.8 |
2.4c± 0.8 |
0.06a± 0.01 |
3.2a± 1.3 |
|
LSD0.05 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
(P<) |
0.001 |
0.01 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
0.001 |
Table 5: Hycorn 984 plant response to 28 days salt stress.
|
Solution Concentration |
Root Length |
Shoot Length |
Fresh Weight (Root) |
Dry Weight (Root) |
Fresh Weight (Stem) |
Dry Weight (Stem) |
Fresh Weight (Leaf) |
Dry Weight (Leaf) |
Leaf area (cm3) |
|
Control (dil.H20) |
28d± 0.5 |
32d± 2 |
6.5d± 0.1 |
3.1e± 0.1 |
7.9d± 0.5 |
4.3e± 0.4 |
6.8a± 0.6 |
4.2a± 0.1 |
20d± 0.2 |
|
0.2% |
13d± 2.3 |
24b± 1 |
5.5c± 0.1 |
2.5d± 0.4 |
6.3c± 0.2 |
3.5d± 0.2 |
5.5b± 0.1 |
3b± 0.1 |
16c± 0.6 |
|
0.6% |
16c± 0.8 |
14b± 0.4 |
4.3b± 0.1 |
1.2c± 0.1 |
5.7c± 0.4 |
2.3c± 0.4 |
4.6bc± 0.7 |
2.1c± 0.4 |
10c± 0.7 |
|
1.2% |
15b± 4 |
11c± 0.2 |
3.2b± 0.1 |
0.9b± 0.07 |
4.2b± 0.2 |
1.1b± 0.2 |
4.1c± 0.2 |
0.9d± 0.5 |
4b± 0.1 |
|
2% |
5a± 0.3 |
6a± 0.6 |
2.3a± 0.1 |
0.2a± 0.1 |
3.6a± 0.4 |
0.1a± 0 |
3.5cd± 0.4 |
0.08e± 0.01 |
2.6a± 0.7 |
|
LSD0.05 |
1.00 |
1.00 |
1.00± |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
(P<) |
0.001 |
0.01 |
0.001 |
0.001 |
0.001 |
0.001 |
0.12 |
0.001 |
0.001 |
as root and shoot length (Table 5), the root length and shoot length were observed to have decreased at varying levels of the salt concentration. Similarly, the fresh and dry weight of the root, shoot, and leaf area of the maize seedlings was also low at a high concentration of salt.
Mean data and standard deviation of the 7 days seedlings of Zea mays (Hycorn 984) were compared through SPSS software to analyze for differences (Table 6) in root and stem weight. The data so analyzed, deduced that the root and stem weight ratio and root stem ratio decreased at a high concentration of sodium chloride stress. Similarly, the specific leaf area of the same seedlings was also low at a high concentration of salt. Contrary to this, the relative growth ratio and net assimilation rate of the seedlings were increased at a high concentration of salt stress which can be attributed to the adaptability of Hycorn 984 to salinity stress.
The data analyzed indicates that the root and stem weight ratio and root stem ratio decreased at a high concentration of sodium salt stress (Table 7). Similarly, the specific leaf area of the seedlings was observed to be low at a high concentration of salt. Contrary to this, the relative growth ratio and net assimilation rate of the seedlings, increased at high concentrations of salt stress. The root and stem weight ratio and root stem ratio decreased at a high concentration of sodium
Table 6: Hycorn 984 plant growth analysis of 7 days salt stress.
|
Parameters |
Control |
0.2% |
0.6% |
1.2% |
2% |
|
Root Weight Ratio |
0.46a± 0.06 |
0.43ab± 0.02 |
0.40ab± 0.07 |
0.36b± 0.02 |
0.31c± 0.09 |
|
Stem Weight Ratio |
0.79a± 0.29 |
0.57b± 0.02 |
0.60c± 0.07 |
0.64c± 0.02 |
0.69d± 0.09 |
|
Root Shoot Ratio |
0.60a± 0.15 |
0.75b± 0.07 |
0.67bc± 0.18 |
0.57c± 0.04 |
0.47b± 0.19 |
|
Specific Leaf Area |
7.24a± 1.6 |
6.54b± 0.6 |
6.94b± 0.09 |
7.34c± 0.6 |
7.65c± 0.2 |
|
Relative Growth Ratio |
0.21a± 0 |
0.20b± 0.1 |
0.19c± 0 |
0.18d± 0.2 |
0.16d± 0 |
|
Net Assimilation Rate |
27.52a± 4.5 |
18.50b± 8 |
12.69c± 0.01 |
8.68d± 5.6 |
3.73e± 1.3 |
Table 7: Hycorn 984 plant growth analysis of 14 days salt stress.
|
Parameters |
Control |
0.2% |
0.6% |
1.2% |
2% |
|
Root Weight Ratio |
0.44a± 0 |
0.46a± 0.02 |
0.45ab± 0.03 |
0.32c± 0.15 |
0.21b± 0.01 |
|
Stem Weight Ratio |
0.56a± 0 |
0.54a± 0.02 |
0.55b± 0.03 |
0.68b± 0.15 |
0.79a± 0.01 |
|
Root Shoot Ratio |
0.79a± 0.1 |
0.85a± 0.07 |
0.83b± 0.1 |
0.52c± 0.36 |
0.26c± 0.2 |
|
Specific Leaf Area |
4.25a± 1.8 |
4.25ab± 1.8 |
4.48b± 1.5 |
3.51ab± 1.53 |
2.96c± 0.76 |
|
Relative Growth Ratio |
0.02a± 0 |
0.02b± 0 |
0.01c± 0.01 |
0.02d± 0.02 |
0.02d± 0.01 |
|
Net Assimilation Rate |
27.28a± 0.13 |
19.41b± 11.26 |
11.81c± 0.51 |
7.57d± 6.52 |
3.14e± 0.30 |
Table 8: Hycorn 984 plant growth analysis of 21 days salt stress.
|
Parameters |
Control |
0.2% |
0.6% |
1.2% |
2% |
|
Root Weight Ratio |
0.55d± 0.02 |
0.54c± 0.04 |
0.50ab± 0 |
0.49b± 0.06 |
0.51a± 0.11 |
|
Stem Weight Ratio |
0.45c± 0.02 |
0.46bc± 0.04 |
0.50bc± 0 |
0.51b± 0.06 |
0.49a± 0.11 |
|
Root Shoot Ratio |
1.21a± 0.1 |
1.20ab± 0.07 |
1ab± 0.1 |
0.96b± 0.36 |
1.1b± 0.2 |
|
Specific Leaf Area |
5.35d± 0.23 |
5.09c± 0.13 |
4.81cd± 0.88 |
10.89b± 1.67 |
51.29a± 10.3 |
|
Relative Growth Ratio |
0.025a± 0 |
0.032ab± 0 |
0.003b± 0.01 |
0.017c± 0.02 |
0.039d± 0.01 |
|
Net Assimilation Rate |
67.5d± 0.2 |
33.7cd± 3.9 |
9.3c± 1.2 |
4.9b± 0.3 |
1.2a± 1 |
Table 9: Hycorn 984 plant growth analysis of 28 days salt stress.
|
Parameters |
Control |
0.2% |
0.6% |
1.2% |
2% |
|
Root Weight Ratio |
0.42d± 0.03 |
0.42c± 0.05 |
0.36b± 0.02 |
0.43a± 0.02 |
0.59a± 0.13 |
|
Stem Weight Ratio |
0.58a± 0.03 |
0.58ab± 0.05 |
0.64b± 0.02 |
0.57c± 0.02 |
0.41c± 0.13 |
|
Root Shoot Ratio |
0.73d± 0.1 |
0.74a± 0.1 |
0.56b± 0.1 |
0.75c± 0.1 |
1.61bc± 0.9 |
|
Specific Leaf Area |
3.65d± 0.1 |
3.58ab± 0.5 |
3.75b± 0.9 |
9.58c± 6.3 |
38.49a± 10 |
|
Relative Growth Ratio |
0.032c± 0 |
0.023c± 0 |
0.026b± 0 |
0.063a± 0 |
0.046a± 0.01 |
|
Net Assimilation Rate |
75.6e± 2.5 |
29.3d± 4.5 |
13.5c± 1.5 |
10.5b± 0.7 |
1.3a± 0.9 |
chloride salt (Table 8). Similarly, the specific leaf area of the same seedlings was also low at a high concentration of salt. Contrary to this, the relative growth ratio and net assimilation rate of the seedlings were increased at a high concentration of salt stress.
The data analyzed deduced that the root and stem weight ratio and root stem ratio was decreased at a high concentration of sodium chloride salt (Table 9). Similarly, the specific leaf area of the same seedlings was also low at a high concentration of salt. Contrary to this, the relative growth ratio and net assimilation rate of the seedlings were increased at a high concentration of salt stress.
Conclusions and Recommendations
Maize plant undergoes modifications to adapt to the applied salt stress by limiting the overall growth of root and shoot, however, increases the production of chlorophyll and relative water content in the leaf. These enable the plant to enhance its net assimilation and water content in the stressed cell. Moreover, the stressed plant also focuses on the root system because the salt application lowers the water potential in the targeted cells such as in this experiment the leaf cell, therefore the developed root system tries to cope with the stress. The striking fact which is to be researched is the specific leaf area as the specific area is smaller in controlled treated while it was larger in stressed maize seedlings. This may conclude that cells due to salt stress were highly affected as compared to unstressed cells thus it may be referred to as adaptation of the Hycorn 984 stressed cells to salinity (Simulation 1).
Based on the findings of the current study it is recommended to use Hycorn 984 maize cultivar in moderately saline soil due to its adaptive advantage. However, maximizing crop yield may require field management interventions focussed on enhancing the soil physiology of the saline soil. These field management interventions coupled with the use of salt-tolerant cultivars may be a good combination to get economic yields under such challenging field conditions.
Novelty Statement
In the current study, the growth and adaptability of maize plant was ana-lyzed under salt stress. The study revealed an interesting factor that the plant growth rate was compromised to make the plant well-adapted to the stress i.e., survival of the plant at the expense of growth.
Author’s Contribution
Muhammad Junaid Yousaf: Conducted the research and drafted the initial manuscript.
Farhad Ali: Critically reviewed and edited the manuscript.
Fawad Ali: Performed statistical analysis.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdelaal, K.A., Mazrou, Y.S. and Hafez, Y.M. 2020. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants, 9(6): 733. https://doi.org/10.3390/plants9060733
Ait-El-Mokhtar, M., Baslam, M., Ben-Laouane, R., Anli, M., Boutasknit, A., Mitsui, T., Wahbi, S. and Meddich, A. 2020. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst., 4: 131. https://doi.org/10.3389/fsufs.2020.00131
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A. and Hayat, S. 2020. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem., 156: 64-77. https://doi.org/10.1016/j.plaphy.2020.08.042
Bogoutdinova, L.R., Lazareva, E.M., Chaban, I.A., Kononenko, N.V., Dilovarova, T., Khaliluev, M.R., Kurenina, L.V., Gulevich, A.A., Smirnova, E.A. and Baranova, E.N. 2020. Salt stress-induced structural changes are mitigated in transgenic tomato plants over-expressing superoxide dismutase. Biology, 9(9): 297. https://doi.org/10.3390/biology9090297
Campos, P.S., nia Quartin, V., chicho Ramalho, J. and Nunes, M.A. 2003. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. plant physiol., 160(3): 283-292. https://doi.org/10.1078/0176-1617-00833
Che-Othman, M.H., Jacoby, R.P., Millar, A.H. and Taylor, N.L. 2020. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol., 225(3): 1166-1180. https://doi.org/10.1111/nph.15713
Deinlein, U., Stephan, A.B., Horie, T., Luo, W., Xu, G. and Schroeder, J.I. 2014. Plant salt-tolerance mechanisms. Trends Plant Sci., 19(6): 371-379. https://doi.org/10.1016/j.tplants.2014.02.001
Farooq, M., Hussain, M., Wakeel, A. and Siddique, K.H. 2015. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev., 35(2): 461-481. https://doi.org/10.1007/s13593-015-0287-0
Himabindu, Y., Chakradhar, T., Reddy, M.C., Kanygin, A., Redding, K.E. and Chandrasekhar, T. 2016. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot., 124: 39-63. https://doi.org/10.1016/j.envexpbot.2015.11.010
Huang, P., de-Bashan, L., Crocker, T., Kloepper, J.W. and Bashan, Y. 2017. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo) bacteria on growth promotion of crop plants. Biol. Fertil. Soil, 53(2): 199-208. https://doi.org/10.1007/s00374-016-1160-2
Huda, K.M.K., Banu, M.S.A., Yadav, S., Sahoo, R.K., Tuteja, R. and Tuteja, N. 2014. Salinity and drought tolerant OsACA6 enhances cold tolerance in transgenic tobacco by interacting with stress-inducible proteins. Plant Physiol. Biochem., 82: 229-238. https://doi.org/10.1016/j.plaphy.2014.06.007
Iqra, L., Rashid, M.S., Ali, Q., Latif, I. and Mailk, A. 2020. Evaluation for Na+/K+ ratio under salt stress condition in wheat. Life Sci. J., 17(7): 43-47. https://doi.org/10.54112/bcsrj.v2020i1.16
Islam, F., Wang, J., Farooq, M.A., Yang, C., Jan, M., Mwamba, T.M., Hannan, F., Xu, L. and Zhou, W. 2019. Rice responses and tolerance to salt stress: deciphering the physiological and molecular mechanisms of salinity adaptation. In Advances in Rice Research for Abiotic Stress Tolerance (pp. 791-819). Woodhead Publishing. https://doi.org/10.1016/B978-0-12-814332-2.00040-X
Kumar, S., Li, G., Yang, J., Huang, X., Ji, Q., Liu, Z., Ke, W. and Hou, H. 2021. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci., 12. https://doi.org/10.3389/fpls.2021.660409
Kumar, S., Li, G., Yang, J., Huang, X., Ji, Q., Zhou, K., Khan, S., Ke, W. and Hou, H. 2020. Investigation of an antioxidative system for salinity tolerance in Oenanthe javanica. Antioxidants, 9(10): 940. https://doi.org/10.3390/antiox9100940
Ma, Y., Wei, Z., Liu, J., Liu, X. and Liu, F. 2021. Growth and physiological responses of cotton plants to salt stress. J. Agron. Crop Sci., 207(3): 565-576. https://doi.org/10.1111/jac.12484
Mata, G.C. and L. Lamattina. 2001. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol., 126: 1196–1204. https://doi.org/10.1104/pp.126.3.1196
Qadir, A., Khan, S.A., Ahmad, R., Masood, S., Irshad, M., Kaleem, F., Kumar, S. and Shahzad, M. 2017. Exogenous Ca2SiO4 enrichment reduces the leaf apoplastic Na+ and increases the growth of okra (Abelmoschus esculentus L.) under salt stress. Sci. Hortic., 214: 1-8. https://doi.org/10.1016/j.scienta.2016.11.008
Rahnama, A., Fakhri, S. and Meskarbashee, M. 2019. Root growth and architecture responses of bread wheat cultivars to salinity stress. Agron. J., 111(6): 2991-2998. https://doi.org/10.2134/agronj2018.12.0795
Shahid, M.A., Sarkhosh, A., Khan, N., Balal, R.M., Ali, S., Rossi, L., Gómez, C., Mattson, N., Nasim, W. and Garcia-Sanchez, F. 2020. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy, 10(7): 938. https://doi.org/10.3390/agronomy10070938
Shiraz, M., Sami, F., Siddiqui, H., Yusuf, M. and Hayat, S. 2020. Interaction of auxin and nitric oxide improved photosynthetic efficiency and antioxidant system of Brassica juncea plants under salt stress. J. Plant Growth Regul., pp. 1-11. https://doi.org/10.1007/s00344-020-10268-0
Shrivastava, P. and Kumar, R. 2015. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Boil. Sci., 22(2): 123-131. https://doi.org/10.1016/j.sjbs.2014.12.001
Slama, I., Ben Rejeb, K., Rouached, A., Jdey, A., Rabhi, M., Talbi, O., Debez, A., Savouré, A. and Abdelly, C. 2014. Presence of proline in salinized nutrient solution re-enforces the role of this amino acid in osmoregulation and protects lipid membrane peroxidation in Arabidopsis thaliana. Aust. J. Crop Sci., 8(10): 1367.
Stasiulewicz, M., Panuszko, A., Śmiechowski, M., Bruździak, P., Maszota, P. and Stangret, J. 2021. Effect of urea and glycine betaine on the hydration sphere of model molecules for the surface features of proteins. J. Mol. Liq., 324:115090. https://doi.org/10.1016/j.molliq.2020.115090
Urano, K., Maruyama, K., Jikumaru, Y., Kamiya, Y., Yamaguchi-Shinozaki, K. and Shinozaki, K. 2017. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J., 90(1): 17-36. https://doi.org/10.1111/tpj.13460
Vaid, N., Pandey, P., Srivastava, V.K. and Tuteja, N. 2015. Pea lectin receptor-like kinase functions in salinity adaptation without yield penalty, by alleviating osmotic and ionic stresses and upregulating stress-responsive genes. Plant Mol. Biol., 88(1-2): 193-206. https://doi.org/10.1007/s11103-015-0319-9
Voronin, P.Y., Myasoedov, N.A., Khalilova, L.A. and Balnokin, Y.V. 2021. Water Potential of the Apoplast in Substomatal Cavity of the Suaeda altissima (L.) Pall. Leaf under Salt Stress. Russ. J. Plant Physiol., 68(3): 519-525. https://doi.org/10.1134/S1021443721030171
Zulfiqar, F. and Ashraf, M. 2021. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. https://doi.org/10.1016/j.plaphy.2021.01.028
To share on other social networks, click on any share button. What are these?







