Effect of Salt Stress on the Germination and Early Seedling Growth in Okra (Abelmoschus esculentus)
Research Article
Effect of Salt Stress on the Germination and Early Seedling Growth in Okra (Abelmoschus esculentus)
Shehzadi Saima1*, Faiza Ghaffar1, Ghulam Yasin1, Muhammad Nawaz2 and Khalid Masood Ahmad1
1Institue of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan; 2Department of Environmental Science, Bahauddin Zakariya University, Multan, Pakistan.
Abstract | Salinization has severe affects on agriculture worldwide. For successful survival and better yield, ability of seeds germinations under high salt concentration is very vital. With this object we observed the effects of various levels of NaCl (0, 25, 50 and 75 mM) on six varieties (Green leaf, Rama posa, Arka anamika, Super green, Okra kashish and Nerali) of Okra. The results clearly indicated that seeds of all varieties can tolerate the lower concentration of salt (25 mM) and higher (50 mM) greatly reduced the seeds germination while at highest concentration (75 mM) no germination was recorded. Salt treatments also reduced the dry and fresh weight of roots and shoots in all varieties considerably (P<0.05). Over all Nerali performed better and thus concluded as tolerant variety while Rama posa was proved to be a sensitive variety.
Received | January 27, 2021; Accepted | November 09, 2021; Published | January 31, 2022
*Correspondence | Shehzadi Saima, Institue of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan; Email: shehzadi.saima@bzu.edu.pk, saimakhalid2012@yahoo.com
Citation | Saima, S., F. Ghaffar, G. Yasin, M. Nawaz and K.M. Ahmad. 2022. Effect of salt stress on the germination and early seedling growth in Okra (Abelmoschus esculentus). Sarhad Journal of Agriculture, 38(2): 388-397.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.2.388.397
Keywords | Salinity tolerance, Varieties, Germination, Stress index
Introduction
In Pakistan due to increase population, more food production is a big challenge for agriculture. Production of crops is not increasing according to the increasing food demand. Abiotic stresses like salinity, drought and heat stress are the main causes of low productivity. Amongst these problems, salinity is the main hurdle for the reduction in agricultural productivity (Nazeer et al., 2021). Worldwide, salinity led to the decline of crop production and plant growth in many cultivated areas (Farahmand and Sadeghi, 2020), resulting in 65% loss of crop yield (Gupta et al., 2021). To date, about 1,125 million hectares of agricultural lands have already been seriously affected by salinity. Therefore, it is considered a serious threat to agriculture (Islam et al., 2019; Sanower-Hossain, 2019). The main causes of salinity include inadequate leaching of salts, ineffective use of chemical amendments to reclaim sodic and saline sodic soils, inadequate drainage and sub-soil water table conditions. To meet the increasing food demand, losses of crops are major areas of concern. In Asia, it is expected that increasing levels of salinization could result in a loss of 50% of cultivated land by 2050 (Khan et al., 2019). In developing countries like Pakistan, these losses are of considerable attention because its economy relies on agriculture. In Pakistan, 6 Mha of cultivated land is affected by salinity, which is a great threat to future food production (Naveed et al., 2020). Every year, nearly 100,000 acres of cultivated lands in Pakistan suffer from salinity and water logging.
Abelmoschus esculentus L. (okra) is known as bhindi in Urdu and lady finger in English. It is a flowering plant of family Malvaceae. It is grown in sub-tropical and tropical regions for its edible seed fibrous pods either perennial or annual. It is rich source of minerals and vitamins; therefore, it is quite popular in both farmers and consumers (Oyelade et al., 2003). Oil of okra is greenish yellow in color that can be used as bio fuel (Anwar et al., 2010; Ayub et al., 2018). A 90% loss (6.5 dSm-1) in okra yield has been reported under high salt levels worldwide (Mushtaq et al., 2020). In Pakistan okra is facing abiotic and biotic stresses like other crops. As the crop irrigation requirements of farmers are not fulfilled by canal system so farmers use underground brackish water by pumps for irrigation (Abbas et al., 2014). Additionally, dispose of sewages to rivers and canals makes it not suitable for irrigation as well as salty. These high concentrations of salts cause nutritional disorders as well as specific ions and osmotic injuries in plants and ultimately decrease the quality and yield.
In the life cycle of plants, for the successful establishment of seedlings and consequent growth, seed germination is a critical phase (Grime and Campbell, 1991). Therefore, seed germination being a sensitive stage, determines the fate of seedlings growth (Luan et al., 2014). Abiotic factors that affects the soil interface and control germination of seeds includes salts, water, light and temperature (Ungar, 1991). These stress factors leads to injury and in extreme cases even death of plants (Jaleel et al., 2007). Salt is the key factor among these that reduced germination of seeds, establishment of seedlings, succeeding growth and finally yield of plants (Ghoulam and Fares, 2001; Naqve et al., 2021). Under non-saline conditions best germination of seeds occurs mostly and it decreases with elevation in levels of salinity (Khan and Gul, 1998; Luan et al., 2014). Seeds germination is influenced by salinity either by toxicity of specific ions i.e. Na+ and Cl− on germinating seeds or maintenance of osmotic potential inside the seeds that reduces/inhibit uptake of water (Khajeh-Hosseini et al., 2003). Diverse types of ions having different concentrations are present in saline soils. Evapotranspiration, changes in water sources, solute availability and drainage fluctuate the concentrations of these ions in soils (Jamil et al., 2006). Under conditions of low osmotic potential and decrease soil moisture, the capability of seeds to germinate and amount of precipitation determines the successful establishment of seedlings (Roundy, 1987; Jamil et al., 2006). Delayed or inhibition of seed germination and establishment of seedlings are caused by both osmotic stress and salts (Almansouri et al., 2001; Nasri et al., 2015).
Determination of seeds’ germination ability under saline situations should appear as the beneficial and simple parameter for selecting salt-resistant populations (Panuccio et al., 2014). Furthermore, salinity stress is broadly said to negatively affect seed germination, plant growth and development, yield and crop productivity (Kim et al., 2014). It is due to the statement that germination typically takes place in surface soils, which gather soluble salts due to evaporation and capillary push of water content in the soil. The identification and development of salt tolerant varieties especially in vegetables is the dire need in Pakistan to meet the food requirements of large growing population. Keeping in view to utilize saline soils and significance of okra to increase its production, this study was designed to screen out salt tolerant cultivars and also determination of level of salt that would not delay/inhibit seeds germination.
Material and Methods
Growth conditions and experimental design
The experiments were conducted in the Green-house of Bahauddin Zakariya University Multan, Pakistan. Seeds of six cultivars of Abelmoshcus esculentus (Green leaf, Rama posa, Arka anamika, Super green, Okra kashish and Nerali) were obtained from Ayub Research Center, Faisalabad, Pakistan. Before germination seeds of all cultivarss were sterilized with 0.1% sodium hypochlorite. Seeds of all cultivars were germinated in plastic trays (70 × 50 × 6 cm), lined with four layers of filter paper supplemented with Hoagland nutrient solution. Plastic trays were not aerated (A thin film of Hoagland’s nutrient solution was applied which was thinner than the seed diameter, i.e., seeds were not immersed in solution and no aeration is required. Filter paper (four layers) moistened with nutrient solution is sufficient to provide water, nutrient and air for respiration of germinating seeds. Moreover, aeration with aquarium pumps or similar type of pumps to germinating seeds cause disturbance rather than any support). Twenty seeds of each variety were sown in two rows (10 seeds in each row; ~6 cm spacing in rows) on filter papers moistened with Hoagland’s nutrient solution along with salt stress (0, 25, 50, and 75 mM NaCl). It is well documented that water evaporate from water surface present in any vessel or body at any temperature. Rate of evaporative water loss depends on surface area, ambient temperature and ambient relative humidity. Since surface area of trays/filter paper is large enough and evaporative water loss per day in these empty trays was ~10 mL. This has been followed by Ashraf and Waheed (1990) and Gibeaut et al. (1997) was applied in Hoagland’s nutrient solution after mixing it with respective amount of NaCl salt.
The experiment was replicated with six times in a completely randomized design (CRD) at ambient temperature (26±3 °C). In order to maintain salinity stress levels due to evapo-transpirational water loss ~10 mL of water was added daily in each tray. For a period of 10 days germination of seeds from each cultivar was counted on daily basis. The seeds were supposed to be germinated when both plumule and radical were about 5mm. Cumulative germination rate through time wasmeasured by the following (Bashir et al., 2016) for each cultivar. After 10 days of experimental period, seedlings were harvested.
Measurments
Fresh weight measurements: After harvest seedlings were separated into shoots and roots. Fresh weights of shoot and root were recorded immediately after harvest (Stavridou et al., 2017).
Dry Weight Measurements: Plants components were placed in heat resistant paper bags. After harvesting the paper bags were placed in a drying oven, adjusted at 800 C. The plants were weighted by using a four-figure digital balance after 72 hours of drying described previously (Kumar et al., 2021). After taking paper bags from the oven weights were measured immediately to ensure that reabsorb ion of water from atmosphere was minimum.
Stress indices: From the seedlings the Promptness index (PI), germination stress tolerance index (GSI), dry matter stress tolerance index (DMSI) were calculated by following Saima et al. (2018).
P.I = nd2 (1.00) +nd4 (0.75) +nd6 (0.5) +nd8 (0.25) where n is the number of seeds germinated at day.

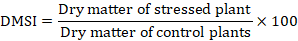
Data analysis
By using analysis of variance treatments and varieties for overall the parameters were compared. Appropriate graphs were drawn to illustrate significant difference derived from Duncan’s (1955), tables. All the statistical analysis was performed by computer statistical package (MINITAB FOR WINDOW).
Results and Discussion
Germination %age
The germination percentage of six varieties of okra and their interactions were significantly affected by salt treatments (Table 1). There was no germination at the first day in six varieties of okra. Increased salt stress concentrations considerably reduced the germination percentage of all varieties relative to their controls. Overall there was reduced germination in 25mM and 50mM salt stress treatments, but no growth in 75mM treatment. After one day of sowing, the germination of all varieties started in control while at 25mM treatment three varieties i.e Green leaf, Rama posa and Nerali were germinated and at 50mM treatment only Green leaf and Nerali were germinated (Figure 1). After two days of sowing at 0mM all varieties showed germination. At 25mM of NaCl Green leaf, Rama posa and Nerali showed little germination and at 50mM only two varieties i.e Green leaf and Nerali showed germination and all other varieties had no germination. At 3rd day of experimental period all varieties showed germination at 0mM and 25mM solution of NaCl, however the magnitude of germination was different in different varieties (Figure 1). At 25mM salt treatment the germination of Rama posa, Okra kashish and Nerali was 40% while the germination of other three varieties i.e. Green leaf, Arka anamika and Super green was 30%. At 50mM salt treatment except Super green all other varieties were germinated. Among these Nerali has 30% germination while, Rama posa, Arka anamika and okra kashish has 10% germination only (Figure 1).
At 4th and 5th day varieties differed significantly between themselves (Table 1). At 0mM treatment the germination of Okra kashish and Nerali was 70% while the germination of the Super green was 40%. The Green leaf, Arka anamika and Rama posa showed 50% germination. At 25mM treatment Nerali showed 50% and Rama posa and Okra kashish showed 40%, while Green leaf, Arka anamika and Super green showed 30% germination. At 50mM treatment Nerali showed 50% germination while varieties Arka anamika and Okra kashish have 30% germination.
Table 1: Analysis of variance (ANOVA) of the data of mean square for germination days in six varieties of Abelmoschus esculentus under salt stress treatments.
|
Source |
Germi-nation day 1 |
Germi-nation day 2 |
Germi-nation day 3 |
Germi-nation day 4 |
Germi-nation day 5 |
Germi-nation day 6 |
Germi-nation day 7 |
Germi-nation day 8 |
Germi-nation day 9 |
Germi-nation day 10 |
|
Treatment (T) |
1.5833 ** |
5.0833 *** |
161.444 *** |
322.889 *** |
284.528 *** |
292.538 *** |
319.083 *** |
360.111 *** |
471.5 *** |
310.67 *** |
|
Varieties (V) |
0.716ns |
2.3167** |
21.044 *** |
105.222 *** |
23.78** |
18.583* |
22.533** |
21.494** |
39.67* |
99.07** |
|
T x V |
0.15ns |
1.05** |
19.678** |
106.9** |
2.449ns |
13.817* |
51.02* |
83.11* |
39.027* |
66.031** |
Table 2: Analysis of variance (ANOVA) of the data of mean square for root fresh weight, root dry weight, shoot fresh weight and shoot dry weight in six varieties of Abelmoschus esculentus under salt stress treatments.
|
Source |
Root fresh weight |
Root dry weight |
Shoot fresh weight |
Shoot dry weight |
GSI |
DMSI |
|
Treatment (T) |
0.0465*** |
0.0297*** |
0.0034*** |
0.0032258** |
115.53*** |
403.01*** |
|
Varieties (V) |
0.00576* |
0.0007ns |
0.000049ns |
0.0001671ns |
87.342** |
211.54* |
|
T x V |
0.00783* |
0.0459** |
0.00016* |
0.00098* |
54.986* |
303.7* |
Green leaf and Super green has 15% and Rama posa has 20% germination (Figure 1).
In germination days of 6thand 7th all varieties showed more germination at 0mM as compared to salt stressed treatments. In 25mM treatment Rama posa and Nerali showed 50% and 60% germination respectively but germination of all other varieties was reduced (Figure 2). In 50mM treatment Okra kashish and Nerali showed more germination; while other varieties showed less germination. At 8th, 9thand 10th day of experimental period treatments had significantly influenced the germination of okra as detected by analysis of variance. Varieties also had highly significant differences between themselves. (Table 1). At these days all varieties showed maximum germination under control conditions. At 25mM salt treatment Nerali showed 70% germination while Rama posa and Okra kashish exhibited 50% germination. Arka anamika and Green leaf showed 50% and 40% germination respectively. At 50mM salt treatment Okra kashish and Nerali showed 60% germination. Arka anamika and Super green showed 45%, Rama posa showed 30% and Green leaf showed 15% germination. (Figure 2).
Fresh and dry weight of shoot
Treatment had significantly influenced the fresh and dry weight of shoots in okra at P<0.05 (Table 2). The fresh weight of shoot was highest in Super green in both control and salt stressed treatments than all other varieties. In Green leaf and Nerali the shoot fresh weight was reduced significantly almost 60% upon 25mM salt treatment and it further reached to minimum level among all varieties at 50mM salt treatment. Okra kashish, Arka anamika and Super green performed better under salt stress and showed about 40% reduction. Rama posa had less shoot fresh weight under control condition among all varieties and salt treatments decreased it further (Figure 3). Similar trends were observed in dry weight of shoot in which Super green has more dry weight in all treatments than other varieties. Arka anamika, Okra kashish and Nerali showed similar magnitude of reduction in dry weights in all salt treatments. Ram posa and Green leaf had less dry weight of shoots under control as well salt treatments among all varieties. All varieties showed great reduction in dry weight of shoots under salt stress therefore the interaction between varieties and treatments was significant in overall analysis of variance (Table 2).
Fresh and dry weight of root
Analysis of variance had showed significant differences (P<0.05) in the root fresh and dry weight of varieties of okra (Table 2). Super green showed the maximum fresh weight of root under control condition as compared to all other varieties. It reduced little at 25mM of salinity and further reduced at 50 mM of salt and hence fresh and dry weight of root in super green was reached to the level of other varieties. Okra kashish and Nerali had almost same root fresh weights under control and 25mM salt treatments but at 50mM salt treatment Nerali showed more severe reduction in fresh weight of root than Okra kashish (Figure 3). Among all varieties Rama posa showed lower fresh weight of root and it further decreased gradually with the increased salt concentration. Similar patterns were observed in root dry weight of all varieties. Among different treatments 50mM salt
treatment decreased the root dry weight more severely as compared to control and 25mM salt treatment in all varieties. Under control Nerali, Super green and Green leaf had highest root dry weight while others had lowest dry weigh. At 25mM salt treatment Nerali and Okra kashish had maximum root dry weight while Super green and Rama posa showed lowest dry weight. Other varieties showed intermediate values of root dry weight at 25mM salt concentration. At 50mM salt treatment Okra kashish had highest dry weight than the rest of all varieties. (Figure 3).
Germination stress index (GSI)
ANOVA had detected significant differences at P<0.05 in GSI of all varieties (Table 2). Nerali had more GSI in all treatments as compared to other varieties. In 25mM treatment Super green has 10% more GSI than Okra kashish. Green leaf and Arka anamika
have the same GSI in 25mM treatment. Rama posa has less GSI as compared to Green leaf, Okra kashish, Arka anamika, Super green and Nerali. In 50mM treatment Arka anamika and Nerali has the same GSI, but GSI in Okra kashish was reduced (Figure 4). On the other hand Green leaf and Rama posa has less GSI as compared to rest of other varieties.
Dry matter stress index (DMSI)
DMSI of Okra varieties was significantly influenced by salt treatments in over all analysis of variance (Table 2). At the 25 mM concentration of salt Okra kashish had maximum DMSI and Super green had minimum than the rest of all varieties. Green leaf and Nerali showed almost same DMSI at 25 mM concentration of salt (Figure 4). In 50mM treatment DMSI of Arka anamika was highest then came Okra kashish, Rama posa and Green leaf. Super green and Nerali showed lowest DMSI among all the varieties.
Plants have great range in terms of their tolerance to soil salinity. Successful seed germination under stress conditions determines the fate of species survival and distribution (Zivkovic et al., 2007). Continuous water evaporation creates the deposition of salts on the surface of soil where the seeds are mostly located (Ungar, 1991). Salinity reduces germination of seeds in two ways: The embryos are sensitive for the toxicity of ions. The osmotic potential of soil is decreased due to salts that inhibit the water uptake required for germination to mobilize the nutrients in the seeds (Kaymakanova, 2009). Although during course of evolution seeds of some crops had adapted changes to germinate under high soil salinity (Luan et al., 2014) but still tolerance varies with osmotic potential of the medium and type of salt.
Okra is a salt sensitive crop, especially in its early growth stage (Kamaluldeen et al., 2014). In the present investigation over all germination in all varieties was delayed as the salt concentration increases. Messaitfa et al. (2014) also suggested that the amount of water in the salt solution had direct influence on seeds germination. Salinity increases the osmotic potential of growth medium and, as a result, seeds require more energy to absorb water, resulting in decreased germination (Habib et al., 2021). Plants showed variations in terms of their salt tolerance to osmotic potential of the medium (Kaymakanova, 2009). Seedling growth was affected by salinity due to less or slow mobilization of food material that suspended cell elongation,division and embryo formation (Messaitfa et al., 2014). So there is negative correlation between germination and salt tolerance. When applying increasing salt concentrations all parameters i.e. germination rate, GSI and DMSI decreased remarkably revealing that increased salt concentrations severely affected seed germination and seedling establishment. Under stress environment different varieties showed different performance. Increased salt stress concentrations considerably reduced the germination percentage of all varieties relative to their controls, however the magnitude of reduction was variety specific. Similar results were predicted in wheat (Gholamin and Khayatnezhad, 2010), sufflower (Mostafavi, 2011), Physalis (Yildirim et al., 2011) and lettuce (Nasri et al., 2015). Over all germination stress index showed that Nerali performed better.
Plant growth consists of potential of germination process, hypocotyle length, fresh and dry Biomass, seedling growth and vegetative growth of plants, mainly caused through cell division, cell differentiation and cell enlargement (Nazeer et al., 2021). In the current study salt stress has affected the fresh and dry weight of root and shoots in all varieties. Two varieties i.e. Rama posa and Arka anamika showed greater reduction in fresh and dry weight of roots while Okra kashish and Nerali was less affected under salinity treatments. This may be due to inhibitory effect of ions that limits the growth of plants. The reduction in shoot and root development may be due to unbalanced nutrient uptake and toxicity of NaCl. Yildirim et al. (2011) also documented that salinity greatly reduced the fresh and dry weight of plants. Datta et al. (2009) demonstrated that different levels of salinity have significant affects on the growth parameters due to reduction in biomass of root and shoot. It can be concluded that Nerali was more tolerant and Rama posa was more sensitive among all varieties.
Conclusions and Recommendations
Salinity reduced germination in all six varieties in concentration dependent manner. Nerali showed better germination as compared to all other varieties while rama posa showed least germination. Among growth parameters i.e. fresh and dry weight of shhot and root were also reduced by salinity. In these parameters Nerali performed better and hence considered as salt tolerant variety. Rama posa was termed as salt sensitive variety.
Novelty Statement
The varieties tested for salt tolerance screening are new varieties and not studied before.
Author’s Contribution
Shehzadi Saima: Conseived, guided the study, analyzed the data and wrote the manuscript.
Faiza Ghaffar: Performed the experiments.
Ghulam Yasin and Muhammad Nawaz: Edited the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbas, T., M.A. Pervez, C.M. Ayyub, M.R. Shaheen, S. Tahseen, M.A. Shahid, R.M. Bilal and A. Manan. 2014. Evaluation of different okra genotypes for salt tolerance. Int. J. Plant Anim. Environ. Sci., 4(3): 23-30.
Almansouri, M., J.M. Kinet and S. Lutts. 2001. Effect of salt and osmotic stresses on germination in Durum wheat (Triticum durum Desf.). Plant Soil, 231: 243-254. https://doi.org/10.1023/A:1010378409663
Anwar, F., U. Rashid, M. Ashraf and M. Nadeem. 2010. Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl. Energy, 87(3): 779-785. https://doi.org/10.1016/j.apenergy.2009.09.020
Ashraf, M., and A. Waheed. 1990. Screening of local/exotic accessions of lentil (Lens culinasis Medic.) for salt tolerance at two growth stages. Plant Soil, 128(2):167-176. https://doi.org/10.1007/BF00011106
Ayub, Q., S.M. Khan, A. Khan, I. Hussain, Z. Ahmad and M.A. Khan. 2018. Effect of gibberellic acid and potassium silicate on physiological growth of Okra (Abelmoschus esculentus L.) under salinity stress. Pure Appl. Biol., 7(1): 8-19. https://doi.org/10.19045/bspab.2018.70002
Bashir, N., S. Mahmood, Z.A. Zafar, S. Rasul, H. Manzoor and H.R. Athar. 2016. Is drought tolerance in maize (Zea mays L.) cultivars at the juvenile stage maintained at the reproductive stage? Pak. J. Bot., 48: 1385–1392.
Datta, J.K., S. Nag, A. Banerjee and N.K. Mondal. 2009. Impact of salt stress on five varieties of wheat Triticum aestivum L. cultivars under laboratory condition. J. Appl. Sci. Environ. Manage., 13: 93-97. https://doi.org/10.4314/jasem.v13i3.55372
Duncan, D.B. 1955. Multiple Range and Multiple F-Test. Biometrics, 11: 1-5. https://doi.org/10.2307/3001478
Farahmand, N. and V. Sadeghi. 2020. Estimating soil salinity in the dried lake bed of Urmia lake using optical sentinel-2 images and nonlinear regression models. J. Indian Soc. Remote Sensing, 48: 675-687. https://doi.org/10.1007/s12524-019-01100-8
Gibeaut, D., Hulett, J., Cramer, G. and Seemann, J. 1997. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol, 115:317 - 319. https://doi.org/10.1104/pp.115.2.317
Gholamin, R. and M. Khayatnezhad. 2010. Effects of Polyethylene Glycol and NaCl stress on two cultivars of Wheat Triticum durum at germination and early seeding stages. Am. Eu. J. Agric. Environ. Sci., 9: 86-90.
Ghoulam, C. and K. Fares. 2001. Effect of salinity on seed germination and early seedling growth of sugar beet (Beta vulgaris L.). Seed Sci. Technol., 29 (2): 357–364.
Grime, J.P. and B.D. Campbell. 1991. Growth rate, habitat productivity, and plant strategy as predictors of stress response,” in Response of Plants to Multiple Stresses, H. A. Mooney, W. E. Winner and E. J. Pell, Eds., pp. 143–159, Academic Press, Orlando, Fla, USA. https://doi.org/10.1016/B978-0-08-092483-0.50012-8
Gupta, A., S. Rai, A. Bano, A. Khanam, S. Sharma and N. Pathak. 2021. Comparative evaluation of different salt-tolerant plant growth-promoting bacterial isolates in mitigating the induced adverse effect of salinity in Pisum sativum. Biointerface Res. Appl. Chem., 11(5): 13141–13154. https://doi.org/10.33263/BRIAC115.1314113154
Habib, S.H., H. Kausar and H.M. Saud. 2021. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed. Res. Int., https://doi.org/10.1155/2016/6284547
Islam, F., J. Wang, M.A. Farooq, C. Yang, M. Jan and T.M. Mwamba. 2019. Rice responses and tolerance to salt stress, in Advances in rice research for abiotic stress tolerance. eds. M. Hasanuzzaman, M. Fujita, K. Nahar and J. Biswas (Cambridge: Woodhead Publishing), 791–819. https://doi.org/10.1016/B978-0-12-814332-2.00040-X
Jaleel, C.A., R. Gopi, P. Manivannan and R. Panneerselvam. 2007. Antioxidative potentials as a protective mechanism in Catharanthus roseus (L.) G. Don. plants under salinity stress. Turkish J. Bot., 31 (3): 245–251.
Jamil, M., D.B. Lee, K.W. Jung, M. Ashraf, S.C. Lee and E.S. Rha. 2006. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetable species. J. Central Eu. Agric., 7(2): 273-282.
Kaymakanova, M. 2009. Effect of salinity on germination and seed physiology in bean (Phaseolus Vulgaris L.). Biotechnol. Biotechnol. Equipment., 23: 326-329. https://doi.org/10.1080/13102818.2009.10818430
Khajeh-Hosseini, M., A.A. Powell and J.J. Bingham. 2003. The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Sci. Technol., 31: 715-725. https://doi.org/10.15258/sst.2003.31.3.20
Khan, A., A.L. Khan, S. Muneer, Y.H. Kim, A. Al-Rawahi and A. Al-Harrasi. 2019. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci., 10: 1429. https://doi.org/10.3389/fpls.2019.01429
Khan, M.A. and B. Gul. 1998. High salt tolerance in germinating dimorphic seeds of Arthrocnemum indicum. Int. J. Plant Sci., 159(5): 826–832. https://doi.org/10.1086/297603
Kim, K., Y.J. Jang, S.M. Lee, B.T. Oh, J.C. Chae and K.J. Lee. 2014. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells, 37: 109. https://doi.org/10.14348/molcells.2014.2239
Kumar, S., G. Li, J. Yang, X. Huang, Q. Ji, Z. Liu, W. Ke and H. Hou. 2021. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci., 12: 660409. https://doi.org/10.3389/fpls.2021.660409
Luan, Z., M. Xiao, Z. Zhou, H. Zhang, Y. Tian, Y. Wu, B. Guan and Y. Song. 2014. Effects of salinity, temperature, and polyethylene glycol on the Seed germination of sunflower (Helianthus annuus L.). Sci. World J. Article ID 170418, 9. https://doi.org/10.1155/2014/170418
Messaitfa, Z.H., Shehata, A., Quraini, F., Hazzani, A.A., Rizwana, H. and Mona S El wahabi, M. 2014. Proteomics analysis of salt stressed Sunflower (Helianthus annuus). Int. J. Pure App. Biosci., 2 (1): 6-17.
Mushtaq, N.U., S. Saleem, A. Rasool, W.H. Shah, K.R. Hakeem and R.U. Rehman. 2020. Salt stress threshold in millets: Perspective on cultivation on marginal lands for biomass. Phyton, 90: 51. https://doi.org/10.32604/phyton.2020.012163
Naqve, M., X. Wang, M. Shahbaz, S. Fiaz, W. Naqvi, M. Naseer, A. and Mahmood, H. Ali. 2021. Foliar spray of alpha-tocopherol modulates antioxidant potential of okra fruit under salt stress. Plants, 10: 1382. https://doi.org/10.3390/plants10071382
Nasri, N., I. Saidi, R. Kaddour and M. Lachaal. 2015. Effect of salinity on germination, seedling growth and acid phosphatase activity in lettuce. Am. J. Plant Sci., 6: 57-63. https://doi.org/10.4236/ajps.2015.61007
Naveed, M., H. Sajid, A. Mustafa, B. Niamat, Z. Ahmad, M. Yaseen, M. Kamran, M. Rafique, S. Ahmar and J.T. Chen. 2020. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability, 12: 846. https://doi.org/10.3390/su12030846
Nazeer, H., H. Gul, M. Rauf, T. Yaseen, K. Rehman, Y. Khan, B. Amin, S. Shah, M. Shah and M. Noor. 2021. Response of wheat varieties to salinity: growth, yield and ion analysis. Plant Sci. Today, 8(2): 301–311. https://doi.org/10.14719/pst.2021.8.2.1074
Oyelade, O.J., B.I.O. Ade-Omowaye and V.F. Adeomi. 2003. Influence of variety on protein, fat contents and some physical characteristics of okra seeds. J. Food Engineer., 57(2): 111-114. https://doi.org/10.1016/S0260-8774(02)00279-0
Panuccio, M.R., S.E. Jacobsen, S.S. Akhtar and A. Muscolo. 2014. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS 6: https://doi.org/10.1093/aobpla/plu047
Roundy, B.A. 1987. Seedbed salinity and the establishment of range plants. In: Frasier, G.W., Evans, R.A. Proc. Sympos. Seed and Seedbed Ecology of Rangeland Plants, Washington, D.C.: USDA-ARS, 1987, pp. 68-71.
Saima, S., Li, G. and Wu, G. 2018. Effects of drought stress on hybrids of Vigna radiataa germination stage. Acta Biologica Hungarica, 69(4): 481–492. https://doi.org/10.1556/018.69.2018.4.9
Sanower-Hossain, M. 2019. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci., 1: 1–3.
Stavridou, E., A. Hastings, R.J. Webster and P.R.H. Robsonn. 2017. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus 3 giganteus. GCB Bioenergy, 9: 92–104. https://doi.org/10.1111/gcbb.12351
Ungar, I.A. 1991. Seed germination and seed-bank ecology of halophytes,” in Seed Development and Germination, J. Kigeland G. Galili, Eds., Marcle and Dekker, New York, NY, USA, 1995.
Yildirim, E., H. Karlidag and A. Dursun. 2011. Salt Tolerance of Physalis during germination and seedling growth. Pak. J. Bot., 43: 2673-2676.
To share on other social networks, click on any share button. What are these?








