Effect of Organic Acids Blend, Micro-Encapsulated Phyto-Essential Oils Individually or in Combination on Growth Performance, Gut Health and Nutrients Utilization of Broilers
Effect of Organic Acids Blend, Micro-Encapsulated Phyto-Essential Oils Individually or in Combination on Growth Performance, Gut Health and Nutrients Utilization of Broilers
Ziaul Islam1*, Asad Sultan1, Sar Zamin Khan1 and Sher Bahader Khan2
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture Peshawar, Pakistan
2College of Veterinary Sciences, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture Peshawar, Pakistan
ABSTRACT
This study was planned to investigate the effect of organic acids blend and micro-encapsulated phyto-essential oils individually or in combination as a replacement of antibiotic growth promoters (AGP) on growth performance, lymphoid organs weight, gut pH, microbiota, apparent metabolizable energy and nutrient digestibility of broilers. A total of six hundred, day-old healthy broiler chicks (Cobb 500) were procured from a local hatchery and were randomly allocated to five different dietary treatments. A basal diet formulated with corn-soybean meal (CON; without any additives) served as control diet and fed to birds in control group. Birds in other four groups were given same basal diet mixed either with zinc bacitracin (150 mg/kg diet; ZB-150); organic acids (200 mg/kg feed; OA-200); essential oil (150 mg/kg feed; EO-150) and or a combination of organic acid (OA) and essential oil (EO) at rate 200 and 150 mg/kg, respectively. Body weight gain was significantly (P<0.05) enhanced by all different dietary treatments compared to birds in controlled group. Lymphoid organs weight was higher (P<0.05) in birds in groups (OA-200, EO-150 and OA+EO). Log CUF count of Lactobacillus were significantly increased and that of Escherichia coli and Salmonella were decreased by birds in group OA-200, EO-150 and OA+EO both at day-21- and 35 compared to control and ZB treated groups. Apparent ileal digestibility of all different nutrients and apparent metabolizable energy investigated in present study was significantly improved by all different dietary treatments compared to control group. These findings demonstrate that the strategic application of organic acids, micro-encapsulated phyto-essential oils individually and or in combination could potentially replace the antibiotic growth promoters in broiler production without compromising production performance and other parameters of economic importance.
Article Information
Received 14 July 2021
Revised 21 August 2021
Accepted 15 September 2021
Available online 27 December 2021
(early access)
Published 15 July 2022
Authors’ Contribution
ZI performed animal trial, laboratory experiment, statistical analysis, study design and writing. AS designed the study and idea, feed formulation, data evaluation and manuscript review. SZK evaluated data and reviewed the manuscript. SK improved main body text and reviewed the manuscript.
Key words
Organic acid, Essential oil, Broiler chicken, microbiota, Nutrient digestibility, Growth performance
DOI: https://dx.doi.org/10.17582/journal.pjz/20210714190714
* Corresponding author: [email protected]
0030-9923/2022/0005-2391 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Broiler production performance is highly related to the gastrointestinal tract due to its vital role in the nutrient digestion and utilization (Rinttila and Apajalaht, 2013) and any deviation from normal gut function can badly impact the growth performance of broiler birds (Morgan, 2017). Antibiotic growth promoters (AGPs) have been extensively used in broiler production from improved growth performance and gut health (Wang et al., 2019). This however, could lead to antimicrobial resistance (AMR) (Golkar et al., 2014; Pourmand et al., 2017) and sever human health consequences in long run (WHO, 2014). It has been reported that if the use of AGPs is not culminated in poultry industry it will cause fetal casualties in human lives and other serious implications (Neill, 2016). Moreover, the consumer’s demand for safe and healthy poultry products have further stressed the need of an effective ways for the replacement of AGPs in poultry diet and ban on AGPs use (Diarra and Malouin, 2014). Due to serious public concerns USA and the European Union (EU) have already imposed bans on the use of antibiotics in poultry feed as a growth promoter (Giannenas et al., 2014). To sustain and fulfill the growing demand of healthy poultry products it is highly imperative to assess different alternatives to AGPs to attain better production performance and gut health (Huyghebaert et al., 2011; Markowiak and Slizewska, 2018). Organic acid (OA) and essential oil (EO) may be suitable non-antibiotic substances to produce same results as AGPs in terms of production performance and health (Zhai et al., 2018).
A number of plant derivatives compounds possessing beneficial implications e.g., antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory, and immune-regulating properties (Swamy et al., 2016) could be effectively used to enhance the growth of poultry production. Certain essential oils thymol, carvacrol and eugenol can potentiate the growth performance and improve gut health (Henri and Bassole, 2012) and can modulate positively the microbial community of intestinal tract by favoring beneficial microbial growth and reducing harmful microorganisms (Stevanovic et al., 2018). These essential oils can stimulate gut digestive enzymes to improve nutrients digestibility, reduce the inflammatory response and enhance immunity (Kim et al., 2013; Kazempour and Jahanian, 2017).
Organic acid are weak and short chain acids viz acetic acids, propionic, benzoic and butyric acids (Dibner and Buttin, 2002) that can effectively reduce gut pH favoring the growth of beneficial gut microbes, improve digestive enzymes functions and nutrients utilization by broilers (Canibe et al., 2001). Outside the digestive system supplementation of organic acids either in drinking water or feed have been reported to decrease pH of water and feed, reduce the growth harmful microbes and maintain its quality (Jarquin et al., 2007; Islam, 2012). Improved gut health of poultry birds with minimum number of harmful bacteria and better lining for the assimilation of nutrients could be achieved with the use of organic acids (Paul et al., 2007). There is great need to examine the potential benefits of organic acids blend and phyto essential oils in combination for effective replacement of antibiotic growth promoters in poultry diet. The combined effect of organic acids blend and phyto essential oils could have synergistic effect (Omonijo et al., 2018) to enhance the growth performance, immunity and nutrients utilization. It is therefore this study was designed to investigate the individual and combined impact of an organic acids blend and essential oil against the Zinc Bacitracin (ZB) that is commonly used as antibiotic growth promoter in poultry feed industry.
MATERIALS AND METHODS
Ethical consideration
All the experimental procedures adopted in this study were pre-approved from the animal welfare and care committee on the use of experimental animals at The University of Agriculture, Peshawar, Pakistan.
Feed additives
Organic acid blend (propionic acid, formic acid, 2 Hydroxy 4 methyl thiobutanoic acid HMTBa) and microencapsulated phyto-essential oils blend containing (oregano, rosemary, cinnamon, and chili pepper extract) as active ingredients (both USA origin) were procured from a commercial feed additive supplier. Feed additives were mixed with micro-ingredients before mixing in final ration for better and uniform mixing.
Experimental layout and bird’s husbandry
A total of 600 (day-old) broiler chicks (Cobb 500) were obtained from a commercial hatchery and reared in open sided house bedded with softwood shavings. All chicks were randomly assigned to five replicated (n=6; 20 birds/rep) dietary groups as CON; without any additives; ZB-150; OA-200; EO-150 and OA + EO, respectively. Birds in CON group was offered a starter (0-21days) and finisher (22-35 days) corn-soybean meal based diet fulfilling all its nutritional requirements as per Cobb 500 nutrients specification guide using Brill Formulation® software (Table I), while the birds in others groups were added Zinc Bacitracin (150 mg/kg diet), organic acids (200 mg/kg feed) micro-encapsulated phyto-essential oil (150 mg/kg feed) and combination of organic acid (OA) and micro-encapsulated phyto-essential oil (EO) (200 mg + 150 mg/kg), respectively given. Birds in all groups had ad libitum access to feed and water. Optimum environmental conditions of temperature, humidity, ventilation and light were maintained as needed at different stages of rearing.
Data collection and measurements
Birds were weighed on day first and then on weekly basis. Initial weight was subtracted from final weight and divided by number of birds for that particular week and adjustment in mortality if any. Cumulative average body weight gain was measured by sum up all weekly weight gains. Feed intake per replicate was determined on weekly basis and cumulative was determined by summing up all data at the end of each phase. From the cumulative average body weight gain and feed intake feed conversion ratio (FCR) was measured (Sultan et al., 2018).
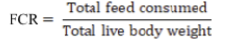
Relative weight of the lymphoid organs
On day 35, five birds from each replicate were randomly selected and live body weight was recorded. All birds were humanly killed, skinned off and dissected. Lymphoid organs thymus, spleen and bursa of Fabricius was carefully removed, trimmed of any foreign tissues and weighed individually and expressed as percent of the live body weight (Yang et al., 2018).

Table I. Diet composition and estimated nutrients used in the present study.
|
Ingredient % |
Starter phase (1-21days) |
Finisher phase (22-35days) |
|
Diet composition |
||
|
Corn |
58.780 |
63.180 |
|
Soybean meal 46 % |
34.470 |
29.630 |
|
Poultry oil/ fat |
2.590 |
3.830 |
|
Salt |
0.370 |
0.370 |
|
Sodium bicarbonate/ soda |
0.100 |
0.100 |
|
Limestone/ chips |
1.470 |
1.360 |
|
DCP |
1.080 |
0.730 |
|
Lysine sulfate |
0.370 |
0.220 |
|
DL methonoine |
0.300 |
0.240 |
|
Threonine |
0.110 |
0.010 |
|
Choline chloride 70 % |
0.100 |
0.100 |
|
Mineral and vitamin premix |
0.250 |
0.220 |
|
Phytase |
0.010 |
0.010 |
|
Calculated nutrients |
||
|
DM (%) |
84.608 |
83.354 |
|
CP (%) |
20.951 |
18.785 |
|
Ash (%) |
5.349 |
4.726 |
|
EE (%) |
5.45 |
6.735 |
|
C. fiber (%) |
2.791 |
2.674 |
|
Ame |
2975 |
3100 |
|
Na (%) |
0.18 |
0.18 |
|
Cl (%) |
0.289 |
0.29 |
|
K (%) |
0.885 |
0.799 |
|
Calcium |
0.9 |
0.76 |
|
Ava. P (%) |
0.45 |
0.38 |
|
Lysine (D) |
1.22 |
1.02 |
|
Methionine (D) |
0.597 |
0.507 |
|
Met +Cys (D) |
0.91 |
0.8 |
|
Tryptophan (D) |
0.227 |
0.202 |
Vitamin and minral premix contained the following per kg of diet: 10,000 IU vitamin A, 4,500 IU vitamin D3, 65 mg vitamin E, 1.5 mg vitamin B1, 12 mg vitamin B2, 3.2 mg vitamin B6, 0.011 mg vitamin B12, 3.0 mg vitamin K3, 18 mg pantothenic acid, 60 mg niacin, 0.18 mg biotin, 1.9 mg folic acid; 20 mg Fe, 16 mg Cu, 110 mg – Zn, 120 mg Mn, 1.25 mg I, 0.9 mg Co, 0.3 mg Se.
Determination of gut pH in different segment of the gastrointestinal tract
The gut pH was measured at four different segments of gastrointestinal tract i.e., crop, ilium, jejunum and ceca immediately after killing the birds. A portable digital pH meter was used as described by (Ndelekwute et al., 2019).
Determination of ileal microbial count
Ileal digesta content were collected both at day 21 and 35 of the experimental period from five birds and pooled. Samples were transferred to sterile plastic air tight test tubes, freezed and stored at -80°C until further analysis. Briefly, for measurement of ileal microbiota 1-gram excreta was diluted in 9 mL of 1% peptone broth and homogenized. Homogenized samples were transferred to selective media for growth. The bacterial counts was performed by serial 10-fold dilutions (10 g/l peptone solution) onto Lactobacillus MRS agar plates, MacConkey agar plates, and Salmonella-Shigella agar plates to isolate the Lactobacillus, Escherichia coli, and Salmonella, respectively. The bacteria colonies was counted immediately after the plates was cultivated at 37°C under anaerobic conditions using a colony counter (Gao et al., 2019).
Apparent ileal digestibility and ileal digestible energy calculation
Birds were selected (n=10) from all replicates at day-35 and shifted to metabolic cages for total excreta collection for final four days till day-42. Feed intake and excreta were collected and weighed daily morning for four days. Representative samples were collected, air and oven dried for further analyses. For determination of ileal nutrients digestibility 0.2% Cr2O3 was used as indigestible marker. Dried samples of excreta, ileal digesta and feed was grind to pass through a 1- mm screen. Gross energy of feed and fecal samples was measured using Adiabatic bomb calorimeter and AME was determined. Proximate analyses of feed and excreta samples were done as outlined in (AOAC, 2005). Chromium concentrations were determined with a UV absorption spectrophotometer (Shimadzu, UV-1201, Shimadzu, Kyoto, Japan) using the method of (Williams et al.,1962). The following formulas were used to calculate the apparent ileal digestibility and ileal digestible energy (Stefanello et al., 2020).
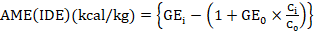
where GEi= gross energy (kcal/kg) in the diet; GEo= gross energy (kcal/kg) in the ileal digesta or excreta; Ci and Co = concentration of marker in the diet and digesta or excreta (%), respectively.
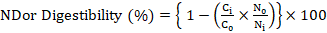
where ND= nutrient digestibility (%); Ci and Co= concentration of marker in the diet and digesta or excreta (%), respectively; Ni and No= concentration of nutrient in the diet and digesta or excreta (%), respectively.
Data analysis
Data were subjected to one-way ANOVA using General Linear Model procedure of SAS 9.3 package (Guide, 2010). Treatments means were compared by LSD.
RESULTS
Growth performance
Table II indicate improved average body weight gain and better FCR both at starter (day-1 to day-21) and finisher phase (day-22 to day-35) that was significantly (P <0.05) affected by all dietary treatments compared to control groups. During starter phase maximum average body weight gain was observed in groups OA+EO (796.91), EO-150 (789.47), OA-200 (789.17) and improved FCR OA+EO (1.41), EO-150 (1.43), OA-200 (1.43). This was followed by group ZB-150 and CON. Overall performance (day-1 to 35) in term of average body weight gain and FCR was improved (p<0.05) in all groups compared to control group. The highest final average body weight gain and better FCR were observed in the treatments groups ZB-150, OA-200, EO-150 and OA+EO as compared to CON. No significant difference (p>0.05) was seen among different groups for feed intake and livability.
Relative weight of lymphoid organs
Lymphoid organs weight, spleen and bursa of Fabricius were significantly improved (P < 0.05) in-group ZB-150, OA-200, EO-150 and OA+EO respectively. Maximum improvement in weight of spleen and bursa of Fabricius was observed in groups OA+EO as compare to other treatment groups. There was a non-significant difference (P > 0.05) between the treatments for the relative thymus weight Table III.
Digesta pH in different section of the digestive tract
pH in different gut section was significantly altered by the dietary treatments in groups that received organic acid blend, micro-encapsulated phyto-essential oil and or both. Maximum change was in pH in crop (4.94), proventriculus (2.36), ileum (6.01), Jejunum (5.12) and Caeca (5.91) was observed in group OA+EO as compared to control, Table IV.
Table II. Effect of an organic acid blend and micro-encapsulated essential oil individually or in combination on production performance of broilers on day 21 and 35.
|
Production traits |
CON |
Treatments1 |
p value |
|||
|
ZB-150 |
OA-200 |
EO-150 |
OA+EO |
|||
|
Starter phase (day 0-21) |
||||||
|
BWG, g |
729.42b± 0.987 |
782.35a± 0.317 |
789.17a±0.815 |
789.47a±0.748 |
796.91a±0.996 |
0.0001 |
|
FI, g |
1129.61±0.230 |
1130.31±0.570 |
1131.92±0.174 |
1129.31±0.650 |
1128.32±0.701 |
0.9665 |
|
FCR |
1.54a± 0.205 |
1.44b± 0.024 |
1.43b± 0.020 |
1.43b±0.664 |
1.41b± 0.011 |
0.0000 |
|
Livability % |
96.16±0.477 |
96.50±.562 |
95.66±0.557 |
96.50±0.562 |
95.66±0.557 |
0.6730 |
|
Finisher phase (day 22-35) |
||||||
|
BWG, g |
1085.22±0.145 |
1061.72± 0.647 |
1080.51±0.601 |
1099.01±0.085 |
1083.31±0.849 |
0.3001 |
|
FI, g |
2254.52±0.466 |
2264.72±0.605 |
2262.81±0.693 |
2259.61±0.592 |
2263.11±0.915 |
0.1540 |
|
FCR |
2.07a± 0.025 |
2.13ab±0.0234 |
2.09bc±0.021 |
2.05c±0.028 |
2.089c±0.012 |
0.2297 |
|
Livability % |
96.50±0.428 |
96.50±0.562 |
96.50±0.670 |
96.50±0.562 |
96.33±0.557 |
0.9993 |
|
Overall period (d 0 to 35) |
||||||
|
BWG, g |
1814.61c±0.971 |
1844.04b±0.240 |
1869.71ab±0.681 |
1888.53a±0.072 |
1880.21a±0.569 |
0.0000 |
|
FI, g |
3384.12± 0.408 |
3395.01±4.939 |
3394.71±0.759 |
3389.01± 0.418 |
3391.41±0.160 |
0.4449 |
|
FCR |
1.86a± 0.014 |
1.841ab±0.027 |
1.81bc±0.503 |
1.79c± 0.013c |
1.80c± 0.133 |
0.0001 |
|
Livability % |
97.00±0.365 |
96.50± 0.562 |
97.16±0.703 |
96.50± 0.562 |
97.33± 0.494 |
0.7438 |
Mean carrying different superscripts are significantly different (p<0.05). Values are presented as mean with standard error mean. BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio. 1Con, basal diet; ZB-150, basal diet+ zinc bacitracin, OA-200, basal diet+ organic acid; EO-150, Basal diet essential oil; OA+EO, basal diet + organic acid + essential oil.
Table III. Effects of an organic acid blend and micro-encapsulated essential oil individually or in combination on lymphoid organs weight of broilers on day 35.
|
Lymphoid organ |
CON |
Treatments |
p value |
|||
|
ZB-150 |
OA-200 |
EO-150 |
OA+EO |
|||
|
Spleen |
0.12b±0.019 |
0.14b±0.011 |
0.17a±0.009 |
0.16a±0.077 |
0.18a ± 0.773 |
0.0000 |
|
Thymus |
0.43±0.023 |
0.42±0.021 |
0.45±0.573 |
0.43±0.019 |
0.42±0.023 |
0.8176 |
|
Bursa of Fabricius |
0.15b ± 0.819 |
0.165b±0.638 |
0.175ab±0.638 |
0.176ab±0.216 |
0.196a±0.014 |
0.0322 |
Mean carrying different superscripts are significantly different (p<0.05). Values are presented as mean with standard error mean. 1C, basal diet; ZB-150, basal diet+ zinc bacitracin; OA-200, basal diet+ organic acid; EO-150, basal diet+ essential oil; OA+EO, basal diet + organic acid + essential oil.
Table IV. Effects of an organic acid blend and micro-encapsulated essential oil individually or in combination on pH value of different gut sections of broilers on day 35.
|
Gut region |
CON |
Treatments |
p value |
|||
|
ZB-150 |
OA-200 |
EO-150 |
OA+EO |
|||
|
Crop |
5.95a±0.040 |
5.92a±0.010 |
5.28b±0.018 |
5.18c±0.054 |
4.94c±0.013 |
0.0000 |
|
Proventriculus |
2.64a±0.011 |
2.63a±0.010 |
2.49b±0.048 |
2.42b±0.570 |
2.36c±0.039 |
0.0000 |
|
Ilium |
7.52a±0.118 |
7.46a±0.120 |
6.71b±0.703 |
6.73c±0.203 |
6.01b±0.30 |
0.0000 |
|
Jejunum |
6.26a±0.011 |
6.25a±0.015 |
5.83b±0.011 |
5.18b±0.903 |
5.12b±0.037 |
0.0000 |
|
Caeca |
6.24a±0.163 |
6.42a±0.031 |
6.01b±0.203 |
6.16b±0.013 |
5.91c±0.042 |
0.0006 |
Means in rows carrying different superscripts are significantly different (p<0.05). Values are presented as mean with standard error mean. 1Con, basal diet; ZB-150, basal diet+ zinc bacitracin; OA-200, basal diet+ organic acid; EO-150, basal diet+ essential oil; OA+EO, basal diet + organic acid + essential oil.
Ileal microbial count (log cfu g-1)
Birds under different treatments showed a significant difference in microbial count of Escherichia coli, Salmonella and Lactobacillus both at starter and finisher phase of rearing, (Table V). Escherichia coli and Salmonella was found lowest in birds of group OA-200, E0-150 (6.20, 6.85, 6.10, 6.70, respectively) and OA+EO (5.25, 6.47) compared to birds in ZB-150 and CON group. However, the birds in same treatment groups had improved count of Lactobacillus (8.71, 8.91, 9.78) respectively, compared to other groups ZB-150 (6.16) and CON (7.68).
Nutrient utilization and ileal apparent metabolizable energy (Kcal kg-1)
Table VI depicts findings of the nutrients digestibility and apparent metabolizable energy of all different treatments. It was interesting to note that digestibility of all different nutrients and energy utilization was significantly altered by organic acid, essential oils and or their combination with significant difference among these groups. Protein digestibility was maximum in group OA+EO (84.20%) and EO-150 (82.70%) compared to all groups with no significant difference among groups (ZB-150, OA-200 and CON). Significantly higher apparent metabolizable energy was recorded for groups OA+EO (2853.34 Kcal kg-1) and EO-150 (2821.71 Kcal kg-1), followed by group OA-200 (2756.23 Kcal kg-1) respectively. The antibiotic treated groups ZB-150 had no significant (p>0.05) impact however numerically higher values compared to controlled group.
DISCUSSION
The unrestricted use at sub-therapeutic levels of feed antibiotics as growth promoters may be associated with the development of antibiotic-resistant human pathogens (AGP) and therefore there is tremendous pressure on the poultry feed sector to phase out its use. Poultry experts across the globe are faced with the challenge of finding effective alternatives to replace AGP’s for optimum poultry production. The use of essential oils and organic acids have shown substantial benefits in poultry production over the last few years (Banday et al., 2015). The additive effects of organic acids and essential oils have been observed on the gut health and growth performance in some previous studies (Liu et al., 2017). The variation of the gut microbiota may be the main mode of action linked to the synergic effects of a blend of organic acids and essential oils (Walia et al., 2017). The supplementation of organic acid and essential oil alone or in combination improved body weight gain in starter phase and overall production period in present study. The blend of organic acids and
Table V. Effects of an organic acid blend and micro-encapsulated essential oil individually or in combination on different microbial count (log cfu g-1) of broilers on day 21 and 35.
|
Gut microbe |
CON |
Treatments1 |
p Value |
|||
|
ZB-150 |
OA-200 |
EO-150 |
OA+EO |
|||
|
Day 21 |
||||||
|
Escherichia coli |
6.78a±0.030 |
6.78a±0.030 |
6.20b±0.036 |
6.10b±0.044 |
5.25c±0.042 |
0.0000 |
|
Salmonella |
6.90a±0.094 |
6.91a+0.30 |
6.85b±0.011 |
6.70c±0.094 |
6.47d± 0.077 |
0.0000 |
|
Lactobacillus |
7.68d±0.219 |
6.16d±0.021 |
8.71c±0.060 |
8.91b±0.025 |
9.78a±0.158 |
0.0000 |
|
Day 35 |
||||||
|
Escherichia coli |
7.11a ±0.065 |
7.18a±0.070 |
7.10a±0.056 |
6.83b±0.033 |
6.52c±0.033 |
0.0000 |
|
Salmonella |
6.78a± 0.021 |
6.75a±0.011 |
6.62b±0.014 |
6.61b±0.064 |
6.44c±0.025 |
0.0000 |
|
Lactobacillus |
7.81c±0.065 |
7.73c±0.079 |
8.15ab±0.064 |
8.04b±0.013 |
8.22a±0.021 |
0.0000 |
Means carrying different superscripts are significantly different (p<0.05). Values are presented as mean with standard error mean. 1con, basal diet; ZB-150, basal diet+ zinc bacitracin; OA-200, basal diet+ organic acid; EO-150, basal diet+ essential oil; OA+EO, basal diet + organic acid + essential oil.
Table VI. Effects of an organic acid blend and micro-encapsulated essential oil individually or in combination on nutrients digestibility and apparent metabolizable energy of broilers on day 42.
|
Digestibility % |
CON |
Treatments |
p value |
|||
|
ZB-150 |
OA-200 |
EO-150 |
OA+EO |
|||
|
Dry matter |
76.50c±0.000 |
76.36c±0.261 |
76.30c±0.000 |
78.70b±0.000 |
80.10a±0.000 |
0.0000 |
|
Crude protein |
78.50d±0.000 |
78.66d±0.315 |
79.60c±0.000 |
82.70b±0.000 |
84.20a±0.000 |
0.0011 |
|
Ether extract |
90.40b±0.000 |
90.68b±0.325 |
90.36b±0.266 |
91.70a±0.000 |
91.10a±0.000 |
0.0000 |
|
Energy |
69.61b±0.000 |
72.34a±0.203 |
72.34a±0.210 |
72.45a±0.044 |
72.45a±0.044 |
0.0003 |
|
Nitrogen |
64.39b±0.000 |
67.26a±0.000 |
67.30a±0.000 |
67.24a±0.016 |
67.260a±0.017 |
0.0000 |
|
AME (Kcal kg-1) |
2688.21c±0.417 |
2689.20c±0.183 |
2756.23b±0.408 |
2821.71a±0.395 |
2853.34a±0.705 |
0.0000 |
Means carrying different superscripts are significantly different (p<0.05). Values are presented as mean with standard error mean. 1C, basal diet; ZB-150, basal diet+ zinc bacitracin; OA-200, basal diet+ organic acid; EO-150, basal diet +essential oil; OA+EO, basal diet + organic acid + essential oil.
essential oils improve the body weight gain and feed efficiency at finisher phase of broiler production (Gheisar et al., 2015). A commercial blend of thyme, carvacrol and organic acids improve the feed conversion ratio and body weight gain in broiler chickens (Pham et al., 2020) and is related to present findings. The essential oils increase the permeability of bacterial membrane, which may expedite the influx of organic acid into the cytoplasm (Basmacioglu et al., 2016). It has been observed that dissociated forms of organic acid have the ability to reduce the intestinal pH and disturb the bacterial metabolism (Edgar and Oviado, 2019) that confirms outcomes of present study of reduced pH in organic acid supplemented group. Improved nutrients digestibly and energy utilization in present study could be attributed to a reduction in bacterial metabolism and population of pathogenic bacteria that could potentially promote digestion and nutrient utilization as has been reported previously (Ricke, 2003). A further improvement in the FCR in present study are similar to the finding of an earlier study in which a commercial blend of thyme, carvacrol and organic acids improved feed conversion ratio and body weight gain in broiler chickens (Yang et al., 2019). In the current study the blended organic acid and essential oil led to increase the development of spleen and bursa of Fabricius that indicate a better impact of these additives on lymphoid organs as has been observed previously (Sultan et al., 2015). Reduction in the digesta pH was significantly reduced by the diet containing organic acid and essential oil alone or their combination confer a better gut health and functioning of digestive tract in all different aspects. Ndelekwute et al. (2019) reported similar findings that supplementation of organic acid and essential oil reduce the gut pH in different sections of the gastrointestinal tract of chicken. Organic acid and essential has the ability to reduce the buffering capacity, thus lower the pH of the feed, and facilitate digestion in the intestinal tract (Garcia et al., 2008). Gut microbiota plays an important role for animal health, performance, and product safety. Decreased numbers of pathogenic bacteria in the gut may improve the ability of epithelial cells to regenerate villus and thus enhance intestinal absorption capacity (Zeng et al., 2015). The current results showed that Escherichia coli and Salmonella population were decreased while Lactobacillus at 21- and 35 day-old broilers tended to be increased by encapsulated blends of essential oil and organic acid. The present results are in agreement with the findings of previous researchers (Gao et al., 2019). The essential oil and organic acid have antimicrobial potential which can be used against different pathogenic microorganism (Adewole et al., 2021). Organic acids and their salts decrease the digesta pH and constrains the replication of gram-negative bacteria like Escherichia coli, Salmonella (Rodjan et al., 2018). The decrease in population of pathogenic bacteria by organic acid and essential oil in chickens is associated with the changes produced by a blend of organic acids and essential oils, which may increase the bacterial resistance capacity of the intestine (Stanley et al., 2012). The use of organic acids and essential oils stimulate the pancreatic secretion and increase the gastric retention time, thus improve the nutrient digestion and absorption (Sethiya, 2016; Stamilla et al., 2020). Improvement in production performance, lymphoid organ weight, nutrients utilization and gut health implicate that organic acid and essential oils are more effective in poultry production.
CONCLUSION
The strategic supplementation of organic acids and essential oils either individually or in combination could significantly improve production performance of broiler birds. Moreover, a reduction in gut pH, harmful microbes and improved beneficial microbes indicates its impact as potential positive gut modulator that enable birds to utilize more nutrients from a given feed.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Adewole, D.I., Oladokun, S. and Santin, E., 2021. Effect of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim. Nutr. J., 7: 1039-1051. https://doi.org/10.1016/j.aninu.2021.02.001
AOAC, 2005. Official methods of analysis of AOAC International.18th ed. AOAC Int., Gaithersburg, MD.
Banday, M.T., Adil, S., Khan, A.A. and Madeha, U., 2015. A study on efficiency of Fumeric Acid supplementation in diet of broiler chickens. Int. J. Poult. Sci., 14: 589-594. https://doi.org/10.3923/ijps.2015.589.594
Basmacioglu, H., Ozdemir, P. and Bagriyanik, H.A., 2016. Influence of an organic acid blend and essential oil blend, individually or in combination, on growth performance, carcass parameters, apparent digestibility, intestinal microflora and intestinal morphology of broilers. Br. Poult. Sci., 57: 227-234. https://doi.org/10.1080/00071668.2016.1141171
Canibe, N., Engberg, R.M. and Jensen, B.B., 2001. An overview of the effect of organic acids on gut flora and gut health. Proceeding of the workshop: Alternatives to Feed Antibiotics and Coccidiostats in Pigs and Poultry (AFAC), Norfa network, Norway 2001.
Diarra, M.S. and Malouin, F., 2014. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microb., 5: 282. https://doi.org/10.3389/fmicb.2014.00282
Dibner, J.J. and Buttin, R.J., 2002. Use of organic acids as a model to study the impact of gut microflora in nutrition and metabolism. J. appl. Poult. Res., 11: 453–463. https://doi.org/10.1093/japr/11.4.453
Edgar, O. and Oviedo, R., 2019. Holistic view of intestinal health in poultry. Anim. Feed Sci. Technol., 250: 1-8. https://doi.org/10.1016/j.anifeedsci.2019.01.009
Gao, Y.Y., Zhang, X.L., Xu, L.H., Peng, H., Wang, C.K. and Bi, Y.Z., 2019. Encapsulated blends of essential oils and organic acids improved performance, intestinal morphology, cecal microflora, and jejunal enzyme activity of broilers. Czech. J. Anim. Sci., 64: 189-198. https://doi.org/10.17221/172/2018-CJAS
Garcia, A., Olmo, B., Lopez-Gonzalvez, A., Cornejo, L. and Ruperez, F.J., 2008. Capillary electrophoresis for short chain organic acids in faeces. References values in a Mediterranean elderly population. J. Pharma. Biomed., 46: 356-361. https://doi.org/10.1016/j.jpba.2007.10.026
Gheisar, M.M., Hosseindoust, A. and Kim, I.H., 2015. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chicken’s diet. J. appl. Poult. Res., 24: 511–519. https://doi.org/10.3382/japr/pfv063
Giannenas, I., Papaneophytou, C.P., Tsalie, E., Pappas, I., Triantafillou, E., Tontis, D. and Kontopidis, G.A., 2014. Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. Asian-Austral. J. Anim. Sci., 27: 225–236. https://doi.org/10.5713/ajas.2013.13376
Golkar, Z., Bagasra, O. and Pace, D.G., 2014. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Count., 8: 129–136. https://doi.org/10.3855/jidc.3573
Guide, SUs., 2010. Statistic (Version 9.3). SAS Institute. Inc, Cary, NC, USA; 2010.
Henri, I. and Bassole, N., 2012. Essential oils in combination and their antimicrobial properties. Molecules, 17: 3989–4006. https://doi.org/10.3390/molecules17043989
Huyghebaert, G., Ducatelle, R. and Immerseel, V.F., 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J., 187: 182-188. https://doi.org/10.1016/j.tvjl.2010.03.003
Islam K.M.S., 2012. Use of citric acid in broiler diets. Worlds Poult. Sci. J., 68: 104-118. https://doi.org/10.1017/S0043933912000116
Jarquin, R.L., Nava, G.M., Wolfenden, A.D., Donoghue, A.M., Hanning, I. and Higgins, S.E., 2007. The evaluation of organic acids and probiotic cultures to reduce Salmonella enteriditis horizontal transmission and crop infection in broiler chickens. Int. J. Poult. Sci., 6: 182-186. https://doi.org/10.3923/ijps.2007.182.186
Kazempour, F. and Jahanian, R., 2017. Effects of different organic acids on performance, ileal microflora, and phosphorus utilization in laying hens fed diet deficient in non-phytate phosphorus. J. Anim. Feed. Sci. Technol., 223: 110-118. https://doi.org/10.1016/j.anifeedsci.2016.11.006
Kim, D.K., Lillehoj, H.S., Lee, S.H., Lillehoj, E.P. and Bravo, D., 2013. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr., 109: 76-88. https://doi.org/10.1017/S0007114512000530
Liu, Y., Yang, X., Xin, H., Chen, S and Yang, C., 2017. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J., 88:1414–1424. https://doi.org/10.1111/asj.12782
Markowiak, P. and Slizewska, K., 2018. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut. Pathog., 10: 21. https://doi.org/10.1186/s13099-018-0250-0
Morgan, N.K., 2017. Managing gut health without reliance on antimicrobials in poultry. Anim. Prod. Sci., 7: 2270–2279. https://doi.org/10.1071/AN17288
Ndelekwute, E.K., Unah, U.L. and Udoh, U.H., 2019. Effect of dietary or- ganic acids on nutrient digestibility, faecal moisture, digesta pH and viscosity of broiler chickens. MOJ Anat. Physiol., 6: 40–43. https://doi.org/10.15406/mojap.2019.06.00242
Neill, J., 2016. Tackling drug-resistant infections globally: Final report and recommendations. In: Review of antimicrobial resistance. HM Government and Wellcome trust, London.
Omonijo, F.A., Ni, L., Gong, J., Wang, Q. and Lahaye, L., 2018. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr., 4: 126–136. https://doi.org/10.1016/j.aninu.2017.09.001
Paul, S.K., Halder, G., Mondal, M.K. and Samanta, G., 2007. Effect of organic acid salt on the performance and gut health of broiler chicken. J. Poult. Sci., 44: 389-395. https://doi.org/10.2141/jpsa.44.389
Pham, V.H., Kan, L., Huang, J., Geng, Y. and Zhen, W., 2020. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotech., 11: 1-18. https://doi.org/10.1186/s40104-019-0421-y
Pourmand, A., Amirshahi, M.M., Jasani, G. and May, L., 2017. Emerging trends in antibiotic resistance: Implications. Emerg. Medic., 35: 1172-1176. https://doi.org/10.1016/j.ajem.2017.03.010
Ricke, S.C., 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci., 82: 632–639. https://doi.org/10.1093/ps/82.4.632
Rinttila, T. and Apajalaht, J., 2013. Intestinal microbiota and metabolites Implications for broiler chicken health and performance. J. appl. Res., 22: 647-658. https://doi.org/10.3382/japr.2013-00742
Rodjan, P., Soisuwan, K., Thongprajukaew, K., Theapparat, Y. and Khongthong, S., 2018. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens. J. Anim. Physiol. Anim. Nutr., 102: 931–940. https://doi.org/10.1111/jpn.12858
Sethiya, N.K., 2016. Review on natural growth promoters available for improving gut health of poultry: An alternative to antibiotic growth promoters. Asian J. Poult. Sci., 10: 1–29. https://doi.org/10.3923/ajpsaj.2016.1.29
Stamilla, A., Messina, A., Sallemi, S., Condorelli, L. and Antoci, F., 2020. Effects of microencapsulated blends of organics acids (oa) and essential oils (eo) as a feed additive for broiler chicken. A focus on growth performance, gut morphology and microbiology. Animals, 10: 442. https://doi.org/10.3390/ani10030442
Stanley, D., Denman, S., Hughes, R.J., Geier, M. and Crowley, T., 2012. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotech., 96: 1361-1369. https://doi.org/10.1007/s00253-011-3847-5
Stefanello, C., Rosa, D.P., Dalmoro, Y.K., Segatto, A.L. and Vieira, M.S., 2020. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci., 6: 491. https://doi.org/10.3389/fvets.2019.00491
Stevanovic, Z.D., Bosnjak, J. and Lijakovic, R., 2018. Essential oils as feed additives future perspectives. Molecules, pp. 23. https://doi.org/10.3390/molecules23071717
Sultan, A., Khan, S., Khan, S.Z., Chand, N., Khan, M.S. and Maris, H., 2018. Effect of organic acid blend on carcass yield, nutrient digestibility and tibia ash during starter phase of broiler chicks. Pakistan J. Zool., 50: 1483-1488. https://doi.org/10.17582/journal.pjz/2018.50.4.1483.1488
Sultan, A., Ullah, T., Khan, S. and Khan, R.U., 2015. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period. Pakistan J. Zool., 47: 635–639.
Swamy, M.K., Akhtar, M.S. and Sinniah, U.R., 2016. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med., https://doi.org/10.1155/2016/3012462
Walia, K., Argüello, H., Lynch, H., Leonard, F.C. and Grant, J., 2017. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med., 137: 28-35. https://doi.org/10.1016/j.prevetmed.2016.12.007
Wang, X., Peebles, E.D., Kiess, A.S., Wamsley, K.G.S. and Zhai, W., 2019. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poult. Sci. J., 98: 2054–2065. https://doi.org/10.3382/ps/pey552
Williams, C.H., David, D.J. and Lismaa, O., 1962. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agric. Sci., 59: 381-385. https://doi.org/10.1017/S002185960001546X
World Health Organization, 2014. Antimicrobial resistance: Global report on surveillance. 2014. Geneva, Switzerland: WHO; 2014.
Yang, X., Li, W., Feng, Y. and Yao, J., 2011. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci., 90: 2740- 2746. https://doi.org/10.3382/ps.2011-01591
Yang, X., Xin, H., Yang, C. and Yang, X., 2018. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr., 4: 388-393. https://doi.org/10.1016/j.aninu.2018.04.005
Zeng, Z., Zhang, S., Wang, H. and Piao, X., 2015. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotech., 6: 7. https://doi.org/10.1186/s40104-015-0004-5
Zhai, H., Liu, H., Wang, S. and Kluenter, J., 2018. Potential of essential oils for poultry and pigs. Anim. Nutr., 4: 179–186. https://doi.org/10.1016/j.aninu.2018.01.005
To share on other social networks, click on any share button. What are these?









